In n=6 the correct sequence for filling of electron will be
Answers
Answer:
If n=6, the correct sequence for filling of electrons will be ns→(n−2)f→(n−1)d→np
Related Questions
when 57.0 g copper are reacted with silver nitrate solution, 138g of silver are obtained. what is the percent yield of silver obtained
Answers
Answer:
86.96
Explanation:
a 76.8 lb 76.8 lb child has a streptococcus infection. amoxicillin is prescribed at a dosage of 45 mg per kg 45 mg per kg of body weight per day given b.i.d.
Answers
The child needs 1,569.25 mg of amoxicillin per day, with 784.63 mg of amoxicillin per dose.
Streptococcus infections are commonly treated with amoxicillin, a broad-spectrum antibiotic.
Amoxicillin is effective against many different types of bacteria, including Streptococcus bacteria.
When a child has a streptococcus infection, amoxicillin may be prescribed at a dosage of 45 mg per kg of body weight per day given b.i.d.
In this case, a 76.8 lb child would be given 1,385.28 mg of amoxicillin per day, divided into two equal doses, for a total of 692.64 mg per dose.
Amoxicillin is a penicillin antibiotic used to treat infections caused by bacteria.
It is effective against many different types of bacteria, including streptococcus bacteria.
The required dosage of amoxicillin for a child is determined by their body weight and the extent of the infection they are experiencing.
In this case, the child weighs 76.8 lbs, which is equivalent to 34.85 kg.
The dosage of amoxicillin is 45 mg per kg of body weight per day, so the child needs 1,569.25 mg of amoxicillin per day.
This dosage is divided into two equal doses, so the child needs 784.63 mg of amoxicillin per dose.
Since amoxicillin is often taken orally, this dosage can be provided in the form of a tablet, suspension, or chewable tablet.
The duration of amoxicillin treatment will depend on the severity of the infection and the response of the child to the treatment. Generally, amoxicillin treatment lasts for 10 to 14 days.
The child should continue taking amoxicillin for the full prescribed course, even if they start feeling better before the treatment is completed.
Learn more about amoxicillin at: https://brainly.com/question/15701486
#SPJ11
2. Calculate the number of moles represented by the following masses.
a. 2.00 × 102 g of silver
b. 37.1 g of silicon dioxide, SiO2
40 POINTS!!!!!!!!!!!!
Answers
a. There are 1.85 moles in 2.00 × 10² g of silver (Ag).
b. There are 0.618 moles in 37.1 g of silicon dioxide (SiO₂)
What is the molar mass?The molar mass is the mass in grams of 1 mole of particles, that is, the mass in grams of 6.02 × 10²³ particles. The units are g/mol.
We want to calculate the number of moles represented by different masses of different substances. In each case, the conversion factor between mass and moles is the molar mass.
a. 2.00 × 10² g of silver (Ag)The molar mass of silver is 107.87 g/mol.
2.00 × 10² g × (1 mol/107.87 g) = 1.85 mol
b. 37.1 g of silicon dioxide (SiO₂)The molar mass of silicon dioxide is 60.08 g/mol.
37.1 g × (1 mol/60.08 g) = 0.618 mol
a. There are 1.85 moles in 2.00 × 10² g of silver (Ag).
b. There are 0.618 moles in 37.1 g of silicon dioxide (SiO₂)
Learn more about molar mass here: https://brainly.com/question/21334167
#SPJ1
Part A Fluoroacetate functions as a poison by what mechanism? - The compound binds very tightly to but does not form a covalent complex with aconitase. - The compound forms a covalent complex with the aconitase. - This compound is first converted to 2-fluorocitrate, which subsequently inhibits aconitase. - Aconitase converts this compound to a reactive species that covalently modifies the next enzyme in the citric acid cycle
Answers
The mechanism by which Fluoroacetate functions as a poison involves the compound being first converted to 2-fluorocitrate, which subsequently inhibits aconitase.
This inhibition disrupts the citric acid cycle, which is necessary for cellular respiration and energy production. Fluoroacetate does not form a covalent complex with aconitase, but rather binds tightly to it. This binding ultimately leads to the inhibition of aconitase and disruption of the citric acid cycle, making it a potent and deadly poison. Fluoroacetate binds very tightly to but does not form a covalent complex with aconitase, preventing it from functioning. This process leads to the accumulation of citrate, which is then converted to 2-fluorocitrate by hydrolysis. 2-fluorocitrate then inhibits aconitase, leading to a decrease in the amount of ATP production. Ultimately, this leads to cellular death due to a decrease in ATP production.
To learn more about Fluoroacetate click here https://brainly.com/question/29340630
#SPJ11
if this atom has one additional neutron but the other subatomic particles remained the same as shwon, this slightly different atoms would be called
Answers
If this atom has one additional neutron but the other subatomic particles remained the same as shown, this slightly different atom would be called an isotope.
What is an isotope?An isotope is a variant of an atom that has a different number of neutrons. Because isotopes of the same element have the same number of protons in the nucleus, they all have the same atomic number. However, they can differ in mass number, and therefore in atomic mass. In a neutral atom, the number of protons in the nucleus equals the number of electrons that orbit the nucleus, giving the atom a net electrical charge of zero.
However, the number of neutrons in the nucleus can differ, implying that isotopes of the same element may have different atomic masses.
Learn more about isotope here: https://brainly.com/question/28039996
#SPJ11
What is the freezing point of a solution that contains 10.0 g of glucose in 100g of H2O?
Answers
The proposed solution, which has 10.0 grams of glucose in 100 gram of water, has a freezing point of 1.034 C.
The freezing point is what?The degree of heat in which a liquid becomes solid. precisely the temperatures at which a material's solid and liquid states are balanced at atmospheric pressure.
How significant is freezing point?If a substance is kept below its freezing point, it may either become more or less dangerous. The freezing point additionally offers an essential safety standard for evaluating the impacts of occupational exposure to cold conditions.
Briefing:m = molality
i = van 'toff factor,
molality = n/w*t of solvent
n = w*t of Glucose/M* w t
= 10/180*1/0.1
=0.555 w* t
= 1.86*0.555*1
= 1.034
T (solvent) - d= 0-1.034
freezing point = -1.034C
To know more about freezing point visit:
https://brainly.com/question/9530198
#SPJ4
please help asap in 10 mins
What are the conditions necessary for electro-chemical corrosion to occur?
Answers
Answer:
Presence of an Electrolyte
Metal Surface
Oxygen or Other Oxidizing Agent
Difference in Potential
Electrochemical Pathway
Explanation:
X rays are used for
tracking storms.
killing bacteria.
screening luggage at airports.
two of the above
Answers
Find the heat produced from an 8.00 L cylinder of propane gas under 5.00 atm at 25.0 oC, if one mole of propane can produce 2220 kJ.
A. 4290 kJ
B. 0.0289 kJ
C. 877 kJ
D. 1.63 kJ
E. 5420 kJ
F. 1750 kJ
G. 8440 kJ
H. 1360 kJ
I. 37.2 kJ
J. 630 kJ
K. 266 kJ
L. 645 kJ
M. 2420 kJ
N. 7.36 x 10-4 kJ
Answers
Answer: 3597 kJ of heat
Explanation:
According to ideal gas equation:
\(PV=nRT\)
P = pressure of gas = 5.00 atm
V = Volume of gas = 8.00 L
n = number of moles = ?
R = gas constant =\(0.0821Latm/Kmol\)
T =temperature =\(25.0^0C=(25.0+273)K=298K\)
\(n=\frac{PV}{RT}\)
\(n=\frac{5.00atm\times 8.00L}{0.0821 L atm/K mol\times 298K}=1.63moles\)
As it is given :
1 mole of propane produces = 2220 kJ of heat
Thus 1.63 moles of propane produces = \(\frac{2200}{1}\times 1.63=3597kJ\)
Thus 3597 kJ of heat is produced
why do water molecules have a stronger attraction than helium?
answer needed before 3:00 June 2nd 2023
Answers
Water molecules have a stronger attraction than helium due to the presence of dipole-dipole interactions resulting from the polarity of the water molecule.
Water molecules have a stronger attraction than helium due to the difference in their intermolecular forces. Intermolecular forces are the attractive forces that exist between molecules and play a crucial role in determining the physical properties of substances.
Water molecules have a polar nature, meaning they have a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom.
This polarity arises from the unequal sharing of electrons in the O-H bonds due to oxygen's higher electronegativity compared to hydrogen. The presence of polar bonds within the water molecule gives rise to a dipole-dipole interaction.
In contrast, helium is a noble gas and exists as individual atoms. Helium atoms are electrically neutral and do not possess a permanent dipole moment.
As a result, helium exhibits weak intermolecular forces known as London dispersion forces or Van der Waals forces. These forces arise due to temporary fluctuations in electron distribution, causing temporary dipoles that induce dipoles in neighboring atoms or molecules.
The dipole-dipole interaction in water is stronger than the London dispersion forces in helium. This is because dipole-dipole forces are more significant when there are permanent dipoles in the molecules.
The stronger attraction between water molecules leads to higher boiling and melting points compared to helium.
For more such question on molecules. visit :
https://brainly.com/question/24191825
#SPJ8
When carbon is completely combusted in the presence of oxygen the only product produced is carbon dioxide as shown in the following equation, C+O2——>CO2 If 12 grams of carbon are burned, how many grams of carbon dioxide will be produced?

Answers
Answer:
C.44g is that one okkkkkkkkkkkkkkkk
Helpppppp please helpppp helpppp

Answers
Chlorine has a total of 17 electrons and 7 Valence electrons. Why are these two numbers
different?

Answers
Chlorine contains a total of 17 electrons which are filled in different orbitals of which 7 electrons are located in the outermost energy level or orbital.
What are valence electron?In an atom, the electrons are surrounding the nucleus through fixed paths or energy levels called orbitals. Electrons are filled in the increasing order of energy level. Thus lower energy levels are filled first.
The last electrons are filled in the outermost energy levels and are called valence electrons. Each shell have a definite capacity for electrons.
Cl is 17the element in periodic table and has 17 electrons of which 7 electrons are located in the outermost shell. Hence, option A is correct.
To learn more about chlorine, find the link below:
https://brainly.com/question/14962130
#SPJ5
If the temperature of a gas in a container is doubled on the Kelvin scale, what will happen to the pressure of the gas?
Answers
Explanation:
Pressure and temperature will both increase or decrease simultaneously as long as the volume is held constant. If temperature were to double the pressure would likewise double.
Chlorine-
What is the mass number of this element?
How many neutrons?
Answers
Answer:
Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic structure. The chemical symbol for Chlorine is Cl. Neutron Number and Mass Number of Chlorine Mass numbers of typical isotopes of Chlorine are 35; 37.
Explanation:
Atomic number(#of protons): 17
To get the number of neutrons you must subtract the atomic number (#of protons) from the atomic mass which is 35.
35-17=18
Therefore the number of neutrons is 18.
This is because each proton and neutron have a relative mass of one unit.
why are hydrocarbons insoluble in water? group of answer choices the majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages. the majority of their bonds are polar covalent carbon-to-hydrogen linkages. they exhibit considerable molecular complexity and diversity. they are hydrophilic. they are less dense than water.
Answers
Hydrocarbons are insoluble in water because the majority of their bonds are polar covalent carbon-to-hydrogen linkages hence option b is the right choice.
A hydrocarbon is an organic molecule in organic chemistry that is made completely of hydrogen and carbon. The majority of hydrocarbons are colorless and hydrophobic, and their scents are either insignificant or best characterized by those of gasoline and lighter fluid. They can be gases, liquids, low-melting solids, or polymers, and they can also exist in a wide variety of molecular configurations and phases.
Hydrocarbons are the naturally occurring petroleum, natural gas, and coal, as well as their hydrocarbon derivatives and refined forms, in the fossil fuel industry. The primary source of energy for the entire planet is the burning of hydrocarbons.
Want to know more about hydrocarbons visit the link which is given below;
https://brainly.com/question/17578846
#SPJ4
Environmental- from the movie lion king:
Name three heterotrophs from the movie (use characters named please). Why are they heterotrophs?
Answers
Answer:
Shenzi
Banzai
Ed
Scar
Simba
Timon
Pumbaa
Explanation:
they don't make their own food but get it from other organisims
Determine the complete, balanced chemical reaction for the following reaction. Selenic acid (H2SeO4) is a powerful oxidizing acid and it dissolve silver and gold: Au + H2SO4 --> Au2(SeO4)3 + H2SO3 + H2O Then, proceed to answer the following questions: Reactants: Enter the coefficient number in front of Au in the reaction: | Enter the coefficient number in front of H2Se04 in the reaction: Products: Enter the coefficient number in front of Auz(SeO4)3 in the reaction: Enter the coefficient number in front of H2SeO3 in the reaction: Enter the coefficient number in front of H20 in the reaction: What type of chemical reaction is this? Enter one of the following: "synthesis", "decomposition", "single displacement", or "double displacement".
Answers
The complete, balanced chemical reaction for the reaction between gold (Au) and selenic acid (H2SeO4) is: 4Au + 3H2SeO4 → 2Au2(SeO4)3 + H2SO3 + H2O. The coefficients for the reactants and products are as follows: 4 for Au, 3 for H2SeO4, 2 for Au2(SeO4)3, 1 for H2SO3, and 1 for H2O. The type of chemical reaction is a single displacement reaction.
The balanced chemical equation for the reaction between gold (Au) and selenic acid (H2SeO4) is:
4Au + 3H2SeO4 → 2Au2(SeO4)3 + H2SO3 + H2O
In this equation, the coefficient in front of Au is 4, indicating that 4 moles of gold are involved in the reaction. The coefficient in front of H2SeO4 is 3, indicating that 3 moles of selenic acid are present.
On the product side, the coefficient in front of Au2(SeO4)3 is 2, indicating the formation of 2 moles of gold selenate. The coefficient in front of H2SO3 is 1, representing the formation of 1 mole of sulfurous acid. The coefficient in front of H2O is also 1, indicating the production of 1 mole of water.
Based on the reactants and products involved, the type of chemical reaction is a single displacement reaction, as gold displaces hydrogen in selenic acid to form gold selenate.
To learn more about reactants click here:
brainly.com/question/30129541
#SPJ11
When a 8 gram slice of bread is burned under a beaker of 4500 grams of water, it heats up the water by 1.3 degrees Celsius. The specific heat of water is 1 cal/g degree C. How many calories does this slice of bread contain
Answers
Answer:
\(5850\ \text{cal}\)
Explanation:
m = Mass of water = 4500 g
c = Specific heat of water = \(1\ \text{cal/g}^{\circ}\text{C}\)
\(\Delta T\) = Change in temperature of water = \(1.3^{\circ}\text{C}\)
Heat is given by
\(q=mc\Delta T\\\Rightarrow q=4500\times 1\times 1.3\\\Rightarrow q=5850\ \text{cal}\)
A slice of the bread contains \(5850\ \text{cal}\).
if a reaction is first order with a rate constant of 0.0450 s⁻¹, how much time is required for 65% of the initial quantity of reactant to be consumed?
Answers
It will take approximately 26.5 seconds for 65% of the initial quantity of reactant to be consumed in this first-order reaction.
The integrated rate law for a first-order reaction is:
ln([A]t/[A]0) = -kt
where [A]t is the concentration of a reactant at time t, [A]0 is the initial concentration, k is the rate constant, and ln is the natural logarithm.
To solve for t when 65% of the initial quantity of reactant is consumed, we can use the following steps:
Determine the concentration of reactant remaining after 65% is consumed:
[A]t = (1 - 0.65) [A]0 = 0.35 [A]0
Rearrange the integrated rate law to solve for time t:
t = -ln([A]t/[A]0) / k
Substitute the given values and solve for t:
t = -ln(0.35) / 0.0450 s⁻¹
t ≈ 26.5 seconds
Therefore, it will take approximately 26.5 seconds for 65% of the initial quantity of reactant to be consumed in this first-order reaction.
To learn more about integrated rate law:
https://brainly.com/question/16021790
#SPJ4
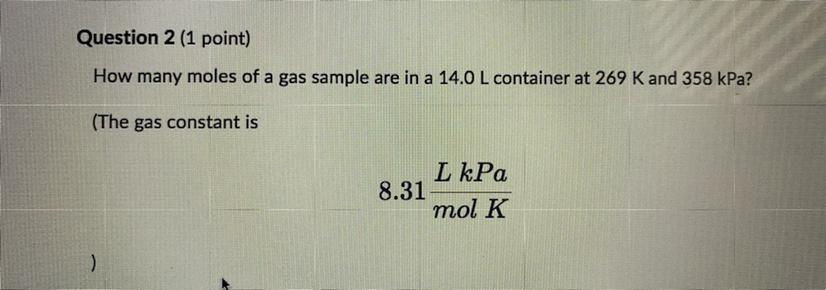
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
Help me pls I will mark you as brain

Answers
Answer:Infection with a pathogen does not necessarily lead to disease. Infection occurs when viruses, bacteria, or other microbes enter your body and begin to multiply. Disease occurs when the cells in your body are damaged as a result of infection and signs and symptoms of an illness appear.
Explanation:
With carbon dioxide, what phase change takes place when pressure
increases from 1 atm to 10 atm at -40°C?
Carbon Dloxide Phase Diagram
Melting
point
Boling
point
20-
15-
Liquid
Pressure (atm)
10-
Solid
5-
Gas
0
0
00
-100
Temperature (°C)
A. A solid changes to a liquid.
B. A liquid changes to a solid.
O C. A liquid changes to a gas.
D. A gas changes to a liquid.
Answers
Answer:B. A liquid changes to a solid.
Explanation:
A Liquid changes to solid when pressure
increases from 1 atm to 10 atm at -40°C
What is the concept for this?The increase in pressure on liquid it forms a solidIf the pressure above the liquid is decreased sufficently, the liquid form a gas How to solve this problem?Here the Freezing point is -78.33°C i.e [-109°F]above this temperature CO2 remains in liquid stateSo, given at -40°C
CO2 will be in liquid state and as per the concept explained above by increasing the pressure on liquid we get the solid state
Thus , With CO2 a liquid phase change to a solid
phase when pressure increases 1 atm to 10atm at -40°C
Learn more about phase diagram here
https://brainly.com/question/1612862
#SPJ2
what is the standard potential of a galvanic (voltaic) cell where x is the anode and y is the cathode?
Answers
The standard potential of a galvanic cell where x is the anode and y is the cathode can be determined using the equation E°cell = E°cathode - E°anode, where E°cell is the standard cell potential, E°cathode is the standard reduction potential of the cathode, and E°anode is the standard oxidation potential of the anode.
The standard potential is a measure of the tendency of the cell to produce a flow of electrons. A positive standard cell potential indicates that the cell will produce a flow of electrons from the anode to the cathode. The magnitude of the potential is dependent on the specific electrode materials and conditions of the cell.
The standard potential of a galvanic (voltaic) cell is determined by the difference in standard reduction potentials between the anode (X) and the cathode (Y). In this cell, X undergoes oxidation, losing electrons, while Y undergoes reduction, gaining electrons. The standard cell potential (E°cell) can be calculated using the formula:
E°cell = E°cathode - E°anode
Here, E°cathode is the standard reduction potential of Y and E°anode is the standard reduction potential of X. To find the values for X and Y, refer to the standard reduction potential table. A positive E°cell indicates a spontaneous reaction, while a negative value implies non-spontaneity.
To know about potential:
https://brainly.com/question/28300184
#SPJ11
Consuela lives on the east coast of Florida. She notices a neap tide occurs twice a
month. Neap tides occur when the Sun, the Moon, and Earth are in specific positions.
Sun
mm
Moon
4
Moon
Moon
Earth
Not to Scale
Moon
Which two of the four Moon positions shown above will cause nean tides 2

Answers
Answer: 2 and 4
Explanation:
Mrs. Scott did a demonstration for her class. She used tongs to hold a piece of steel wool in the flame of a Bunsen burner. The steel wool caught on fire and began to burn. Mrs. Scott removed the steel wool from the flame and allowed it to burn for 20 minutes.
Later, Mrs. Scott took a piece of the burned steel wool and held it in the flame of the Bunsen burner. It did not catch on fire.
Make a claim about whether the steel wool underwent a chemical reaction while burning. Support your claim with evidence from the demonstration.
Answers
The demonstration shows that steel wool burned chemically.
From the demonstration, steel wool burned chemically. The supporting evidence is:
1. Combustion: Bunsen burner flame ignited steel wool. A material combines with oxygen to produce heat, light, and gases or new compounds in combustion. Steel wool burning implies a chemical reaction.
2. Steel wool burned for 20 minutes. This prolonged burning signals a chemical process. Shape and size alterations rarely last this long.
3. Failure to Ignite: A piece of charred steel wool held in the flame again did not ignite. This implies that the initial chemical reaction that caused the steel wool to ignite was irreversible. After a chemical reaction consumes the reactants, it may not happen again without replacing them.
Learn more about combustion, here:
https://brainly.com/question/31123826
#SPJ1
a chemist prepares a solution of calcium sulfate by measuring out 0.53 umol of calcium sulfate into a 350 ml volumetric flask and filling the flask to the mark with water. calculate the concentration in of the chemist's calcium sulfate solution. round your answer to significant digits.
Answers
The chemist prepares a solution of calcium sulfate by measuring out 0.53 umol of calcium sulfate into a 350 ml volumetric flask and filling the flask to the mark with water. The concentration of a solution is 1.5 uM.
The concentration or molarity of a solution is defined as the number of moles present in the specific volume of a solution. It is calculated as
Concentration=moles/volume(in L)
The volume of a solution is in mL. Convert the given volume in L
350 ml×(1 L/1000 mL)=0.35 L
Plug the value of moles and volume in the formula
Concentration=0.53 umol/0.35 L
Concentration=1.51429 umol/L
Concentration=1.5 uM (∵M=mol/L)
Therefore, the concentration of a solution is 1.5 uM.
I have answered the question in general as given question is incomplete. The complete question is
a chemist prepares a solution of calcium sulfate by measuring out 0.53 umol of calcium sulfate into a 350 ml volumetric flask and filling the flask to the mark with water. calculate the concentration in uM of the chemist's calcium sulfate solution. round your answer to two significant digits.
To know more about concentrations
https://brainly.com/question/13872902
#SPJ4
There is 1 mole of propane (C3H8) for every 3 moles CO2. How many moles of CO2 would there be if you started with 2 moles of propane instead of 1?
Answers
Answer:
\(1 \: mole \: of \: propane \: reacts \: with \: 3 \: moles \: of \: carbondioxide \\ 2 \: moles \: of \: propane \: react \: with \: \frac{2 \times 3}{1} moles \\ = 6 \: moles \: of \: carbondioxide\)
a phase change that occurs at the normal melting point by the addition of very small amounts of heat at constant temperature and pressure will have
Answers
The phase change that occurs at the normal melting point by the addition of very small amounts of heat at constant temperature and pressure is called a "fusion" or "melting" transition.
Fusion is the transition of a solid into a liquid state. It is usually accompanied by an increase in temperature and an increase in pressure.
Fusion occurs when energy is added to a solid and the intermolecular forces are overcome, allowing the solid particles to move freely. The temperature of the solid at which fusion occurs is called the melting point. It is the same as the normal freezing point, but with the addition of heat.
When fusion occurs, the solid particles begin to move more freely and the solid take on a liquid form. The solid particles remain in contact with each other, but they can now move freely. The solid particles are now able to move past each other, allowing the solid to take the shape of the container in which it is held.
In summary,
Fusion is the phase change that occurs at the normal melting point by the addition of very small amounts of heat at constant temperature and pressure. The energy added causes the intermolecular forces to be overcome, allowing the solid particles to move freely and form a liquid.
To know more about Fusion transition, refer here:
https://brainly.com/question/29853732#
#SPJ11
Easyyy plz
What Is the Reactivity Series? ...
Answers
Answer:
In chemistry the reactivity series is an empirical, calculated, and structurally analytical progression of a series of metals, arranged by their "reactivity" from highest to the lowest.
Answer:
In a reactivity series, the most reactive element is placed at the top and the least reactive element at the bottom. More reactive metals have a greater tendency to lose electrons and form positive ions.
A reactivity series of metals could include any elements. For example,
A list of elements from most reactive to least reactive: potassium, sodium, lithium, calcium, magnesium, aluminum, zinc, iron, copper, silver, and gold.
A good way to remember the order of a reactivity series of metals is to use the first letter of each one to make up a silly sentence. For example, People Say Little Children Make A Zebra Ill Constantly Sniffing Giraffes.
Observations of the way that these elements react with water, acids, and steam enable us to put them into this series.
The tables show how the elements react with water and dilute acids:
Element Reaction with water
Potassium Violently
Sodium Very quickly
Lithium Quickly
Calcium More slowly
Element Reaction with dilute acids
Calcium Very quickly
Magnesium Quickly
Zinc More slowly
Iron More slowly than zinc
Copper Very slowly
Silver Barely reacts
Gold Does not react
Note that aluminum can be difficult to place in the correct position in the reactivity series during these experiments. This is because its protective aluminum oxide layer makes it appear to be less reactive than it really is. When this layer is removed, the observations are more reliable.
Non-metals in the reactivity series
It is useful to place carbon and hydrogen into the reactivity series because these elements can be used to extract metals.
Here is the reactivity series including carbon and hydrogen:
A list of elements from most reactive to least reactive: potassium, sodium, lithium, calcium, magnesium, aluminum, carbon, zinc, iron, hydrogen, copper, silver, and gold.
Note that zinc and iron can be displaced from their oxides using carbon but not using hydrogen. However, copper can be extracted using carbon or hydrogen. Displacement reactions of metal oxides
A more reactive metal will displace a less reactive metal from a compound. The thermite reaction is a good example of this. It is used to produce white-hot molten (liquid) iron in remote locations for welding. A lot of heat is needed to start the reaction, but then it releases an incredible amount of heat, enough to melt the iron.
aluminium + iron(III) oxide → iron + aluminium oxide
2Al + Fe2O3 → 2Fe + Al2O3
Because aluminum is more reactive than iron, it displaces iron from iron(III) oxide. The aluminum removes oxygen from the iron(III) oxide:
iron is reduced
aluminum is oxidized
Reactions between metals and metal oxides allow us to put a selection of metals into a reactivity series. Using metals A, B, and C:
Metal A Metal B Metal C
A oxide X Displaces A Displaces A
B oxide No reaction X No reaction
C oxide No reaction Displaces C X
Metal A cannot displace either B or C - so it must be the least reactive and be at the bottom of this reactivity series.
Metal B displaces both A and C - so it must be the most reactive and be at the top of this reactivity series.
Metal C displaces A but cannot displace B - so it must be more reactive than A but less reactive than B, and be in between them in this reactivity series.
In general, the greater the difference in reactivity between two metals in a displacement reaction, the greater the amount of energy released.
Aluminum is much higher than iron in the reactivity series, so the thermite reaction releases a lot of energy. Magnesium is very high in the reactivity series, and copper is very low - so the reaction between magnesium and copper oxide is more violent.
Therefore, the order is:
A list of letters from most reactive to least reactive: B, C and A,
Displacement reactions of solutions
A more reactive metal will displace a less reactive metal from a solution of one of its salts. For example:
magnesium + copper(II) sulfate → copper + magnesium sulfate
Mg(s) + CuSO4(aq) → Cu(s) + MgSO4(aq)
In this reaction, the blue color of the copper(II) sulfate fades as it is used up (magnesium sulfate solution is colorless). We would also see copper metal forming.
Reactions between metals and solutions of metal salts allow us to put a selection of metals into a reactivity series. Using metals J, K, and L:
Metal J Metal K Metal L
J sulfate X No reaction No reaction
K sulfate Displaces K X Displaces K
L sulfate Displaces L No reaction X
Metal J displaces both K and L - so it must be the most reactive and be at the top of this reactivity series.
Metal K cannot displace either J or L - so it must be the least reactive and be at the bottom of this reactivity series.
Metal L displaces K but cannot displace J - so it must be more reactive than K but less reactive than J, and be in between them in this reactivity series.
- sorry I'm late and it's is long -_-||