Answers
The formula of the ionic compound consisting of the positive ion to the left of the box and the negative ion above the box are as follows:
MgCl₂, MgS, MgF₂, Mg₃N₂, MgO, Mg₃P₂
CsCl, Cs₂S, CsF, Cs₃N, Cs₂O, Cs₃P
CrCl₃, Cr₂S₃, CrF₃, CrN, Cr₂O₃, CrP
NaCl, Na₂S, NaF, Na₃N, Na₂O, Na₃P
ZnCl₂, ZnS, ZnF₂, Zn₃N₂, ZnO, Zn₃P₂
AlCl₃, Al₂S₃, AlF₃, AlN, Al₂O₃, AlP
What are ionic compounds?Ionic compounds are pure substances made up of chemically bound ions. Examples include his binary compounds such as table salt and polyatomic compounds such as sodium sulfate. All ionic compounds form crystal lattices. Ionic compounds are pure substances formed mainly by positive and negative ionic attraction. Compounds are formed mainly by ions, although a small amount of electron sharing always occurs.
Ionic compounds are pure substances formed from chemically bound ions. Ionic bonds form ionic compounds. Covalent bonds form molecular bonds.Most atoms connect through covalent bonds, in which shared electrons form directional bonds. These are molecules.Ionic compounds are electrically neutral and consist of a few atoms (ions) or many atoms (ions). Diatomic and polyatomic compounds are equally common.To know more about ionic compounds, visit:
https://brainly.com/question/9167977
#SPJ1
Related Questions
ASAPP Which electron below is furthest from the nucleus?

Answers
3) An electron in the 4s orbital
Hope it helps
Account for the change when NO2Cl is added using the reaction quotient Qc. Match the words in the left column to the appropriate blanks in the sentences on the right.
1. decreases
2. loss
3. Increases
4. greater
A. Disturbing the equilibrium by adding NO2Cl______Qc to a value_____than Kc.
B. To reach a new state of equilibrium, Qc therefore______which means that the denominator of the expression for Qc______.
C. To accomplish this, the concentration of reagents______, and the concentration of products_______.
Answers
Answer:
A. Disturbing the equilibrium by adding NO2Cl decreases Qc to a value less than Kc.
B. To reach a new state of equilibrium, Qc therefore increases which means that the denominator of the expression for Qc decreases.
C. To accomplish this, the concentration of reagents decreases, and the concentration of products increases.
Explanation:
Hello,
In this case, for the equilibrium reaction:
\(NO_2Cl(g)+NO(g)\rightleftharpoons NOCl(g)+NO_2(g)\)
Whose equilibrium expression is:
\(Kc=\frac{[NO_2][NOCl]}{[NO_2Cl][NO]}\)
The proper matching is:
A. Disturbing the equilibrium by adding NO2Cl decreases Qc to a value less than Kc, since the denominator becomes greater, therefore, Qc decreases.
B. To reach a new state of equilibrium, Qc therefore increases which means that the denominator of the expression for Qc decreases, since the lower the denominator, the higher Qc as it has the concentration of reactants.
C. To accomplish this, the concentration of reagents decreases, and the concentration of products increases, since the reactants must be consumed in order to reestablish equilibrium by shifting the reaction towards the products.
Best regards.
Scientific method practice hypothesis construction & experimental design
Answers
Best example of a new substance being formed
Answers
Best example of a new substance being formed is the reaction between hydrogen gas and oxygen gas to form water.
In this reaction, two hydrogen molecules (H₂) react with one oxygen molecule (O₂) to form two water molecules (H₂O). The resulting substance, water, has entirely different properties from its constituent elements. For example, water is a liquid at room temperature, while both hydrogen and oxygen are gases.
Water also has different chemical and physical properties, such as boiling point, melting point, density, and solubility. These changes in properties indicate that a new substance with a different chemical composition has been formed. The process of hydrogen and oxygen reacting to form water is an example of a chemical reaction, which involves the breaking and forming of chemical bonds between atoms to create new substances.
To learn more about chemical reaction, here
https://brainly.com/question/29039149
#SPJ1
pls answer my questions i need them asap
The preferred arrangement of electrons in a molecular structure can be determined by calculating the _______ of each atom in the molecule.
If the dipole moments from polar covalent bonds don't cancel, then the molecule will be _______.
When a molecule can have multiple structures with _______ in different positions, the molecule has resonance.
Repulsion between _______ and electrons in covalent bonds causes some molecules to have a bent shape.
Respond to the following based on your reading.
Explain the relationship between the polarity of a molecule, its dipole moment, and intermolecular bonding.
Explain why ionic compounds are always polar.
pls answer my questions i need them asap
Answers
The preferred arrangement of electrons in a molecular structure can be determined by calculating the electronic configuration of each atom in the molecule.
What is electronic configuration?
The electronic configuration of an atom is determined by the number of protons in the nucleus and the specific rules governing the filling of energy levels and orbitals. The electronic configuration is usually represented using a notation that indicates the number of electrons in each energy level or orbital.
what is delocalized electron ?
Delocalized electrons are electrons that are not restricted to a specific location or region in a molecule or material, but instead are spread out over multiple atoms or molecules. In other words, delocalized electrons are free to move throughout a larger region instead of being confined to a single atom or bond.
To know more about polar visit:-
https://brainly.com/question/1433127
#SPJ1
Define biotechnology. } List two advantages in the use of biotechnology
Answers
Advantages of biotechnology:
Improvement of plants and animal breeds to give a high yield of their products.
Pests and pathogen control in agriculture which will reduce the loss of yield in food crops.
Synthesis of biocatalyst which can be used for enhancing the reactions which can be carried out in vitro or laboratory conditions.
Sewage treatment or water recycling can be done with the help of transgenic microbes which have better efficiency and speed.
Biotechnology is the use of living organisms or other biological systems in the manufacture of drugs or other products or for environmental management, as in waste recycling: includes the use of bioreactors in manufacturing, microorganisms to degrade oil slicks or organic waste, genetically engineered bacteria to produce human hormones, and monoclonal antibodies to identify antigens.
Biotech offers the possibility of improving human health, the environment, and agriculture while creating more sustainable modes of production.
What is the percent yield when 1.72 g of H2O2 decomposes and produces 375 mL of O2 gas measured at 42 oC and 1.52 atm
Answers
Answer:
87.0%
Explanation:
Step 1: Write the balanced reaction
H₂O₂ ⇒ H₂O + 0.5 O₂
Step 2: Calculate the real yield of oxygen, in grams
We have 375 mL (0.375 L) of O₂ at 42 °C (315 K) and 1.52 atm. First, we will calculate the number of moles using the ideal gas equation.
P × V = n × R × T
n = P × V / R × T
n = 1.52 atm × 0.375 L / (0.0821 atm.L/mol.K) × 315 K = 0.0220 mol
The molar mass of oxygen is 32.00 g/mol.
0.0220 mol × 32.00 g/mol = 0.704 g
Step 3: Calculate the theoretical yield of oxygen, in grams
According to the balanced equation, the mass ratio of H₂O₂ to O₂ is 34.01:16.00.
1.72 g H₂O₂ × 16.00 g O₂/34.01 g H₂O₂ = 0.809 g O₂
Step 4: Calculate the percent yield of oxygen
We will use the following expression.
%yield = real yield / theoretical yield × 100%
%yield = 0.704 g / 0.809 g × 100% = 87.0%
Considering the reaction stoichiometry and the ideal gas law, the percent yield when 1.72 g of H₂O₂ decomposes and produces 375 mL of O₂ gas measured at 42 °C and 1.52 atm is 86.96%.
Theoretical yield of oxygenThe balanced reaction is:
2 H₂O₂ → 2 H₂O + O₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
H₂O₂: 2 moleH₂O: 2 mole O₂: 1 molesThe molar mass, this is the amount of mass a substance contains in one mole, of H₂O₂ is 34 \(\frac{g}{mole}\). Then, the amount of moles of H₂O₂ that decomposes when 1.72 grams of H₂O₂ reacts is calculated as:
\(1.72 gramsx\frac{1 mole}{34 grams}= 0.0506 moles\)
Then you can apply the following rule of three: if by stoichiometry 2 moles of H₂O₂ produce 1 moles of O₂, 0.0506 moles of H₂O₂ will produce how many moles of O₂?
\(amount of moles of O_{2} =\frac{0.0506 moles of H_{2} O_{2} x1 mole of O_{2} }{2 moles of H_{2} O_{2}}\)
amount of moles of O₂= 0.0253 moles
Real yield of oxygenOn the other side, an ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of the gases:
P× V = n× R× T
In this case, for O₂ gas you know:
P= 1.52 atmV= 375 mL= 0.375 L (being 1000 mL= 1 L)n= ?R= 0.082 \(\frac{atmL}{molK}\)T= 42 °C= 315 °K (being 0°C= 273°K)Replacing:
1.52 atm× 0.375 L = n× 0.082 \(\frac{atmL}{molK}\)× 315 K
Solving:
\(n=\frac{1.52 atmx 0.375 L}{0.082\frac{atmL}{molK}x 315 K }\)
n= 0.022 moles
Percent yield of oxygenThe percent yield is calculated as
\(Percent yield= \frac{real yield}{theoretical yield} x100\)
In this case, for oxygen the percent yield is calculated as
\(Percent yield of oxygen= \frac{0.022 moles}{0.0253 moles} x100\)
Percent yield of oxygen= 86.96 %
Finally, the percent yield when 1.72 g of H₂O₂ decomposes and produces 375 mL of O₂ gas measured at 42 °C and 1.52 atm is 86.96%.
Learn more about:
the reaction stoichiometry: brainly.com/question/16487206?referrer=searchResults brainly.com/question/14446695?referrer=searchResults brainly.com/question/11564309?referrer=searchResults brainly.com/question/4025026?referrer=searchResults brainly.com/question/18650135?referrer=searchResultsideal gas lawhttps://brainly.com/question/4147359?referrer=searchResultsWhat happens to the total mass during a chemical reaction
Answers
Explanation:
That the mass can neither be created nor destroyed in a chemical reaction.
10. Although lidocaine is marketed as its hydrochloride salt, it doesn’t exhibit the same level of physiological activity as the free amine. The free amine is more lipophilic and diffuses across a neuron cell membrane more rapidly than the ionic salt, resulting in a more rapid onset of anesthesia. Therefore, sodium bicarbonate (NaHCO3) is added to a solution of lidocaine prior to injection. How does the addition of sodium bicarbonate promote a faster anesthetic effect?
Answers
Answer:
Bicarbonate neutralizes the acidity of Lidocaine and hence reduce the pain
Explanation:
Alkalinization by addition of sodium bicarbonate causes buffering of local anesthetics and hence produce faster anesthetic effect such as pain control, pain reduction while injecting the patient and faster onset of local anesthetics.
Lidocaine along with epinephrine results into acidic compound wit respect to subcutaneous tissue. Hence, when bicarbonate is added, it neutralizes the acidity of Lidocaine and hence decrease the pain.
When C2H4(g) reacts with H2(g), the compound C2H6(g) is produced, as represented by the equation above. The reaction is correctly classified as which of the following types?
answer choices
a. Acid-base, because two hydrogen atoms are donated to
b. C2H4(g).
c. Precipitation, because two reactant species form a single product.
d. Decomposition, because pure H2(g) is consumed.
e. Oxidation-reduction, becauseH2(g) is oxidized.
Answers
When C2H4(g) reacts with H2(g), the compound C2H6(g) is produced, as represented by the equation above. The reaction is reduction reaction.
What is reduction reaction?
Because it involves the addition of electrons, which lowers the atom's oxidation number, reduction is known as what it is.
any of a range of chemical processes where an atom or group of atoms' associated electron count is enhanced. Another substance, which is oxidized as a result, supplies the electrons that are taken up by the reduced molecule. Check out the oxidation-reduction reaction.
A good reducing agent is hydrogen. It can react with both free oxygen and oxygen-containing molecules.
C2H4+H2-> C2H6
Read more about reduction reaction:
https://brainly.com/question/21851295
#SPJ4
How many grams of Ca(OH)2are required to make 1.5 L of a 0.81 M solution?
A 40 grams
B 0.016 grams
C 89 grams
D 12 grams
Answers
Answer:
Mass = 90.28 g
Explanation:
Given data:
Mass of Ca(OH)₂ = ?
Volume of solution= 1.5 L
Molarity of solution = 0.81 M
Solution:
First of all we will calculate number of moles.
Molarity = number of moles / volume in L
by putting values,
0.81 M = Number of moles / 1.5 L
Number of moles = 0.81 M × 1.5 L
Number of moles = 1.22 mol
Mass of Ca(OH)₂ in gram:
Mass = number of moles × molar mass
Mass = 1.22 mol × 74.09 g/mol
Mass = 90.28 g
other m Ammonium nitrate decomposes to nitrogen(1) oxide and water. 9. Some oxides of nitrogen are atmospheric pollutants. and oxygen. Revision Exercise When compound X is heated, a red-brown gas is evolved and a yellow residue is left on cooling. Name: (i) The red-brown gas. (ii) The ions present in the residue. (ui) Compound X.
Answers
We can determine the following based on the provided information:
Metal nitrate A is a compound that, when heated, transforms into colourless gas, brown gas B, and a metal oxide with a yellowish brown hue. B. C: Colourless petrol C. B: Brown petrol C. D: Compound D, a yellow precipitate produced by the reaction of potassium iodide with an aqueous solution of compound A.
We may deduce that A is probably lead nitrate (Pb(NO3)2) because lead is frequently used in soldering alloys and the metal contained in A is utilised in an alloy for soldering purposes.
Identifications:
Lead nitrate, or Pb(NO3)2,
N2O: Nitrogen dioxide
B: Carbon (CO)
D: PbI2, or lead iodide.
Thus, this can be concluded regarding the given scenario.
For more details regarding metals, visit:
https://brainly.com/question/29404080
#SPJ1
Your question seems incomplete, the probable complete question is:
A metal nitrate A on heating gives a yellowish brown coloured metal oxide along with brown gas B and a colourless gas C. An aqueous solution of A on reaction with potassium iodide forms a yellow precipitate of compound D. Identify A, B, C and D. Also, identify the types of reactions taking place. Metal present in A is used in an alloy which is used for soldering purposes.
Which of the following is an example of an unbalanced force?
A stroller being pushed
A ball on the grass
A chair leaning on the wall
A book sitting on a table
Answers
Answer:
A. A stroller being pushed
Explanation:
The person pushing the stroller is putting more force than the stroller is.
I need a quick answer please

Answers
We can determine the relative atomic ratios in a compound using an empirical formula.
What sort of empirical formula would that be?The chemical structure of glucose is C6H12O6. Every mole of carbon and oxygen is accompanied by two moles of hydrogen. Glucose has the empirical formula CH2O. Ribose has the chemical formula C5H10O5, which can be simplified to the empirical formula CH2O.We can determine the relative atomic ratios in a compound using an empirical formula. The ratios also hold true at the molar level. H2O is made up of two hydrogen atoms and one oxygen atom.The complete question is,
How does the empirical formula inform us?
To learn more about Empirical formula refer to:
https://brainly.com/question/1603500
#SPJ1
How many atoms are in 12 g of Carbon-12 (12C)?
Answers
There are approximately 6.022 × 10^23 atoms in 12 grams of Carbon-12 (12C).
The number of atoms in a given amount of a substance can be calculated using Avogadro's number, which represents the number of atoms or molecules in one mole of a substance. Avogadro's number is approximately 6.022 × 10^23.
Carbon-12 is a specific isotope of carbon, with an atomic mass of 12 atomic mass units (amu). One mole of Carbon-12 has a mass of 12 grams. Since one mole of any substance contains Avogadro's number of particles, in the case of Carbon-12, it contains 6.022 × 10^23 atoms.
Therefore, if we have 12 grams of Carbon-12, which is equal to one mole, we can conclude that there are approximately 6.022 × 10^23 atoms in this amount of Carbon-12.
In summary, 12 grams of Carbon-12 contains approximately 6.022 × 10^23 atoms. Avogadro's number allows us to relate the mass of a substance to the number of atoms or molecules it contains, providing a fundamental concept in chemistry and enabling us to quantify and understand the microscopic world of atoms and molecules.
for such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
Is a burning log an exothermic or endothermic event if the log is the (system) ?
Answers
Answer:
Exothermic Reaction
Explanation:
Burning wood in air is an exothermic process (it releases heat), but there is an energy barrier, so it requires a bit of heat in the beginning to get the reactions started.
Wood reacts with oxygen in the air to form (mostly) carbon dioxide and water vapor. The process involves many different individual chemical reactions, and it requires some energy to initiate the reactions. This is because it's usually necessary to break some chemical bonds (endothermic) before new stronger bonds can be formed (exothermic). Overall, though, more heat is released in forming the final products than is consumed in starting new reactions.
Have a great day <3
In the given question, a burning log is an exothermic event if the log is the system.
An exothermic reaction releases energy to the surroundings, usually in the form of heat. The change in enthalpy is negative.
When a log burns, it reacts with oxygen in the air to produce carbon dioxide, water vapor, and heat. This heat is released to the surroundings, making the burning log an exothermic event.
Therefore, a burning log is an exothermic event if the log is the system. This is due to the fact that the reaction emits heat into the environment, making it an exothermic reaction.
Learn more about exothermic reaction here:
https://brainly.com/question/28546817
#SPJ6
Natural gas is almost entirely methane. A container with a volume of 2.65L holds 0.120mol of methane. What will the volume be if an additional 0.182mol of methane is added to the container under constant temperature and pressure? Give your answer in three significant figures.
Answers
The final volume of the methane gas in the container is 6.67 L.
The given parameters;
initial volume of gas in the container, V₁ = 2.65 Linitial number of moles of gas, n₁ = 0.12 moladditional concentration, n = 0.182 molThe total number of moles of gas in the container is calculated as follows;
\(n_t = 0.12 + 0.182 = 0.302 \ mol\)
The final volume of gas in the container is calculated as follows;
\(PV = nRT\\\\\frac{V}{n} = \frac{RT}{P} \\\\\frac{V_1}{n_1} = \frac{V_2}{n_2} \\\\V_2 = \frac{V_1 n_2}{n_1} \\\\V_2 = \frac{2.65 \times 0.302}{0.12} \\\\V_2 = 6.67 \ L\)
Thus, the final volume of the methane gas in the container is 6.67 L.
Learn more here:https://brainly.com/question/21912477
Arjuna stood at Krishna feet with " rgppsmk Arjuna aet "arms folded what aspect of Arjuna character does this gesture show
Answers
The gesture of Arjuna standing at Krishna's feet with folded arms represents the aspect of Arjuna's character known as
Humility is an aspect of Arjuna's character that is represented by his gesture of standing at Krishna's feet with folded arms. Humility is the quality of being humble, which is the ability to show modesty, kindness, and an appreciation of the worth of others.
According to the Bhagavad Gita, humility is a highly regarded virtue and is one of the essential qualities that a person should have. It is said that by cultivating humility, a person can overcome many of the obstacles and difficulties that life throws their way. Humility is also believed to be the key to true knowledge and wisdom.
Know more about aspect here:
https://brainly.com/question/13095294
#SPJ8
What is the only entirely liquid layer of the earth
Answers
Answer:
i am pretty sure that it is the inter core
What is the temperature (in K) of 16.45
moles of methane gas in a 70.7 L
container at 3,557 torr?
Answers
At 3,557 torr, 16.45 moles of methane gas are at a temperature of T=17.15 K in a 70.7 L container.
According to the ideal gas law: PV=nRT, where R=0.082 and T is temperature in Kelvin (K). P is pressure, V is volume, n is the amount in moles.
(4.68)*(4.95)=(16.45)*(0.0821)*
T=17.15, therefore calculate it.
What's called gas?An object that is in the gaseous, or vaporous, condition of matter, is referred to as a gas. When referring to material with characteristics of a gaseous substance, the word "gas" can also refer to the condition itself. Along with liquid, solid, and plasma, gases are one of the four natural states of matter. The shape or volume of a gas can change.
To know more about Gas visit:
https://brainly.com/question/10725862
#SPJ1
Answer:
245
Explanation:
everyone needs to chill don't answer if you can't get it right it wastes you time and others as well not trying be a hater but its true
Which of the following accurately represents the relationship between ceramic and
metal?

Answers
Relationship between metal and ceramic is accurately represented by the statement that metal is a better conductor than ceramics.
What are conductors?Conduction is defined as a process as a means of which heat is transferred from the hotter end of the body to it's cooler end.Heat flows spontaneously from a body which is hot to a body which is cold. Substances which enable conduction are called conductors.Conductors allow passage heat and electricity through the material.
In the process of conduction,heat flow is within the body and through itself.In solids the conduction of heat is due to the vibrations and collisions of molecules while in liquids and gases it is due to the random motion of the molecules .
When conduction takes place, heat is usually transferred from one molecule to another as they are in direct contact with each other.There are 2 types of conduction:1) steady state conduction 2) transient conduction.According to the type of energy conduction is of three types:
1) heat conduction
2) electrical conduction
3)sound conduction
Learn more about conductors,here:
https://brainly.com/question/8426444
#SPJ6
HELP WITH SIMPLE ELECTROCHEM QUESTION

Answers
How many moles are in 1.81x10^26 molecules of H2O
Answers
According to mole concept and Avogadro's number , there are 300.5 moles in 1.81×10²⁶ molecules of water.
What is a mole?Mole is defined as the unit of amount of substance . It is the quantity measure of amount of substance of how many elementary particles are present in a given substance.
It is defined as exactly 6.022×10²³ elementary entities. The elementary entity can be a molecule, atom ion depending on the type of substance. Amount of elementary entities in a mole is called as Avogadro's number.
As 1 mole= 6.023×10²³ molecules ,therefore 1.81×10²⁶ molecules= 1.81×10²⁶ /6.023×10²³=300.5 moles.
Thus, there are 300.5 moles in 1.81×10²⁶ molecules of water.
Learn more about mole,here:
https://brainly.com/question/26416088
#SPJ1
The isotope calcium-41 decays into potassium-41, with half-life of 1.03 x 105 years. There is a sample of calcium-41 containing 5 x 10° atoms. How
many atoms of calcium-41 and potassium-41 will there be after 4.12 › 105 years?
A.
3.125 x 108 atoms of calcium-41 and 4.375 x 10° atoms of potassium-41
6.25 x 108 atoms of calcium-41 and 4.6875 x 10° atoms of potassium-41
•
6.25 x 108 atoms of calcium-41 and 4.375 10° atoms of potassium-41
O D. 3.125 x 108 atoms of calcium-41 and 4.6875 › 10° atoms of potassium-41
Answers
3.125 × 10^8 atoms of calcium-41 and 4.6875 × 10^9 atoms of potassium-41.
Option D is correct
Isotope 41 of calcium is it?A rare and long-lived radioactive isotope of calcium is calcium-41 (41Ca).
# Given-
Half life = 1.03×10^5 years
- After 1.03×10^5 years (1 half life)
calcium-41 will be 50%
potassium-41 will be 50%
- After 2.06×10^5 years (2 half lives)
calcium-41 will be 25%
potassium-41 will be 75%
- After 3.09×10^5 years (3 half lives)
calcium-41 will be 12.5%
potassium-41 will be 87.5%
- After 4.12×10^5 years (4 half lives)
calcium-41 will be 6.25%
potassium-41 will be 93.75%
After 4.12×10^5 years,
Calcium-41 = 6.25/100 × 5 × 10^9 = 3.12×10^8 atoms
Potassium-41 = 93.75/100 × 5 × 10^9 = 4.69×10^9 atoms
To know more about Isotope 41 visit:-
https://brainly.com/question/29427598
#SPJ1
A ramp is at an angle of elevation of 15° . The distance from the top of the ramp to the ground is 20 feet. What is the length of the ramp?
Answers
Answer:
Length of ramp = 77.27ft
Explanation:
Step 1 - Sketch a diagram
Make a line about 15 deg. above the horizontal x-axis. Draw the horizontal and vertical components to form a right triangle. See image if you don’t understand.
Step 2 - Label your diagram
Your triangle creates a 15 deg. angle with the x-axis and we know the vertical distance from the top of the ramp to the ground is 20 ft.
Step 3 - Use trig to make an equation and solve
SOH CAH TOA
I’m this problem we know the angle, know “opposite, and we are solving for the hypotenuse of the triangle. Therefore, it would be easiest to use SOH or sin(theta)= (opp./hyp.) Plug in the numbers and solve for the hypotenuse.
All work is shown in the attached image.

Gaseous butane (CH₂(CH₂)₂CH₂) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 48. g of butane is mixed with 54.6 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.
Answers
The maximum mass of carbon dioxide that could be produced by the chemical reaction is 46.2 g
How do i determine the mass of of carbon dioxide produced?First, we shall determine the limiting reactant. This obtained as follow
2CH₃(CH₂)₂CH₃ + 13O₂ -> 8CO₂ + 10H₂O
Molar mass of CH₃(CH₂)₂CH₃ = 58 g/molMass of CH₃(CH₂)₂CH₃ from the balanced equation = 2 × 58 = 116 g Molar mass of O₂ = 32 g/molMass of O₂ from the balanced equation = 13 × 32 = 416 gFrom the balanced equation above,
116 g of CH₃(CH₂)₂CH₃ reacted with 416 g of O₂
Therefore,
48 g of CH₃(CH₂)₂CH₃ will react with = (48 × 416) / 116 = 172.14 g of O₂
From the above calculation, we can see that a higher amount (i.e 172.14 g) of O₂ than what was given (i.e 54.6 g) is needed to react with 48 g of CH₃(CH₂)₂CH₃
Thus, the limiting reactant is O₂
Finally, we shall determine maximum mass of carbon dioxide, CO₂ produced. Details below:
2CH₃(CH₂)₂CH₃ + 13O₂ -> 8CO₂ + 10H₂O
Molar mass of O₂ = 32 g/molMass of O₂ from the balanced equation = 13 × 32 = 416 gMolar mass of CO₂ = 44 g/molMass of CO₂ from the balanced equation = 8 × 44 = 352 gFrom the balanced equation above,
416 g of O₂ reacted to produce 352 g of CO₂
Therefore,
54.6 g of O₂ will react to produce = (54.6 × 352) / 416 = 46.2 g of CO₂
Thus, the maximum mass of carbon dioxide, CO₂ produced is 46.2 g
Learn more about mass produced:
https://brainly.com/question/9526265
#SPJ1
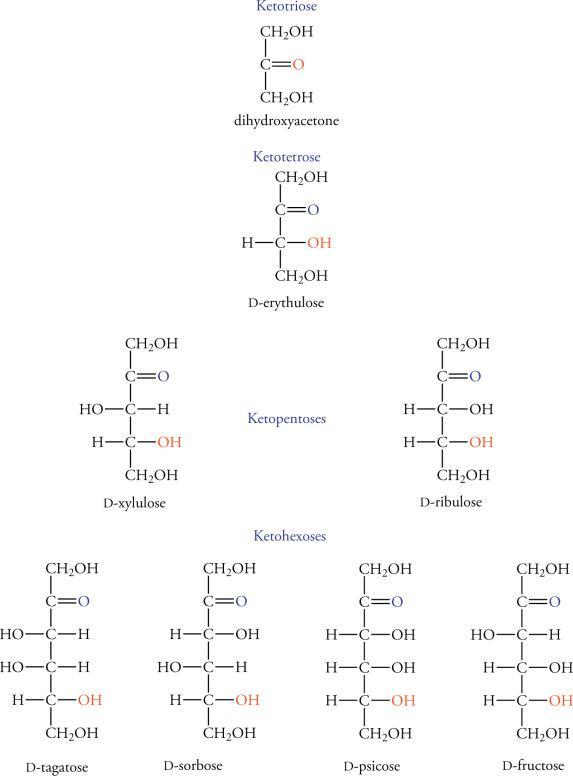
1. List the ketose carbon(s) that is(are) reduced with NaBH4.
Answers
Answer:
ehh im nit sure but here!
Explanation:
What are the reactant in the following chemical equation 2h2o o2 +2h2
Answers
Answer:
In Explanation
Explanation:
The reactants in the following chemical equation:
2H2O + O2 → 2H2 + 2O2
are H2O and O2. They are substances that are present at the beginning of the reaction and are consumed during the reaction to form the products. In this case, 2 water molecules (H2O) and 1 molecule of Oxygen (O2) are the reactants.
Determine the [H+] , [OH−], and pOH of a solution with a pH of 7.41
at 25 °C. [H+]=
M
[OH−]=
M
pOH=
Answers
Answer:
Explanation:
H+ = 1 X 10^-7.41 = 3.89 X 10^ -8
POH = 14-7.41 = 6.59
OH- = 1 x 10 ^-6.59 = 2.57 X 10^ -7
The [H+] and [OH−] concentrations of the solution are approximately 2.38 × 10^(-7) M, and the pOH is 6.59.
The pH of a solution is a measure of the concentration of hydrogen ions ([H+]) in the solution. The pH scale ranges from 0 to 14, with a pH of 7 considered neutral. A pH of 7.41 indicates that the solution is slightly basic. To calculate the [H+], [OH−], and pOH of the solution, we can use the relationship:
pH + pOH = 14
Given that the pH is 7.41, we can subtract it from 14 to find the pOH:
pOH = 14 - 7.41 = 6.59
Since pH + pOH = 14, we can also determine the [OH−] by taking the antilogarithm of the pOH value:
[OH−] = 10^(-pOH)
[OH−] = 10^(-6.59)
[OH−] ≈ 2.38 × 10^(-7) M
Since the solution is neutral, the concentration of [H+] will be equal to the concentration of [OH−]:
[H+] = [OH−] ≈ 2.38 × 10^(-7) M
Therefore, the [H+] and [OH−] concentrations of the solution are approximately 2.38 × 10^(-7) M, and the pOH is 6.59.
For more question on concentrations
https://brainly.com/question/30766678
#SPJ11
Which choice describes the function of the pancreas gland?
A. It secretes hormones that regulate blood sugar levels.
B. It secretes adrenaline to help the body respond to fear.
C. It secretes hormones that regulate water concentration levels.
D. It secretes thyroxine, which is involved in growth.
Answers
Answer:
A. It secretes hormones that regulate blood sugar levels.
Explanation:
The pancreas is the organ that helps in the process of digestion of the food. The location of the pancreas is in the abdomen. It helps in the process of digestion of the food and regulating the blood sugar level in the body. It helps in regulating and controlling the glucose level in the body during the process of digestion. Pancreas produces enzymes that are useful in the process of digestion.
Answer:
A
Explanation:
