Answers
Answer:
atomic number =17
mass number = 17+18= 35
Related Questions
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
where is Allahabad ?
Answers
Answer: Prayagraj
Uttar Pradesh, India
i hope this helps :) Can i get brainiest ?
Answer:
Prayagraj
Explanation:
Uttar Pradesh, India
pls help I’ll give brainly

Answers
Please help me do this

Answers
The total mass of the balloon and its content is 1521.17 g, the number of moles of CO₂ in the balloon is 34.15 mol, and the number of CO₂ molecules in the balloon is 2.06 x 10²⁵ molecules.
a) The molar mass of CO₂ is 44.01 g/mol. To find the total mass of the balloon and its content, we need to add the mass of the balloon (20g) to the mass of the CO₂ inside the balloon.
Mass of CO₂ = number of moles of CO₂ x molar mass of CO₂
Since the balloon is at STP (standard temperature and pressure), we can use the molar volume of a gas at STP (22.4 L/mol) to find the number of moles of CO₂ in the balloon:
Volume of CO₂ = Volume of balloon = 765 L (at STP)
Number of moles of CO₂ = volume of CO₂ / molar volume of a gas at STP
= 765 L / 22.4 L/mol
= 34.15 mol
Mass of CO₂ = 34.15 mol x 44.01 g/mol
= 1501.17 g
Total mass of balloon and its content = 20 g + 1501.17 g
= 1521.17 g
b) Number of moles of CO₂ in the balloon is 34.15 mol
c) To find the number of CO₂ molecules in the balloon, we need to use Avogadro's number (6.02 x 10²³ molecules/mol).
Number of CO₂ molecules = number of moles of CO₂ x Avogadro's number
= 34.15 mol x 6.02 x 10²³ molecules/mol
= 2.06 x 10²⁵ molecules
To know more about the Mass, here
https://brainly.com/question/22104139
#SPJ1
10. When dissolved in water, most Group 1 metal salts can be described as
strong electrolytes.
strong acids.
weak electrolytes.
A
B
C
D
non-electrolytes.
(1)
Answers
When dissolved in water, most Group 1 metal salts can be described as strong electrolytes.
When Group 1 metal salts are dissolved in water, they can be described as strong electrolytes. This is because Group 1 metals, such as lithium (Li), sodium (Na), potassium (K), and so on, readily lose their outermost valence electron to form positive ions (cations). These cations then dissociate completely in water, separating from the anions to which they were originally bonded.
The dissociation of Group 1 metal salts in water results in the formation of positively charged metal ions and negatively charged non-metal ions (anions). These ions are free to move and conduct electric current, making the solution a good conductor of electricity. The complete dissociation of Group 1 metal salts in water and the presence of freely moving ions make them strong electrolytes.
Strong electrolytes are substances that ionize completely or almost completely in solution, producing a high concentration of ions. This is in contrast to weak electrolytes, which only partially ionize and produce a lower concentration of ions.
In summary, when Group 1 metal salts are dissolved in water, they form strong electrolytes due to their ability to dissociate completely into ions, leading to a high concentration of freely moving ions in the solution, thus enabling efficient electrical conductivity.
Know more about Group 1 metal salts here:
https://brainly.com/question/13277375
#SPJ8
What is the answer? Please
Answers
Answer:
Carbon Dioxide
Explanation:
Because plants take in Carbon Dioxide, then let out Oxygen
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
Select the correct terms to complete this statement about charged particles.
Like charges attract | repel, and opposite charges attract repel. According to Coulomb's law, as the distance between two charged particles decreases, the force between the particles decreases I increases. As the magnitude of the charges decreases, the force decreases | increases.
Answers
Like charges repel each other, while opposite charges attract each other. This principle is one of the fundamental aspects of electrostatics. According to Coulomb's law, the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
As the distance between two charged particles decreases, the force between them increases. This is because the closer the particles are, the stronger the electric field they create, leading to a stronger force of interaction.
On the other hand, as the magnitude of the charges decreases, the force between the particles also decreases. This is because the force is directly proportional to the product of the charges. If one or both of the charges are smaller, the force they exert on each other will be weaker.
In summary, according to Coulomb's law, decreasing the distance between charged particles increases the force between them, while decreasing the magnitude of the charges decreases the force. This understanding of the relationship between charge, distance, and force is crucial in explaining the behavior of charged particles and the interactions between them.
Know more about Coulomb's law here:
https://brainly.com/question/26892767
#SPJ8
Why do we study chemistry?
Answers
Answer:
The study of chemistry provides global work opportunities.
Explanation:
Answer:
2 answers.
Explanation:
1. The study of chemistry provides global work opportunities. Chemistry underpins understanding and progress in almost every science, technology, and industry sphere. It also makes a vital contribution to the economy, commerce and industry.
2. Because teachers want us to be bored and make time feel like it's stuck in honey.
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
What are the coefficients when the following reaction is properly balanced?
Si4C3 +o2 -> si2o3+ c
Answers
The balanced equation for the given reaction is:
4 Si4C3 + 15 O2 → 8 Si2O3 + 3 C
What is Balanced Chemical Equation?
The coefficients in a balanced chemical equation represent the stoichiometric relationship between the reactants and products. They show the relative amounts of each substance that are involved in the reaction. In the given chemical equation, Si4C3 + O2 -> Si2O3 + C, the coefficients can be determined by balancing the number of atoms of each element on both sides of the equation.
Starting with Si, there are 4 Si atoms on the left and 2 Si atoms on the right, so a coefficient of 2 is needed in front of Si2O3 to balance the number of Si atoms.
Moving on to C, there are 3 C atoms on the left and 1 C atom on the right, so a coefficient of 3 is needed in front of C to balance the number of C atoms.
Finally, for O, there are 2x3=6 O atoms on the left and 2x2=4 O atoms on the right, so a coefficient of 3 is needed in front of O2 to balance the number of O atoms.
The balanced equation is thus: Si4C3 + 3O2 -> 2Si2O3 + 3C, with coefficients of 1, 3, 2, and 3 for Si4C3, O2, Si2O3, and C, respectively.
Learn more about Balanced Chemical Equation from given link
https://brainly.com/question/29367108
#SPJ1
A sample of excited atoms lie 3.314×10−19 J above the ground state. Determine the emission wavelength of these atoms.
Answers
The emission wavelength of the atoms excited above the ground state is 599.8 nm.
Emission wavelength of the atomsThe emission wavelength of the atom is determined by using the following formulas as shown below;
E = hf
E = hc/λ
where;
E is the energy of the atomh is Planck's constantc is speed of lightλ is the emission wavelengthλ = hc/E
λ = (6.626 x 10⁻³⁴ x 3 x 10⁸) / (3.314 x 10⁻¹⁹)
λ = 5.998 x 10⁻⁷ m
λ = 599.8 x 10⁻⁹ m
λ = 599.8 nm
Thus, the emission wavelength of the atoms is 599.8 nm.
Learn more about wavelength here: https://brainly.com/question/10728818
Are homogeneous mixtures only able to be chemically separated into their own individual components?
Answers
Answer:
Are the components of a homogeneous mixture are chemically combined?
Mixtures are physically combined structures that can be separated into their original components. A chemical substance is composed of one type of atom or molecule. ... A homogeneous mixture is a type of mixture in which the composition is uniform and every part of the solution has the same properties.
Explanation:
if .709 j of heat is added to water and cause the temperature to go up by .036 degrees C what mass of water is present
Answers
Answer:
0.00471 grams H₂O
Explanation:
To determine the mass, you need to use the following equation:
Q = mcΔT
In this equation,
-----> Q = energy/heat (J)
-----> m = mass (g)
-----> c = specific heat capacity (J/g°C)
-----> ΔT = temperature change (°C)
The specific heat capacity of water is 4182 J/g°C. You can plug the given values into the equation and simplify to isolate "c".
Q = 0.709 J c = 4182 J/g°C
m = ? g ΔT = 0.036 °C
Q = mcΔT <----- Equation
0.709 J = m(4182 J/g°C)(0.036 °C) <----- Insert values
0.709 J = m(150.552) <----- Multiply 4182 and 0.036
0.00471 = m <----- Divide both sides by 150.552
How many molecules are there in 985 mL of nitrogen at 0.0 degrees C and 1.00x10^-6 mm Hg?
(It would be better if work or steps to solve problem are given but if not its fine).
Answers
Answer:
3.48 x 1013 N2 molecules
step-by-step explanation:
Lets set up our equation first
P = 1.00 x 10-6 mm Hg T = 0.0° C + 273 = 273 K
We are given the V = 985 mL ,
R = 0.0821 L·atm/mol·K
Now use the ldeal gas law, but we are solving n, amount of substanvce
PV = nRT, we will change this equation to ;
n = PV/RT
n = 1.00 x 10-6 mm x 1 atm/760 mm x 985 mL x 1 L/103
mL/
(0.0821 L·atm/mol·K x 273 K) = 5.78 x 10-11 moles N2
nmolecules = 5.78 x 10-11 moles N2 x 6.02 x 1023 N2 molecules/1 mol N2
The milliliter is a metric unit for
A.
temperature.
B.
length.
C.
mass.
D.
volume.
Answers
Answer:
d. Volume
Explanation:
The milliliter is a metric unit used for measuring the capacity or volume of an object.
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
How does a magnet separate mixtures like sand and iron filings?
Answers
Answer:
because iron is magnetic and sand is not
Explanation:
I need help with this please fast

Answers
4) The volume of the HCl used is 9.500 mL while the volume of the NaOH used is 3.800 mL.
5) Molarity of sodium hydroxide is obtained from; Molarity of HCl * 1/2
What is titration?By reacting an unknown component with a known quantity of a different chemical known as a titrant, titration is a laboratory procedure used to measure the concentration of an unknown substance, often a solute dissolved in a liquid.
The endpoint of a titration can be detected in a number of ways, depending on the specific titration being performed.
4)
Volume of the Acid used = Initial reading - Final reading = 25.00 - 15.50 = 9.500 mL
Volume of the base used = 8.80 - 5.00 = 3.800 mL
5)
We know that the mole ratio is 1:2 and the implication of this is that the set up to obtain the molarity of the sodium hydroxide solution is Molarity of HCl * 1/2
Learn more about titration:https://brainly.com/question/31271061
#SPJ1
Which of the following explains why a longer bond is also a weaker bond? Help plz

Answers
Answer:
Longer bonds have lower attractive force
What new combined branch of study was developed from the
study of matter within living systems?
Answers
According to the research, the correct option is Biochemistry. It is a new combined branch of study that was developed from the study of matter within living systems.
What is Biochemistry?It is an integrating science or scientific discipline that studies at the molecular level the characteristics and functions of the chemical components of living beings.
In this sense, it is responsible for the study of substances such as proteins, lipids, carbohydrates that are present in living organisms and the fundamental chemical reactions for vital processes.
Therefore, we can conclude that according to the research, the correct option is Biochemistry. It is a new combined branch of study that was developed from the study of matter within living systems.
Learn more about Biochemistry here: https://brainly.com/question/2916594
#SPJ1
What would be the best way to measure the volume of a small ?
Answers
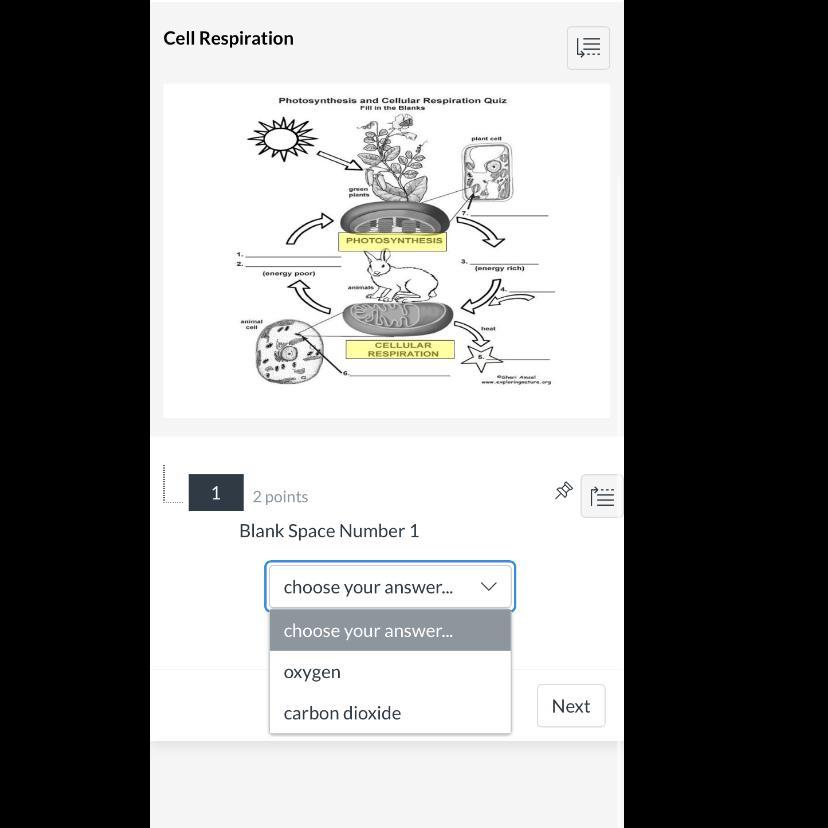
1. How does cellular respiration add carbon to the
atmosphere?
Answers
ch4(g) + h2o(g) 3h2(g) + co(g) enthalpy of formation of CH4
Answers
Answer:
Kc=[[CO][H2]3[CH4][H2O]
3.90=(0.30)(0.10)3[CH4]×0.02
[CH4]=0.023.90×0.30×(0.10)3=5.85×10−2 M
Thus, the concentration of methane in the mixture is 5.85×10−2 M.
What is the tissue that allows for flexibility of bones?
Answers
Answer:
Bone Marrow, or Cartilage
Hope this helps :)
Cattails A Scientist noticed that cattails grew only in swampy parts of his backyard. He decided to try to find out why. He went to the library and found out the following facts: Cattails are not found in deserts, Cattails are usually found in many swamps, Cattails sometimes grow in rivers and streams. The scientist thought for awhile, then said, " I think I have figured out the answer. Cattails need a lot of water to grow." He then went into his yard and dug up 100 cattails. He divided them into four groups. Each group contained 25 cattails. All of the groups were grown in the same type of soil, they all received the same amount of light, and they were all kept at the same temperature. There was only one difference between the groups. Group 1 received 4 mL of water a day. Group 2 received 3 mL of water a day. Group 3 received 2 mL of water a day. Group 4 received 1 mL of water each day. Every day he went out and measured the plants. After 30 days he observed that the plants in group 1 had grown an average of 8 cm. The plants in group 2 had grown an average of 4 cm. The plants in group 3 had grown an average of 2 cm. The plants in group 4 had grown an average of only 1 cm. He then decided that the amount of water that a cattail receives affects its growth. Plants that receive more water, grow more. The scientist then repeated his experiment using another 100 cattails
Answers
The dependent variable in this case is the length of the cattails while the independent variable is the volume of water received.
What is an experiment?An experiment is a carefully controlled study which establishes a cause and effect relationship between variables. There must be a dependent variable and an independent variable.
The dependent variable in this case is the length of the cattails while the dependent variable is the volume of water received. The control group is the group that did not receive any water.
Learn more about experiment: https://brainly.com/question/9199868
16. A sequence of star colors frorn hottest to coolest is
O f. blue, yellow, orange, red
g. red, orange, yellow, blue
0 h. blue, red, yellow, orange
1 yellow, blue, orange, red
Answers
Answer:
g. red, orange, yellow, blue
Explanation:
red is the hottest color and blue is the coolest. :)
In an alternate dimension, Promethium (Pm; Z = 61) is stable. It has 3 stable isotopes
145pm (144.96 amu; 68.47%), 146Pm (145.98 amu; 21.39%), and 147Pm (146.99
amu; 10.14%). What is the average atomic mass of Promethium in this alternate
universe?
Answers
Explanation multiple
Classify each of the substances as an atomic element, molecular element, molecular compound, or ionic compound. Provide one sentence explanation for each. a. fluorine b. N2 O c. silver d. K2 O e. Fe2 O3 g
Answers
Answer and Explanation:
a. fluorine ⇒ atomic element
Fluorine (F) is a chemical element because it is a pure substance that cannot be decomposed into simpler substances.
b. N₂O ⇒ molecular compound
We can see that N₂O (nitrous oxide) is a molecule composed by two different atomic elements: nitrogen (N) and oxygen (O). Thus, it is a molecular compound.
c. silver ⇒ atomic element
Silver is a chemical element with the symbol Ag. It is a pure substance which cannot be decomposed into simpler substances.
d. K₂O ⇒ ionic compound
Potassium oxide (K₂O) is composed by a metallic element (potassium, K) and a non-metallic element (O). Thus, there is a difference in the electronegativity of the chemical elements, so the substance can dissociate into ions. In consequence, it is an ionic compound.
e. Fe₂O₃ ⇒ molecular compound (with ionic character)
Iron(III) oxide (Fe₂O₃) is composed by iron element (Fe), which is a metal, and oxygen element (O), which is a non-metal. Since it is a Metal- Non-Metal combination, it would be an ionic compound. The difference in electronegativity between Fe and O is not high (<2.0) in comparison with other ionic compounds, so Fe₂O₃ is considered as a polar covalent compound (it is between an ionic compound and a molecular compound).