In radioactive decay, with each successive half-life, half the remaining sample decays to form another
a. nucleus
b. proton
c. element
d. life-form
Answers
In radioactive decay, with each successive half-life, half the remaining sample decays to form another element.
In most cases, a new element is created from the destroyed nuclei with each new half-life.
The same element is created during the decay of an isotope since the daughter nucleus has the same amount of protons (same atomic number).
A new element is created when a daughter nucleus has the same number of neutrons as the parent nucleus, or an isotone (new atomic number)
Isobar: daughter nucleus has identical mass number; this might be an example of beta decay; the decayed nucleus retains its mass number but adds a proton in place of a lost electron, resulting in the formation of a new element with an increased atomic number by one.
Learn more about radioactive decay:
brainly.com/question/1770619
#SPJ4
Related Questions
the formula for a molecule formed from n and cl would be?
Answers
The formula for a molecular formed form N and Cl would be N\(Cl_{3}\).
According to the given configuration of Nitrogen(N) and Chlorine(Cl) we have,
N needs 3 electrons and Cl needs 1 electron to complete their octet.
Therefore, one atom of N will combine with three atoms of Cl.
So the molecular formula of the compound formed will be N\(Cl_{3}\).
The addition reaction is:
N + Cl -------> N\(Cl_{3}\)
(nitrogen) (chlorine) ( Nitrogen(III) chloride)
Therefore the formula for a molecule formed from N and Cl would be N\(Cl_{3}\).
To know more about the chemical formula refer to the link given below:
https://brainly.com/question/11574373
#SPJ4
Which actions are part of a system of methodical tests and refinements specific to technological design? 1 documenting, tinkering, testing 2 tinkering, presenting, testing 3 testing, documenting, pr esenting 4 presenting, tinkering, testing
Answers
Answer:
1. documenting, tinkering, testing
Explanation:
Technological design is defined as the process of study, design and development of new technologies.
There are some action in the methodical tests and refinements specific to technological design include documenting, tinkering, testing.
Documenting includes collecting all the information about the design and develop the product, tinkering involves repairing or adjust the issues found in the development, and testing helps to evaluate if the product is ready to work as it is supposed to.
Hence, the correct answer is "1."
How many kg of chlorine gas should be dissolved in 5 million liters of water to result in a concentration of 3.4 ppm
Answers
Approximately 17 metric tons of chlorine gas should be dissolved in 5 million liters of water to achieve a concentration of 3.4 ppm.
To determine the amount of chlorine gas that should be dissolved in 5 million liters of water to achieve a concentration of 3.4 ppm (parts per million), we need to convert the volume of water into the corresponding mass.
1 liter of water has a mass of approximately 1 kilogram, so 5 million liters of water would have a mass of 5 million kilograms (5 × 10^6 kg).
The concentration of 3.4 ppm means that there are 3.4 parts of chlorine gas for every million parts of water. Therefore, to find the amount of chlorine gas needed, we multiply the concentration by the mass of water:
Amount of chlorine gas = (3.4 ppm) × (5 × 10^6 kg) = 17 × 10^6 g = 17 metric tons.
Thus, to obtain a concentration of 3.4 ppm in 5 million liters of water, approximately 17 metric tons of chlorine gas would need to be dissolved.
You can learn more about chlorine gas at
https://brainly.com/question/30459995
#SPJ11
how do you converse 1.45 days to seconds
Answers
Answer:
125280
Explanation:
86400 seconds are in a day so just multiply 86400 by 1.45 to get your answer
Use the web to determine the safety of the spring water sample. explain if a particular web component of the water could be hazardous for consumption.
Answers
The safety of the spring water sample is said to be that in an untreated state, it is said to be undrinkable.
What is spring water?The EPA is one that tells that the spring water is seen as a kind of any water that is said to have its origin from any kind of underground aquifer and is said to be taken as it flows naturally to the earth's surface or through the use of a borehole that lunch into the underground water source.
Note that studies shows that when a spring water is said to be examined, the different parameters of health safety were said to be analyzed by the use of laboratory tests and the outcome of the study reveals that water is not good for drinking because it has higher concentrations of ammonium ion as well as others.
Hence, The safety of the spring water sample is said to be that in an untreated state, it is said to be undrinkable.
Learn more about spring water from
https://brainly.com/question/2375655
#SPJ1
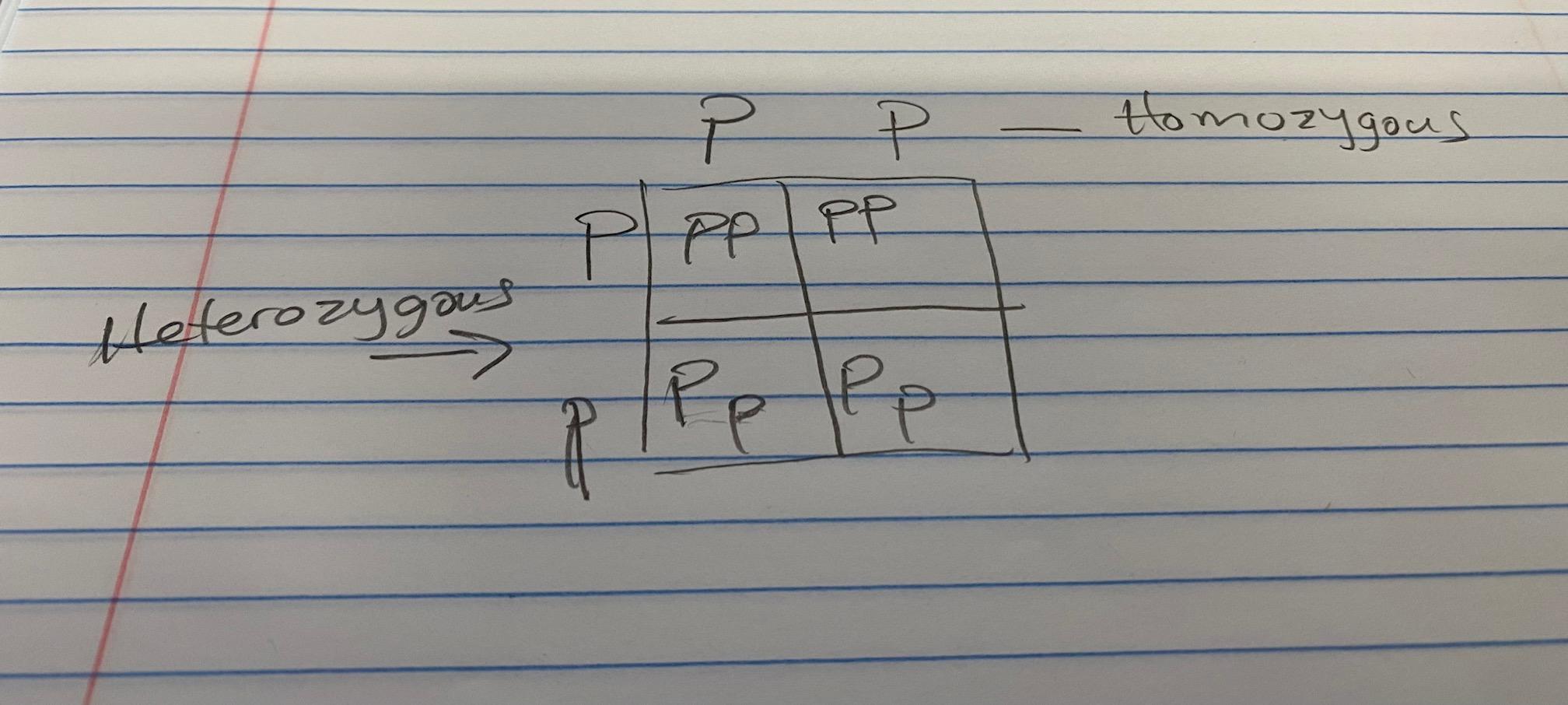
This is the picture btw because it wont let me say all the words.

Answers
Answer:
see image
D is the answer
Explanation:
see image
the box is like a mini multiplication table
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed. (i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution. Initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction
Answers
Answer:
it is an endothermic reaction
Explanation:
This is because there is a rise in temperature from 20 to 46. this means that the reaction takes in heat from the suuroundings
What kind of reaction is this ?

Answers
Draw the major organic product of each reaction. Assume a one-to-one ratio of reagents and benzene.For the functional groups added, be sure to draw out all bonds, lone pairs, and formal charges. (Note: the short-cut for NO2 will not be accepted for this question) Did you draw just one compound in each box? Does the organic product contain all bonds, lone pairs and nonzero formal charges? Which electrophile is formed in each route?
Answers
Secondary amine is what it is. Option (B3-dimethylbutan-2-amine )'s is the primary organic product created during the following reaction. Thus, if more hydrogen is added to carbon, we will obtain the main product, according to the Markovnikov Rule.
As a result, the main product produced by adding hydrogen to carbon-1, which has a higher concentration of hydrogen, and adding bromine to carbon-2 is 2-bromopropane. When a reaction follows Markovnikov's rule, a chemical or product is said to be a major organic product. The tertiary alkyl iodide is the largest byproduct of the second process, which transforms alcohol into alkyl iodide, while the secondary alkyl iodide is the minor byproduct.
To learn more about hydrogen here,
https://brainly.com/question/24433860
#SPJ4
Guided Practice
Old-fashioned windmills are not aerogenerators because
Answers
Answer:
What is the main problem with windmills?
Image result for Guided Practice Old-fashioned windmills are not aerogenerators because
As with all energy supply options, wind energy can have adverse environmental impacts, including the potential to reduce, fragment, or degrade habitat for wildlife, fish, and plants. Furthermore, spinning turbine blades can pose a threat to flying wildlife like birds and bats.
Why are the windmills not moving?
Why do the turbines not spin at times? The most common reason that turbines stop spinning is because the wind is not blowing fast enough. Most wind turbines need a sustained wind speed of 9 MPH or higher to operate. Technicians will also stop turbines to perform routine maintenance or repairs.
Suppose that it rains when the temperature of the ground, and the air just above the ground, is below the freezing point of water (0 C). The rain freezes. Will the freezing lower or raise the temperature near the ground? Explain.
Answers
Rain freezing is a condition of "exothermic" process. The freezing rain emits thermal energy, which is then transmitted to the earth. The earth will receive thermal energy if the rain loses thermal energy. As a result, the ground's temperature rises.
Due to the salt in saltwater, it freezes at a lower temperature than fresh water, approximately 28.4 degrees Fahrenheit (ca. -2 °C). However, because only the water portion of saltwater freezes, very little salt is present in the ice when it is formed. Water may be made from it by melting it down.
Learn more about freezing, here:
https://brainly.com/question/23066238
#SPJ1
Task
01
Perform a literature
survey to find out the methods of simulating general aerofoil shape
including experimental and numerical techniques. Student may use
about 2000 words (± 10%) to elaborate
Answers
The methods of simulating general aerofoil shape include experimental techniques such as wind tunnel testing, and numerical techniques such as Computational Fluid Dynamics (CFD) simulations.
Simulating general aerofoil shapes involves both experimental and numerical techniques. Experimental methods include wind tunnel testing, where scaled-down models of the aerofoil are tested in controlled airflow to measure aerodynamic forces. Numerical techniques, such as Computational Fluid Dynamics (CFD) simulations, involve solving fluid flow equations on a computer to analyze flow characteristics and aerodynamic forces. CFD simulations are cost-effective, flexible, and can handle complex aerofoil shapes, but require accurate modeling and validation. A combination of experimental and numerical methods enhances our understanding of aerofoil aerodynamics and helps optimize their design.
To know more about Computational Fluid Dynamics (CFD) click here,
https://brainly.com/question/30578986
#SPJ11
Which of the following characteristics best describe the properties of liquids? Select all that apply.
Answers
Answer:
No definite shape
Explanation:
A gas in a balloon at constant pressure has a volume of 120. 0 mL at -123C. What is its volume at 27C?
Answers
At 27°C and constant pressure, the volume of the gas in the balloon is approximately 240.0 mL.To solve this problem, we need to use the combined gas law, which relates the pressure, volume, and temperature of a gas:\((P_1V_1)/T_1 = (P_2V_2)/T_2\)
Where \(P_1\)and \(T_1\) are the initial pressure and temperature, \(V_1\)is the initial volume, \(P_2\) and \(T_2\) are the final pressure and temperature, and \(V_2\) is the final volume we are trying to find.We are given the initial volume \(V_1\) = 120.0 mL, the initial temperature \(T_1\)= -123C, and the pressure is constant, so we can assume \(P_1 = P_2\). We need to convert the temperatures to Kelvin, so \(T_1\) = 150 K and \(T_2\)= 300 K.
Using the combined gas law, we can solve for \(V_2\):
\((P_1V_1)/T_1 = (P_2V_2)/T_2\)
\((P_1)(120.0 mL)/(150 K) = (P_2)(V_2)/(300 K)\)
Simplifying, we can cancel out the pressures and cross-multiply:
\(V_2\) = (120.0 mL)(300 K)/(150 K)
\(V_2\) = 240.0 mL
Therefore, the volume of the gas in the balloon at 27C is 240.0 mL.To answer your question, we'll use Charles's Law, which states that the volume of a gas is directly proportional to its temperature in Kelvin, as long as the pressure remains constant.Charles's Law formula: \(V_1/T_1 = V_2/T_2\)
Where:
\(V_1\) = initial volume = 120.0 mL
\(T_1\) = initial temperature = -123°C
\(V_2\)= final volume (what we want to find)
\(T_2\) = final temperature = 27°C
First, we need to convert the temperatures from Celsius to Kelvin:
\(T_1\)(K) = -123°C + 273.15 = 150.15 K
\(T_2\)(K) = 27°C + 273.15 = 300.15 K
Now, we can plug in the values into Charles's Law formula:
(120.0 mL / 150.15 K) = (\(V_2\) / 300.15 K)
To find \(V_2\), we'll rearrange the equation and multiply both sides by 300.15 K:
\(V_2\) = (120.0 mL / 150.15 K) × 300.15 K
\(V_2\)≈ 240.0 mL
For more such questions on pressure
https://brainly.com/question/24719118
#SPJ11
If acrylamide is present in final cooked product of a
food, and the amount of acrylamide can vary depending on the
consumer’s cooking preference (eg: how crispy the fries are cooked
to), should the
Answers
Yes, it is recommended to minimize the formation of acrylamide in food products as it is considered a potential carcinogen.
Food manufacturers and consumers should follow guidelines and cooking practices that help reduce the formation of acrylamide, such as avoiding overcooking or burning foods, using lower cooking temperatures, and employing cooking methods that produce less acrylamide formation. Any drug, radionuclide, or radiation that encourages carcinogenesis (the development of cancer) is considered a carcinogen. This may be as a result of the possibility of genomic damage or cellular metabolic processes being upset. The radiation that certain radioactive compounds release, such gamma rays and alpha particles, is what is thought to be responsible for their carcinogenic activities.
To know more about carcinogen
https://brainly.com/question/29422741
#SPJ11
If a plant has a total of 17g of carbon dioxide and water reacting in photosynthesis, then how much glucose and oxygen will the plant produce?
A. 8.5g
B. 34g
C. 17g
(Take your time)
Answers
Answer:
Basically it would be 8.5g. Not total sure.
Explanation:
If its right plz give brainlest.
Answer:
A. 8.5g
Explanation:
Have a great day!
Discussion Topic
Both Josef Loschmidt and Amedeo Avogadro contributed to our understanding of basic
molecular numbers, sizes, and reaction ratios. Neither scientist discovered Avogadro's
number in the form we use it today (6.02 x 10^23). Still, there's controversy over the
name of this number. Research the contributions of these two scientists and how
Avogadro's number got its name. Note the name you think this number should becalled, provide key details about each scientist's contributions to this concept, and give
a solid rationale for your case in naming the number.

Answers
Josef Loschmidt and Amedeo Avogadro were both scientists who made significant contributions to the understanding of basic molecular numbers, sizes, and reaction ratios.
What is the rational behind their contributions?In 1811, Amedeo Avogadro proposed that equal volumes of gases at the same temperature and pressure contain the same number of molecules. This became known as Avogadro's law, which laid the foundation for the concept of the mole.
Josef Loschmidt, on the other hand, made important contributions to determining the size of molecules. In 1865, Loschmidt used kinetic theory to calculate the number of molecules in one cubic centimeter of gas at standard temperature and pressure (STP). He estimated the number to be about 2.7 x 10¹⁹, which is close to the modern value of Avogadro's number.
The term "Avogadro's number" was not coined until the early 1900s, long after Avogadro's death. The name was proposed by French physicist Jean Baptiste Perrin in honor of Avogadro's contributions to the concept of the mole.
In my opinion, the name "Avogadro's number" is appropriate because Avogadro's law was the first concept to establish a relationship between the volume of a gas and the number of molecules it contains. Moreover, Avogadro's law played a crucial role in the development of the mole concept, which is essential in chemical calculations. While Loschmidt's contributions were also significant, he did not propose a fundamental law like Avogadro did. Therefore, I believe that naming the number after Avogadro is appropriate to recognize his contributions to this fundamental concept in chemistry.
Learn more on Avogadro here: https://brainly.com/question/1513182
#SPJ1
the isotope has a half life of 5715 years. now there are 40g of the isotope. How much will remain after 1600 years. round to 4 decimal places
Answers
Category ------------ Nuclear chemistry
Sub-category --------- Radioactivity
ANSWER
The remaining amount of isotope after 1600 years is 32.9444 grams
EXPLANATION
Given that;
The half life of the isotope is 5715 years
The initial mass of the isotope is 40 grams
The decay time is 1600 years
Follow the steps below to find the remaining mass after 1600 years
\(\text{ A\lparen t\rparen= A}_o(\frac{1}{2})^{\frac{t}{t_{\frac{1}{2}}}}\)Where
A(t) is the amount of mass remaining after time t
Ao is the initial amount of mass of the substance
t1/2 is the half-life of the substance
t is the decay time
Substitute the given data into the above formula
\(\begin{gathered} \text{ A\lparen t\rparen = 40 \lparen}\frac{\text{ 1}}{\text{ 2}})^{\frac{1600}{5715}} \\ \\ \text{ A\lparen t\rparen = 40 \lparen}\frac{\text{ 1}}{\text{ 2}})^{0.279965} \\ A(t)\text{ = 40 \lparen0.5\rparen}^{0.279965} \\ \text{ A\lparen t\rparen = 40 }\times\text{ 0.8236109} \\ \text{ A\lparen t\rparen = 32.9444 grams} \end{gathered}\)Therefore, the remaining amount of isotope after 1600 years is 32.9444 grams
Which of the following is a natural source of CO2 in the atmosphere?
oil spilled into tropical ocean waters
emissions produced by burning fossil fuels
gases released when plants make food
warm water released from nuclear power plants
Answers
If you have 1kg of carbon and 1 kg of gold, they will weigh the same.
Answers
What mass of liquid mercury would fill a glass tube with a diameter of 16.0 mm to a height of 25.0 cm? the density of mercury is 13.53gcm3.
Answers
The mass of liquid mercury that can fill glass tube is 680.365 gm.
We know that ,
Volume of cylinder = π\(r^{2} h\)
where r is the radius of cylinder and h is the height
Here diameter of glass tube is 16mm
Thus the radius r = 8 mm
= 0.8 cm
Volume of glass tube = π \(0.8^{2}\)\(*25\) \(cm^{3}\)
Density of mercury given is \(13.53gm/cm^{3}\)
We know that
Density = Mass / Volume
∴ \(13.53gm/cm^{3}\) = Mass/ π\(0.8*0.8 * 25\)
Mass = 13.53 *π0.8*0.8*25
= 680.36 gm
To know more about density
https://brainly.com/question/24815199
#SPJ4
now suppose hcl was added to the reaction mixture from the previous question. hcl(aq) naal(oh)4(aq) → ??? based on your answers to the previous questions, what do you expect to happen?
Answers
The addition of HCl to the reaction mixture would alter the chemical equilibrium and shift the reaction towards the formation of aluminum hydroxide.
If HCl (hydrochloric acid) is added to the reaction mixture from the previous question, it would react with the NaAl(OH)4 (sodium tetrahydroxoaluminate) present in the mixture. The balanced chemical equation for this reaction would be:
HCl(aq) + NaAl(OH)4(aq) → Al(OH)3(s) + NaCl(aq) + H2O(l)
Here, the HCl would donate a proton to the NaAl(OH)4, resulting in the formation of Al(OH)3 (aluminum hydroxide), NaCl (sodium chloride), and H2O (water). This would be an acid-base reaction where HCl acts as an acid and NaAl(OH)4 acts as a base.
Since aluminum hydroxide is an insoluble solid, it would precipitate out of the solution as a white solid. This would be observed as a white cloudy appearance in the reaction mixture. Additionally, the solution would become more acidic due to the addition of HCl.
The presence of excess HCl in the solution could also lead to the dissolution of some of the aluminum hydroxide precipitate, resulting in a clearer solution.
To know more about aluminum hydroxide refer here:
https://brainly.com/question/29422382#
#SPJ11
what are standard conditions when working with gases?
Answers
Answer:
STP in chemistry is the abbreviation for Standard Temperature and Pressure. STP most commonly is used when performing calculations on gases, such as gas density. The standard temperature is 273 K (0° Celsius or 32° Fahrenheit) and the standard pressure is 1 atm pressure.
The process of injecting small amounts of air into the vial at a time to prevent leaking is called: Select one: Coring Decoding Milking Scooping
Answers
The process of injecting small amounts of air into the vial at a time to prevent leaking is called milking.
Milking is a process used to withdraw liquid from a vial without allowing air to enter the syringe, which can cause the formation of air bubbles or contamination of the sample.
It involves injecting small amounts of air into the vial at a time to create a positive pressure that forces the liquid out. This is particularly important when dealing with viscous or volatile liquids that are prone to clogging or evaporation.
To milk a vial, the needle is inserted into the septum at an angle and a small amount of air is injected into the vial. This is repeated until the desired volume of liquid is withdrawn.
Milking is a common technique used in various scientific applications, including analytical chemistry, biotechnology, and pharmaceutical research, where precise and accurate liquid handling is crucial.
To know more about the milking refer here :
https://brainly.com/question/29360367#
#SPJ11
which of the following elements does not form an ion with a charge of +1
Answers
hope this helps x
Which of the following compounds do not contain an sp3 hybridized oxygen atom? _ A A) ketones B) alcohols C) ethers D) esters E) water
Answers
Which of the following compounds do not contain an sp3 hybridized oxygen atom is option E) water. The oxygen atom in an sp3 hybridized state has a tetrahedral arrangement of its bonds, which is achieved through the hybridization of one s orbital and three p orbitals.
This creates four identical sp3 hybrid orbitals that point to the vertices of a tetrahedron. Each of the listed chemical substances has an oxygen atom bonded to other atoms or functional groups.
The following is a brief explanation of each of them: Ketones contain a carbonyl group, which consists of a carbon atom with a double bond to an oxygen atom, and two additional carbon-containing groups. As a result, the oxygen atom in a ketone is sp2 hybridized, with one lone pair in an unhybridized p orbital.Alcohols have an -OH functional group, which consists of an oxygen atom covalently bonded to a hydrogen atom and a carbon-containing group. The oxygen atom in an alcohol is also sp3 hybridized.
Ethers have two carbon-containing groups connected to an oxygen atom. The oxygen atom in an ether is sp3 hybridized.Esters have a carbonyl group and an alkoxy group (-OR). The oxygen atom in an ester's carbonyl group is sp2 hybridized, while the oxygen atom in the -OR group is sp3 hybridized. Water, on the other hand, is composed of two hydrogen atoms and an oxygen atom, with no other atoms connected to the oxygen atom. In water, the oxygen atom is also sp3 hybridized, with two lone pairs and two single bonds to hydrogen atoms. Therefore, Option E is correct.
To know more about Alcohols visit-
https://brainly.com/question/29268872
#SPJ11
Compound A has a molar mass of 20g/mol and compound B has a molar mass of 30g/mol.
1. How many moles of compound B are needed to have the same mass as 6.0 mol of compound A? (Please give explanation not only the answer)

Answers
Answer:
9 moles
Explanation:
To find out how many moles of compound B are needed to have the same mass as 6.0 mol of compound A, we need to use the molar mass of each compound and set up a proportion:
Moles of A / Molar mass of A = Moles of B / Molar mass of B
We know that the molar mass of compound A is 20 g/mol and that we have 6.0 mol of it, so:
6.0 mol A / 20 g/mol A = Moles of B / 30 g/mol B
Simplifying this equation:
0.3 mol A = Moles of B / 30
Multiplying both sides by 30:
9 mol B = 0.3 mol A
So, we need 9 moles of compound B to have the same mass as 6.0 mol of compound A.
determine the values of k by taking into account the volume of water used to make he saturated solution
Answers
The values of k by taking into account the volume of water used to make the saturated solution is \(Ksp = (sV)(m + n)^m\)
In order to determine the values of K by taking into account the volume of water used to make the saturated solution, we need to use the following equation:
\(Ksp = [M+]^m [X^-]^n\)
where Ksp is the solubility product constant, M+ is the cation of the salt, \(X^-\) is the anion of the salt, m is the stoichiometric coefficient of M+ in the balanced chemical equation, and n is the stoichiometric coefficient of \(X^-\)in the balanced chemical equation.
When the salt dissolves in water to form a saturated solution, the concentration of M+ and \(X^-\) in the solution will be equal to their solubility values. We can express the solubility of \(M+X^-\) in terms of the molar solubility s, which is defined as the number of moles of the salt that dissolve per liter of solution.
Therefore, we can rewrite the Ksp expression as:
Ksp = s(m + n)^m
Since we want to take into account the volume of water used to make the saturated solution, we can multiply the molar solubility s by the volume of water used to make the solution, which we will call V. The number of moles of the salt that dissolves will then be equal to sV.
Therefore, we can rewrite the Ksp expression again as:
Ksp = (sV)(m + n)^m
Learn more about saturated solution here:
https://brainly.com/question/1851822
#SPJ11
To view the daily appointments, you would first click the BLANK icon
A) Appts
B) Select Patient
C) New File
D) Reports
Answers
How many meters are present in the 12.45 miles? Please show your work, and report your answer with the correct number of significant figures and appropriate units.
Answers
Explanation:
We know that,
1 mile = 1609.34 m
We need to find how many meters are present in the 12.45 miles. To find it use unitary method as follows :
12.45 mile = 1609.34 × 12.45
12.45 mile=20036.283 meters
or
\(12.45\ mile=2.0036\times 10^4\ m\)
Hence, this is the required solution.