In the preparation of primary amines, how can direct nucleophilic substitution between nh3 and alkyl halide be made more practical than reacting nh3 and the alkyl halide in a 1:1 ratio?
Answers
Direct nucleophilic substitution between NH₃ and alkyl halide be made more practical than reacting NH₃ and the alkyl halide in a 1:1 ratio By Using a large excess of NH₃.
Why is direct nucleophilic substitution of an haloalkane with NH₃ not a very useful method for preparing primary amines?
Polyalkylation of the amine will end in multiple products
What happens when haloalkane reacts with NH₃?Ch₂₂: RX + NH₃. Ammonia reacts as a nucleophile with alkyl halides to offer primary amines in a nucleophilic substitution reaction. Yields are often poor because the product, a primary amine, RNH₂, is itself a nucleophile and may react with more alkyl halide.
Is NH₃ a robust nucleophile?
NH₃(Ammonia) has the stronger nucleophilic character than water molecule. Because the Oxygen within the water molecule doesn't likely to bond with any carbon atom compared to Nitrogen atom. Since Oxygen has more electronegativity than Nitrogen. Hence it holds onto its lone pair tightly than Nitrogen.
Learn more about substitution reaction :
brainly.com/question/10143438
#SPJ4
Related Questions
LOTS OF POINTS (75) I WILL GIVE BRAINLIEST!!!!!
Whats the Difference between a chemical and physical change
Answers
Answer:
In a physical change the appearance or form of the matter changes but the kind of matter in the substance does not. However in a chemical change, the kind of matter changes and at least one new substance with new properties is formed. The distinction between physical and chemical change is not clear cut.
Explanation:
A copper rod connects two insulated beakers that contain liquids. The temperature of the liquid in Beaker A is 85°C and
the temperature in Beaker B is 15°C. Over the next 60 seconds, what will occur?
Answers
Explanation:
Over the next 60 seconds, the water in the beaker will try to attain thermal equilibrium by bringing the temperature in both beaker to the same level.
Heat generally moves over a gradient from a place of higher temperature to one with lower temperature. The copper rod is a metallic conductor. Heat will flow from the beaker at 85°C to the beaker at 15°CDuring the passage of time, the liquids in both beaker will reach thermal equilibrium and the temperature will be the same.why does the sloth have both a scientific name and common name
Answers
Choose the BEST answer from the four options A, B, C, or D: Due to which property of LPG are we asked to close the valve of the gas cylinder, in the kitchen in our homes? A. It is inflammable B. It is a liquid C. It has a bad smell D. It is a mixture
Answers
Answer:
A
Explanation:
We are usually asked to close the valve of the gas cylinder in our various kitchens at home due to the inflammable property of the Liquified Petroleum Gas.
Without closing the valve, the LPG would diffuse into our homes, and any form of spark would cause an explosion and lead to a fire. Lives and properties could be lost in the process.
The correct option is A.
The term hydrate can be applied to ionic compounds thatA. place hydrogen in the last formulaB. contains trapped water moleculesC. is not reduced down to its simplest formulaD. can be represented by both the stock system and prefix systems of nomenclature
Answers
ANSWER
The term hydrate contains trapped water molecules
STEP-BY-STEP EXPLANATION:
The term hydrate means a substance that contains water molecules
It also means that water is loosely bonded to the ionic compound
According to the options provided, The correct answer is option B ( contains trapped water molecules
hydrochloric acid can be prepared by dissolving hydrogen chloride gas in water. assuming ideal behavior, how many liters hcl gas are required to make concentrated hydrochloric acid (11.6 mol/l) at 25oc and 1 atm pressure?
Answers
The volume of HCl gas required to prepare 11.6 moles/liter of concentrated hydrochloric acid is 26.99 litres.
The volume of hydrochloric acid (HCl) gas required to prepare a certain concentration of hydrochloric acid,calculate the moles of HCl required.
The concentration of hydrochloric acid is given as 11.6 moles/liter.
Therefore, for a given volume of the solution, we can calculate the number of moles of HCl required by multiplying the volume of the solution (V) by 11.6 moles/liter.
Using the Ideal Gas Law, we can calculate the volume of HCl gas (Vg) required to produce the desired concentration of HCl:
Vg = (nRT) / P
Where:
n = number of moles of HCl (calculated above)
R = gas constant (0.0821 L atm/mol K)
T = temperature (25 °C = 298 K)
P = pressure (1 atm)
Therefore, the volume of HCl gas required to prepare 11.6 moles/liter of concentrated hydrochloric acid is:
Vg = (11.6 * 0.0821 * 298) / 1 = 26.99 liters
In conclusion, to prepare a concentrated hydrochloric acid solution of 11.6 moles/liter at 25 °C and 1 atm pressure, you need to dissolve 26.99 liters of HCl gas in water.
to know more about hydrochloric acid refer here:
https://brainly.com/question/15231576#
#SPJ11
3. Ammonium hydroxide, known for its pH control abilities, is used in food production as an effective antimicrobial agent. What is the correct formula for ammonium hydroxide?
Answers
Ammonium is NH4+
Hydroxide is OH-
The two react in a one to one ratio to form the compound.
The study of chemicals and bonds is called chemistry.
The correct answer is \(NH_4(OH)\).
Ammonium hydroxide is the base that helps to increase the ph of the solution and helps to neutralize the acidic effect.
The uses of ammonium hydroxide are as follows:-
Reduce the acidic effectIn bakeryIn medicineThese are the use of ammonium hydroxide.
Hence, the correct answer is \(NH_4(OH)\).
For more information, refer to the link:-
https://brainly.com/question/12985618
A 67.2 g sample of a gold and palladium alloy contains 3.45×1023 atoms .
a) What is the mass percentage of the gold in the alloy?
b) What is the mass percentage of the palladium in the alloy?
Answers
a) Mass percentage of gold in the alloy = 68.75% and,
b) Mass percentage of palladium in the alloy = 31.25%
a) Mass percentage of gold in the alloy Formula for mass percentage is :Mass % of gold = mass of gold / mass of alloy × 100Now, we have to find the mass of gold in the alloy. Let's assume the mass of gold be x g .Given, the mass of alloy = 67.2 g Therefore, the mass of palladium in the alloy = 67.2 - x g Number of gold atoms = 3.45 × 1023 atoms Number of palladium atoms = Total number of atoms - Number of gold atoms = 3.45 × 1023 - Number of gold atoms .
Formula for the number of atoms is:
Number of atoms = (number of moles of atoms) × Avogadro’s number Number of moles of atoms = Number of atoms / Avogadro’s number Avogadro’s number = 6.022 × 1023Number of gold atoms = (mass of gold / molar mass of gold) × Avogadro’s number Number of palladium atoms = (mass of palladium / molar mass of palladium) × Avogadro’s number Given, mass of alloy = mass of gold + mass of palladium + mass of other elements Now, mass of other elements = mass of alloy - (mass of gold + mass of palladium)
b) Mass percentage of palladium in the alloy Formula for mass percentage is: Mass % of palladium = mass of palladium / mass of alloy × 100Therefore,Mass % of palladium = (mass of palladium / (mass of gold + mass of palladium)) × 100.
To learn more about Mass percentage visit
https://brainly.com/question/7730336
#SPJ11
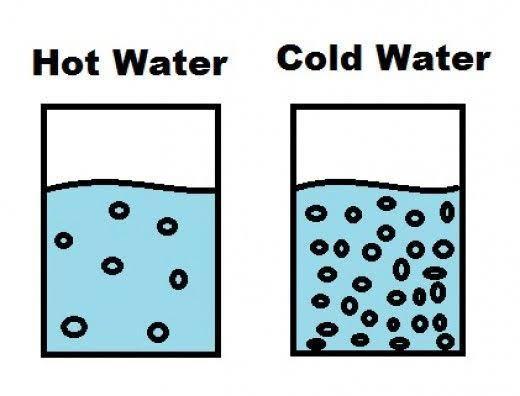
3- You saw a jar of hot water placed upside down over a jar of cold water. The hot
water stayed on top of the cold water without mixing. Why did the hot water stay on
top of the cold water?
Answers
Hot water stays on the top of cold water and doesn't mix. This is because of difference in DENSITY of water at different temperatures.
When we heat water, then the atoms gain energy from the heat and start moving away from each other. Now, when the atoms move away from each other than the (mass per unit volume) i.e. the density decreases. This makes water lighter.
When the temperature is less i.e. cold water then the atoms are more compactly packed and therefore the density of water is more at lower temperatures.
Since, hot water is lighter than cold water that is why it stays on top of the cold water.
which of the following types of radiation causes the formation of damaging chemical bonds in dna?
Answers
The type of radiation that causes the formation of damaging chemical bonds in DNA is ionizing radiation. This type of radiation has enough energy to remove an electron from an atom or molecule, creating charged particles called ions.
These ions can then react with the atoms in DNA, forming chemical bonds that can cause damage such as breaks in the DNA strands or changes to the DNA sequence. This can lead to mutations, cell death, or even the development of cancer. It's important to limit exposure to ionizing radiation, which can come from sources such as X-rays, gamma rays, and radioactive materials.
The type of radiation that causes the formation of damaging chemical bonds in DNA is ionizing radiation. Ionizing radiation includes alpha particles, beta particles, gamma rays, and X-rays.
This type of radiation can remove tightly bound electrons from atoms, creating ions that can form harmful chemical bonds and lead to DNA damage, increasing the risk of mutations and potential health problems.
To know more about DNA, refer
https://brainly.com/question/21992450
#SPJ11
The reaction a(g)⇌b(g) has an equilibrium constant of 5.8 and under certain conditions has q = 336. part a what can you conclude about the sign of δg∘rxn and δgrxn for this reaction under these conditions?
Answers
Answer:
The answer is "As \(Q=336\), at high-temperature \(\Delta G_{rxn}>0\) and When\(K>1,\)\(\Delta G^{\circ}_{rxn}>0\)."
Explanation:
The equation for the reaction is:
\(A(g) \leftrightharpoons B(g)\)
\(K=5.8\\\\Q=336\)
At equilibrium,
\(\Delta G^{\circ}_{rxn}>0\)\(=-RT \ln \ K\)
When k=5.8(>1), the value of \(\ln k\) would be positive
So, \(\Delta G^{\circ}_{rxn}\) is negative (< 0)
So if K > l, \(\Delta G^{\circ}_{rxn}<0\)
If the reaction is not in equilibrium so the equation is :
\(\Delta G_{rxn}>0\)=\(\Delta G^{\circ}_{rxn}\)\(+RT \ln Q\)
Substituting the expression:
\(\Delta G_{rxn}>0\)\(= (-RT \ln K) + RT \ln Q\)
\(= RT(\ln Q- \ln K)\\= RT(\ln (336)-\ln (5.8))\\= RT(4.06)\)
It is the positive value for all temperatures.
So, As Q = 336, at the high temperature \(\Delta G_{rxn}>0\).
How will you calculate the partial pressure of gas using Dalton's Law of partial pressures?
Answers
The overall pressure exerted by a mixture of non-reactive gases is equal to the sum of the partial pressures of the individual gases, according to Dalton's Law, also known as Dalton's Law of Partial Pressures.
The partial pressure law of Dalton states that
The sum of the partial pressures of the various gases determines the overall pressure that a mixture of gases exerts.
mathematical formula:
P(total) = P1 + P2 +... P (n)
P1 = One gas's partial pressure
P2 is the second gas's partial pressure.
Pn is the partial pressure of n gases.
so to find the patial pressure can be cal culate as follows
P (n)= P(total) - P1 + P2+....
Learn more about dalton's law at https://brainly.com/question/14119417
#SPJ4
Which of the following statements must be true for a spontaneous process? Choose all that apply:
ΔGsys < 0
ΔSuniv > 0
ΔSsys > 0
ΔHsys - TΔSsys < 0
ΔHsys < 0
Answers
When we talk about the spontaneous process the following is considered true
ΔSsys > 0 is true for a spontaneous processΔHsys - TΔSsys < 0 is true under the condition temperature and pressure is constantA spontaneous process refers to the working of a reaction that doesn't require any input from the external environment to facilitate the current system. It is considered an irreversible process and can only be brought back to its prime state using the help of some external agents.
some well-known examples of spontaneous processes are
Formation and initiation of cloudsIntermixing of two or more two gasesEvaporation of water in the season of summerRusting of ironMelting of ice at a room temperatureTo learn more about spontaneous processes,
https://brainly.com/question/2855292
#SPJ4
Why were chlorofluorocarbons first developed?
Answers
I need help with this. Thank youuuuuuu

Answers
The number of atoms in 3 moles K of the particle is 1.806 x 10²⁴ atoms.
What is the number of atoms in the given moles?
The value of one mole of an atom is equal to exactly 12 grams of pure carbon-12.
12.00 g (C-12 ) = 1 mol C-12 atoms = 6.02 × 10²³ atoms .
The number of particles in 1 mole is called Avogadro's Number (6.0221421 x 10²³).
The number of atoms in 3 moles K of the particle is calculated as follows;
= 3 moles x 6.02 × 10²³ atoms / mol
= 1.806 x 10²⁴ atoms
Learn more about number of atoms here: https://brainly.com/question/6258301
#SPJ1
how many moles are in 3.67g of s
Answers
Answer:
32.07 g
Explanation:
One atom of sulfur has a mass of 32.07 amu; one mole of S atoms has a mass of 32.07 g.
32.07 grams
How much heat must be transferred to 55 g of ice to change the ice's
temperature from -13°C to -5.0°C? (The specific heat capacity of ice is 2.11
J/g.°C)
Answers
Q= m x c x t
M= 55 g
C=2.11
T=8
You have a mixture of the gases Cl₂ and O₂ along with some N₂ in a container at STP. The moles of Cl₂ and O₂ in the mixture are equivalent and the density of the mixture is 2.063 g/L. Assuming ideal behavior, what is the mole fraction of N₂ in the mixture?
Answers
The mole fraction of N₂ in the mixture, given the data is 0.225
How to determine the mass of the mixtureDensity of mixture = 2.063 g/LVolume (at STP) = 22.4 LMass of mixture =?Density = mass / volume
Cross multiply
Mass = Density × volume
Mass of mixture = 2.063 × 22.4
Mass of mixture = 46.2112 g
How to determine the mole fraction of N₂We'll begin by calculating the mole of N₂ in the mixture. This can be obtained as follow:
Mass of mixture = 46.2112 gLet the mole of Cl₂ = yLet the mole of O₂ = Mole of Cl₂ = yTotal mole = 1 moleLet the mole of N₂ = x =?Total mole = Mole of N₂ + Mole of Cl₂ + Mole of O₂
1 = x + y + y
1 = x + 2y
2y = 1 - x
y = (1 - x) / 2
Mass of mixture = mass of N₂ + mass of Cl₂ + mass of O₂
Recall
mass = mole × molar mass
Thus,
Mass of mixture = (mole of N₂ × molar mass) + (mole of Cl₂ × molar mass) + (mole of O₂ × molar mass)
46.2112 = 28x + 71y + 32y
But
y = (1 - x) / 2
46.2112 = 28x + 71(1 - x) / 2 + 32(1 - x) / 2
Multiply through by 2
92.4224 = 56x + 71(1 - x) + 32(1 - x)
92.4224 = 56x + 71 - 71x + 32 - 32x
Collect like terms
92.4224 - 71 - 32 = 56x - 71x - 32x
-10.5776 = -47x
Divide both sides by -47
x = -10.5776 / -47
x = 0.225 mole
Finally, we can determine the mole fraction of N₂. This can be obtained as follow:
Mole of N₂ = x = 0.225 moleTotal mole = 1 moleMole fraction of N₂ = ?Mole fraction = mole / total mole
Mole fraction of N₂ = 0.225 / 1
Mole fraction of N₂ = 0.225
Learn more about mole fraction:
https://brainly.com/question/2769009
#SPJ1
How many moles of kbr will be produced from 7 moles of babr2.
Answers
When 7 moles of BaBr2 is reacted with excess K2SO4, how many moles of KBr are produced?BaBr2 + K2SO4 → 2KBr + BaSO4Mass of BaBr2 = 7 × molar mass of BaBr2 = 7 × (137.33 + 2 × 79.90) g mol−1 = 1375.31 gMass of K2SO4 required to react with 7 moles of BaBr2 = 7 × molar mass of K2SO4 = 7 × (39.10 + 2 × 32.06) g mol−1 = 847.42 g.
We know that K2SO4 is in excess, so it doesn't get used up in the reaction. The limiting reactant here is BaBr2.Therefore, the number of moles of BaBr2 = 7 moles and the number of moles of KBr = 2 × 7 = 14 moles.
Molar mass of KBr = 39.10 + 79.90 g mol−1 = 119.00 g mol−1Mass of 14 moles of KBr = 14 × 119.00 g mol−1 = 1666 gThe answer to the problem is that 14 moles of KBr will be produced from 7 moles of BaBr2.
To know more about reacted visit:
https://brainly.com/question/14168723
#SPJ11
which basic postulates of Dalton's atomic theory are modified by the modern atomic theory
Answers
Answer:
Dalton's atomic theory has been modified as
Explanation:
Atoms of same element may not have same atomic mass. For example, some atoms of chlorine have atomic mass 35 amu while others have atomic mass 37 amu. Such atoms of same element having different atomic masses are called isotopes
Answer:
On the basis of new experimental facts, Dalton's atomic theory has been modified as: ... Atoms of same element may not have same atomic mass. For example, some atoms of chlorine have atomic mass 35 amu while others have atomic mass 37 amu. Such atoms of same element having different atomic masses are called isotopes.
Explanation:
Plz mark brainliest thanks
A cycle of moon phases can be seen from earth because the
Answers
the Sun light from different parts of the Moon as the Moon revolves around the Earth
Analyzing a company's _______ ratio is one way in which an investor can tell if the company can pay off its short-term debts if there is a drop in sales revenue. acid test activity current leverage (debt)
Answers
Analyzing a company's "acid test" ratio is one way in which an investor can tell if the company can pay off its short-term debts if there is a drop in sales revenue.
The acid test ratio, also known as the quick ratio, measures a company's ability to meet its immediate financial obligations without relying on the sale of inventory. It is calculated by dividing the sum of a company's cash, cash equivalents, and accounts receivable by its current liabilities. A higher acid test ratio indicates a greater ability to cover short-term debts, which is particularly important during times of reduced sales revenue.
learn more about:- acid test here
https://brainly.com/question/30764975
#SPJ11
I need help with the questions in the picture. Please show workings.

Answers
Explanation:
you need to review these formulas

which has greater dissolved oxygen, lake water or groundwater? why? hint: think about the atmosphere. more dissolved oxygen: [ select ] why?: [ select ]
Answers
Lake water typically has a higher dissolved oxygen content compared to groundwater. This difference can be attributed to their exposure to the atmosphere and various factors that influence oxygen dissolution.
Lake water is directly exposed to the atmosphere, allowing it to absorb oxygen more effectively. The air-water interface at the surface of the lake facilitates the diffusion of oxygen from the atmosphere into the water. Additionally, natural processes like photosynthesis by aquatic plants, as well as wind and wave action, contribute to the mixing of oxygen within the lake water.
On the other hand, groundwater is found below the Earth's surface, typically within soil pore spaces and rock formations. Due to its subsurface location, groundwater has limited exposure to the atmosphere. As a result, the oxygen diffusion process is less efficient in groundwater than in lake water. Moreover, since groundwater moves relatively slowly through soil and rock layers, it has less opportunity to be replenished with oxygen from the atmosphere. Some dissolved oxygen in groundwater can be consumed by microorganisms or used in geochemical processes, further reducing its oxygen content.
In summary, lake water generally has a greater dissolved oxygen content than groundwater due to its direct exposure to the atmosphere, which enables efficient oxygen diffusion, as well as natural mixing processes that facilitate oxygen distribution in the water. In contrast, groundwater's subsurface location and limited interaction with the atmosphere result in lower dissolved oxygen concentrations.
To learn more about dissolved oxygen, refer:-
https://brainly.com/question/26113345
#SPJ11
INACCURATE STATEMENT: At the time of the big bang, all the matter and energy in the universe was in a tiny corner of space. Since then, it has expanded to fill up the whole universe.
Choose why this statement is inaccurate using the EVIDENCE that refutes it (proves it wrong).
1 EVIDENCE: Scientists believe the temperature of the universe immediately after the big bang was 100 billion *C. Today, the temperature of the universe is -275*C.
2 EVIDENCE: Scientists believe the very first galaxies began forming about 1 billion years after the big bang.
3 EVIDENCE: Blue light has shorter wavelengths than red light.
4 EVIDENCE: Scientists have observed galaxies are moving away from us.
5 EVIDENCE: The big bang marks the beginning of space and time.
choose only one
Answers
The evidence that refutes the statement is: 4 EVIDENCE: Scientists have observed galaxies are moving away from us.
According to the observations made by astronomers, galaxies in the universe are not only moving away from each other, but they are also moving away from us.
This phenomenon is known as the expansion of the universe, and it contradicts the idea that all matter and energy in the universe was initially concentrated in a tiny corner of space during the time of the big bang and has since filled up the entire universe.
The observation that galaxies are moving away from us suggests that the universe is expanding in all directions. This expansion implies that the universe was not initially confined to a specific location but rather underwent a rapid expansion from a highly dense and hot state.
Therefore, the idea that all matter and energy in the universe was initially concentrated in a small corner of space and then expanded to fill up the whole universe is inaccurate based on the evidence of the observed expansion of galaxies. Evidence 4
For more such questions on galaxies visit:
https://brainly.com/question/29357062
#SPJ8
Help me
Do this for me ???

Answers
Answer:
I was just wondering if you want to be a good day at work and I don't know the answer sorry
One mole of an ideal monatomic gas initially at 300 K and a pressure of 15 atm expands to a final pressure of 1 atm. The expansion can occur via any one of four different paths: 1. isothermal and reversible, 2. isothermal and irreversible, 3. adiabatic reversible, and 4. adiabatic irreversible. In irreversible processes, the expansion occurs against an external pressure of 1 atm. for each case calculate the values of q, w, ΔU, and ΔH. Please show all work trying to understand how everything interplays with each other.
Answers
One mole of an ideal monatomic gas initially at 300 K and a pressure of 15 atm expands to a final pressure of 1 atm. The expansion can occur via any one of four different paths: 1. isothermal and reversible, 2. isothermal and irreversible, 3. adiabatic reversible, and 4. adiabatic irreversible.
In irreversible processes, the expansion occurs against an external pressure of 1 atm. For each case, we can calculate the values of q, w, ΔU, and ΔH using the following equations:
1. Isothermal and reversible:
q = -w = nRTln(V2/V1)
ΔU = 0 (since the temperature is constant)
ΔH = 0 (since the enthalpy is a function of temperature)
2. Isothermal and irreversible:
q = -w = nRTln(V2/V1)
ΔU = 0 (since the temperature is constant)
ΔH = 0 (since the enthalpy is a function of temperature)
3. Adiabatic reversible:
q = 0 (since the process is adiabatic)
w = -nCvΔT = -nCv(T2-T1)
ΔU = nCvΔT = nCv(T2-T1)
ΔH = nCpΔT = nCp(T2-T1)
4. Adiabatic irreversible:
q = 0 (since the process is adiabatic)
w = -PextΔV = -Pext(V2-V1)
ΔU = nCvΔT = nCv(T2-T1)
ΔH = nCpΔT = nCp(T2-T1)
Using these equations, we can plug in the given values for n, R, T1, P1, P2, V1, and V2 to calculate the values of q, w, ΔU, and ΔH for each case. Note that for an ideal monatomic gas, Cv = 3/2R and Cp = 5/2R.
To know more about isothermal click on below link:
https://brainly.com/question/12023162#
#SPJ11
two. Power Generation Type Nuclear Geothermal Renewable or Non-renewable? paring the Reason For Choice 21. Find the energy released or absorbed when one mole of helium-4 is produced in this reaction. Show your work including any formulas use, substitutions, and record your answer to the correct number of significant digits including the appropriate unit. Also include whether the reaction released or absorbed energy. He + H He + H+ 'n -
Answers
The energy released when one mole of helium-4 is produced in the reaction H + H → He + H⁺ + n is approximately -3.598 × 10⁻¹² Joules per mole.
The reaction of producing one mole of helium-4 (He) from a hydrogen atom (H) and a hydrogen ion (H⁺) with the release of a neutron (n) can be represented as:
H + H → He + H⁺ + n
The energy released or absorbed in this reaction can be calculated using the mass defect method.
The mass of one mole of helium-4 (He) is approximately 4.0026 g/mol, and the mass of one mole of a hydrogen atom (H) and a hydrogen ion (H⁺) is approximately 1.0078 g/mol each. The mass of a neutron (n) is approximately 1.0087 g/mol.
Using these values, we can calculate the energy released or absorbed:
Mass of reactants = 2 × (1.0078 g/mol) = 2.0156 g/mol
Mass of products = 4.0026 g/mol + 1.0078 g/mol + 1.0087 g/mol = 6.0191 g/mol
Mass defect = Mass of reactants - Mass of products = 2.0156 g/mol - 6.0191 g/mol = -4.0035 g/mol
Energy released or absorbed (E) = Mass defect × (c²)
E = -4.0035 g/mol × (2.998 × 10⁸ m/s)²
E ≈ -3.598 × 10⁻¹² J/mol
learn more about mass defect here:
https://brainly.com/question/11624098
#SPJ4
what are thermosetting plastics???
Answers
Explanation:
Thermosetting plastic are made up of long chain of molecules which are cross-linked and can be moulded into different shape and size.
Answer:
Hey!
THERMOSETTING PLASTICS are plastics or polymers that become IRREVESIBLY SET/RIGID when it has been heated and cooled...
Explanation:
This means that if you heat up a thermosetting plastic, you only have one chance to mould it or shape it before it cools down...if not you cannot correct this and will have to start again! Which WILL be sad!
AKA : THERMOSET
HOPE THIS HELPS!!
What is the pH of a solution of 0. 25M K3PO4, potassium phosphate? Given
Ka1 = 7. 5*10^-3
Ka2 = 6. 2*10^-8
Ka3 = 4. 2*10^-13
I know there is another post here with the same question but nobody explained anything. Where does the K3 go? Why does everyone I see solve this just ignore it and go to H3PO4?
Answers
The pH of a 0.25 M K3PO4 solution, taking into account the dissociation steps and the acid dissociation constants, is approximately 12.17.
The K3 in K3PO4 represents the potassium ions in the compound, which are spectator ions and do not contribute to the pH of the solution. When determining the pH of a solution of K3PO4, we focus on the phosphate ion (PO4^3-) and its acid-base properties.
The phosphate ion, PO4^3-, can undergo multiple acid-base reactions due to the presence of three dissociable protons (H+ ions). Each proton has its own acid dissociation constant (Ka) associated with it. In this case, we have three Ka values: Ka1, Ka2, and Ka3.
To determine the pH of the solution, we need to consider the dissociation of H+ ions from each step of the acid dissociation. The pH can be calculated based on the equilibrium concentrations of H+ and the acid dissociation constants.
The dissociation reactions for the three steps are as follows:
Step 1: H3PO4 ⇌ H+ + H2PO4-
Step 2: H2PO4- ⇌ H+ + HPO4^2-
Step 3: HPO4^2- ⇌ H+ + PO4^3-
The concentration of H+ ions from each step will depend on the initial concentration of K3PO4 and the relative magnitudes of the Ka values.
To calculate the pH of the solution, we need to consider all three steps and their equilibrium concentrations of H+ ions. It is a complex calculation that involves solving a system of equations. Here, I will provide you with the final result:
The pH of a 0.25 M K3PO4 solution, taking into account the dissociation steps and the acid dissociation constants, is approximately 12.17.
Learn more about dissociation constants here
https://brainly.com/question/28197409
#SPJ11