in acidic medium dichromate Ion oxidized ferrous sulphate to ferric sulphate and and it self reduced to chromium 3 + ions write a balanced equation to represent this this redox reaction use Ion electron method
Answers
Answer:
Cr
2
O
7
2−
+14H
+
+6Fe
2+
→2Cr
3+
+6Fe
3+
+7H
2
O
During the reaction, the oxidation number of Cr decreases from +6 to +3. Net decrease in the oxidation number of one Cr atom is 6−3=3. For 2 Cr atoms (in dichromate ion), the total decrease in the oxidation number is 2×3=6
The gram equivalent weight =
Total change in the oxidation number
gram molecular weight
=
6
294
=49
Related Questions
If you are given a piece of rock sugar about 2.5 cm in diameter, describe three steps you can take to dissolve it in a beaker of water in the shortest time.
Answers
Answer:
1. Crush the sugar into powder.
2. Heat the water.
3. Dissolve it by stirring continuously
Explanation:
1. Crushing the sugar into powder increases surface area. So it increases the changes of dissolving
2. Heating the water increases the capacity of water to dissolve sugar.
3. Stirring continuously increases randomness of particles so eases mixing up thus increasing dissolving tendency.
If you are given the amount of MOLES CONSUMED of a compound, and it asks you for the mass percent of said compound, how do you find the mass percent of said compound?
Answers
Answer:
When we talk about a compound's mass percent, we want to know how much of that chemical is in a combination or sample. To calculate the mass percent, we must first know how much of the compound we have and how much of everything else we have.
Assume we have a dish of fruits that weights 100 grammes. We'd want to know how much of the fruit is made up of kiwis. We count the kiwis and discover that there are twenty of them.
To calculate the mass % of kiwis, we must first determine how much they weigh in comparison to the rest of the fruits. Assume the kiwis weigh 40 grammes in total. We may use this data to compute the percentage of kiwis in the fruit:
Mass percent of kiwis = (mass of kiwis ÷ total mass of fruits) × 100%
Mass of kiwis = 40 grams
Total mass of fruits= 100 grams
Mass percent of kiwis= (40 grams ÷ 100 grams) × 100% = 40%
So we can say that the fruits is 40% kiwis.
Similarly, when we want to find the mass percent of a compound in a mixture or sample, we need to know how much of that compound we have and how much of everything else we have. We can use the formula I gave earlier to calculate the mass percent of the compound.
which is Mass percent = (mass of compound consumed ÷ total mass of sample) × 100%
Below here is the answer, but i suggest you should give it a try first :)
Molar mass of the compound = 20 grams/mole
Mass of the compound consumed = 2 moles × 20 grams/mole = 40 grams
Total mass of the sample or mixture = 50 grams
Mass percent of the compound = (40 grams ÷ 50 grams) × 100% = 80%
So the mass percent of the compound in the sample is 80%.
How much is 1 mole of water molecules?
Answers
Answer:
Hey mate,
Here is your answer. Hope it helps you.
18 grams.
Explanation:
The mass of one mole of any atom/molecule is equal to its atomic molecular mass in grams.
The molecular formula of water is
H
2
O
The atomic mass of
H
= 1
The atomic mass of
O
= 16
In a sense, the formula means
H
+
H
+
O
So... 1 + 1 + 16 = 18
Therefore, the mass of one mole of water = 18 milliletres
1. Determinati masa moleculara, raportul atomic si raportul de masa pentru oxidul de aluminiu 2. Determinati compozutia procebtuala al hidroxidului de sofiu sau sofa cauxica, 3. Determinati ce cantitate calciu este cuprinds in 112 grame oxid de calciu 4. Determinati ce cantiate de sulf in 490 de grame de acid sulfuric
Answers
Answer:
im solving now
Explanation:
Fe(OH)3->Fe2O3->Fe->FeCl2->Fe(OH)2
Answers
\(Fe(OH)3- > Fe2O3- > Fe- > FeCl2- > Fe(OH)2\), represents a set of possible chemical reactions that Fe(OH)3, or iron(III) hydroxide, can undergo.
In the first reaction, Fe(OH)3 is converted into Fe2O3, or iron(III) oxide, through the process of thermal decomposition. This reaction occurs when Fe(OH)3 is subjected to high temperatures, causing it to break down into Fe2O3 and water vapor.
In the second reaction, Fe2O3 is reduced to Fe, or elemental iron, through the process of electrolysis. This reaction involves passing an electric current through a solution of Fe2O3, causing the Fe2O3 to be reduced to Fe at the cathode and oxygen to be produced at the anode.
In the third reaction, Fe is converted into FeCl2, or iron(II) chloride, through the process of chlorination. This reaction involves adding chlorine gas to a solution of Fe, causing the Fe to be converted into FeCl2 and hydrogen gas.
In the fourth reaction, FeCl2 is converted into Fe(OH)2, or iron(II) hydroxide, through the process of acidification. This reaction involves adding an acid to a solution of FeCl2, causing the FeCl2 to be converted into Fe(OH)2 and hydrochloric acid.
Read more about Iron(Fe):
https://brainly.com/question/23408174
The correct question is:
Explain this chemical reaction \(Fe(OH)3- > Fe2O3- > Fe- > FeCl2- > Fe(OH)2\)
what are the reactants in the following equation: hcl(aq) nahco₃(aq)→ co₂(g) h₂o(l) nacl(aq)
Answers
Hydrogen chloride (HCl) and sodium bicarbonate (NaHCO3) are the reactants. The substances produced as a result of this reaction are CO2(g), H2O(l), and NaCl(aq).
The reactants in the following equation:
HCl (a q) + NaHCO3(a q) → CO2(g) + H2O(l) + Na C l (aq) are hydrogen chloride (HC l) and sodium bicarbonate (NaHCO3).
Explanation:
A reactant is a substance that undergoes change during a chemical reaction. A reaction equation includes the symbols and formulas of reactants and products, along with the physical states of the substances, as they appear before and after the reaction.
The chemical reaction in this question is: HC l(a q) + NaHCO3(a q) → CO2(g) + H2O(l) + Na Cl (a q)In this equation, the reactants are H Cl (a q) and NaHCO3(a q), which are in an aqueous state.
Therefore, hydrogen chloride (H Cl) and sodium bicarbonate (NaHCO3) are the reactants. The substances produced as a result of this reaction are CO2(g), H2O(l), and Na Cl (a q).
to know more about reactants visit :
https://brainly.com/question/29035733
#SPJ11
Calculate the bond dissociation energy for the breaking of all the bonds in a mole of methane, ch4.
Answers
397.2 kcal. is the bond dissociation energy for the breaking of all the bonds in a mole of methane, CH4.
What is CH4?
One carbon atom makes up the molecule methane(CH4), which has four hydrogen atoms bound to the carbon by single bonds. It is a odorless, colorless, non-toxic, flammable gas and has a melting point of -161°C. CH4 performs three functions: fossil fuel, greenhouse gas, and bacterial metabolite.
For calculating the bond dissociation energy for the breaking of all the bonds in a mole of methane, CH4.
do as follows.
heat of atomization of solid carbon =170.9kcal
and heat of atomization of four gaseous hydrogen is =4 × 52.1kcal
So, energy for breaking four carbon-hydrogen bonds
= 170.9 + (4 × 52.1) + 17.9 = 397.2 kcal.
Hence, 397.2 kcal. is the bond dissociation energy for the breaking of all the bonds in a mole of methane, CH4
To know more about CH4 visit
https://brainly.com/question/27926556
#SPJ4
What is the acceleration acquired by an object that has a mass of 50kg and is pushed with a force of 20N.
Answers
If a 20 N force is applied to a 50 kg mass, then the acceleration acquired by the body is 0.4 m/s².
¿How to calculate the acceleration of a body?It is possible to know the acceleration of a body from Newton's second law, which states that the acceleration is defined as:
a = F/mWhere:
A = accelerationF = forceM = massTroubleshooting:We proceed to find the acceleration of the body, such that:
a = F/ma = 20N / 50kga = 0.4m/s²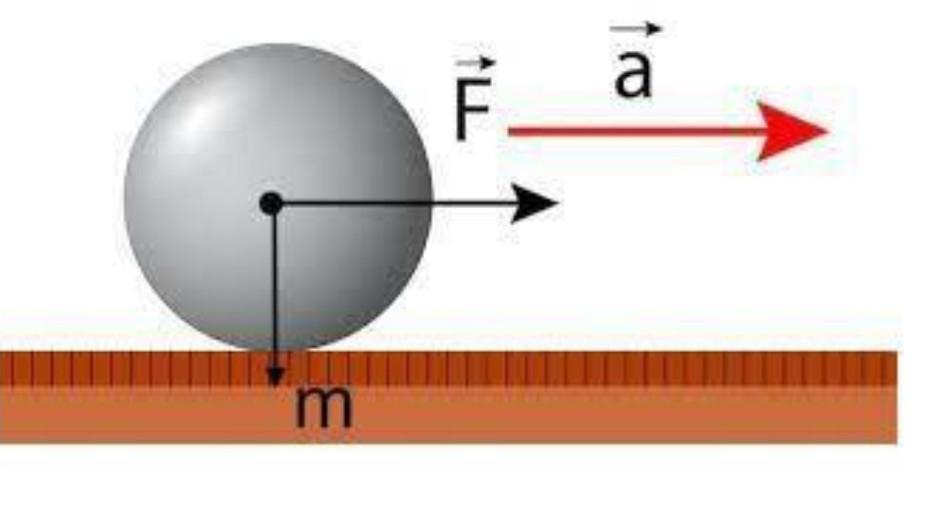
what product is obtained when an acid reacts with a metal?
Answers
Answer:
salt and hydrogen gas
Explanation:
acid + metal = salt + hydrogen
Answer:
hope this helps
Explanation:
hydrogen gas
An acid will react with a metal to form a salt and hydrogen gas. The general word equation for the reaction between an acid and a metal is as follows: acid + metal → salt + hydrogen.
5. Which contains more nitrogen: 60g of urea, (NH2)2CO, or 100g of ammonium sulphate, (NH4)2SO4
Answers
Answer:
Urea contains more nitrogen
Explanation:
1 mole of Urea contains 2 moles of Nitrogen and 1 mole of ammonium sulfate contains, also, 2 moles of nitrogen.
60g of urea (Molar mass: 60g/mol) contains:
60g × (1mol / 60g) = 1 mole. As 1 mole of urea contains 2 moles of nitrogen, moles of nitrogen are 2.
100g of ammonium sulfate (Molar mass: 132g/mol) contains:
100g × (1mol / 132g) = 0.758 moles.
As 1 mole of urea contains 2 moles of nitrogen, moles of nitrogen are 0.758×2 = 1.516 moles.
That means, urea contains more nitrogen.
which of the following compounds is soluble in water? a. a) bas b. b) pbco3 c. c) pb cl2 d. d) pbso4
Answers
The compound that is soluble in water is a) BaS. The compounds b) PbCO3, c) PbCl2, and d) PbSO4 are insoluble or only slightly soluble in water.
To determine the solubility of the compounds in water, we need to consider the solubility rules and the nature of the ions present in each compound.a) BaS (barium sulfide): BaS is generally considered to be soluble in water. Compounds containing group 1 metals (alkali metals) and ammonium ions are usually soluble, as are sulfates except for a few exceptions. Therefore, BaS is likely to be soluble in water.b) PbCO3 (lead(II) carbonate): PbCO3 is generally insoluble in water. Carbonates are typically insoluble, except for those of alkali metals and ammonium. Therefore, PbCO3 is expected to be insoluble in water.c) PbCl2 (lead(II) chloride): PbCl2 is moderately soluble in water. Chlorides are generally soluble, except for those of silver, lead, and mercury(I). Therefore, PbCl2 can dissolve to some extent in water.d) PbSO4 (lead(II) sulfate): PbSO4 is insoluble in water. Sulfates are typically soluble, except for those of barium, strontium, lead, and a few other exceptions. Therefore, PbSO4 is expected to be insoluble in water.
for more such questions compound
https://brainly.com/question/29108029
#SPJ11
what is a chemical bond? an attractive force between 2 molecules
Answers
Answer:
The chemical bond between 2 molecules is called an intermolecular force.
Answer:
Chemical Bond:
Attraction between two atoms results in the formation of a bond which is called a chemical bond.
Inter molecular Forces:
Attractive Force that in between 2 molecules is called Inter molecular Forces. (Inter : Between)
What is the latent heat of vaporization of boiling water?
a. 955 Btu/lb
b. 540 cal/gm
c. 2557 Kj/kg
d. 144 Btu/lb
Answers
The latent heat of vaporization of boiling water is 540 cal/gm. Option b is the correct answer.
It is the amount of heat needed to convert 1 gram of water from liquid to vapor state at atmospheric pressure at a constant temperature. The latent heat of vaporization for water is relatively high compared to other liquids because of the strong hydrogen bonding between water molecules. This means that water requires a lot of energy to break these bonds and change from a liquid to a gaseous state. The latent heat of vaporization is also responsible for the cooling effect of evaporation. When sweat evaporates from our skin, it absorbs heat from our body, which helps to cool us down. In conclusion, the latent heat of vaporization of boiling water is 540 cal/gm, which represents the amount of heat required to convert 1 gram of water from liquid to vapor state at atmospheric pressure at a constant temperature.
know more about latent heat
https://brainly.com/question/23976436
#SPJ11
If you want to produce 6.83 mol of Al;Os, with how many grams of Al must you start
Answers
We must start with 368.82 grams of Al to produce 6.83 mol of \(Al_{2} O_{3}\) .
The balanced reaction for the equation as follows:
\(4Al + 3O_{2}\) ⇒ \(2Al_{2} O_{3}\)
As we can see that 4 moles of Al reacts with 3 moles of \(O_{2}\) to produce 2 moles of \(Al_{2} O_{3}\), which means 108 grams of Al reacts with 96grams of \(O_{2}\) to produce 204 grams of \(Al_{2} O_{3}\).
Now,
2 mol of \(Al_{2} O_{3}\) react with 108 grams of Al
So,
6.83 mol of \(Al_{2} O_{3}\) react with = \(\frac{108}{2} *6.83\) grams of Al
6.83 mol of \(Al_{2} O_{3}\) react with = 368.82 grams of Al
Therefore, we must start with 368.82 grams of Al to produce 6.83 mol of \(Al_{2} O_{3}\) .
Learn more about mol here:
https://brainly.com/question/25281156
#SPJ9
When thermal energy is added to an object, what happens to the motion of the particles?
Answers
1. Which type of matter has the molecules packed closer together?
a. Solid
b. Liquid
c. Gas
d. Plasma
2. Matter is made up of __________.
a. Molecules
b. Particles
c. Atom
d. All of them
3. Particles with more energy move ________ than particles with less
energy.
a. Slower and closer together
b. Slower and farther apart
c. Faster and farther apart
d. Faster and closer together
4. Which best describes the particles in a liquid?
a. More freely moving than a gas
b. More freely moving than a
solid
c. Less freely moving than a
solid
d. Completely unmoving
5. Which of the following statements describes a mass?
a. The force of attraction between two bodies.
b. The force of gravity exerts on a body of mass.
c. The amount of matter in an object.
d. The weight of the object from place to place.
Answers
Answer:
1. Solid
2. atom
3. faster and farther apart
4.more freely moving than a solid
5.The amount of matte in an object
Explanation:
Answer:
1. Solid
2. Atom
3. Faster and farther apart
4. More freely moving than a solid
5. The amount of matter in an object.
What is the chemical equation of the boiling of water?.
Answers
Answer:
Boiling of pure water is not a chemical reaction.
It is a physical change from liquid state to gaseous state.
Explanation:
A 5.2 molal aqueous solution of methyl alcohol, CH3OH, is supplied. What is the mole fraction of methyl alcohol in the solution?
Answers
Answer:
0.086
Explanation:
A 5.2 molal aqueous solution of methyl alcohol indicates that 5.2 moles of methyl alcohol are present in 1 kilogram (or 1000 g) of water. Water has a molecular weight of 18 g/mol.
(100g)/18g/mol=55.56 mol
5.2 mol/(5.2mol+55.56 mol)=0.086
If 0.452 moles of OH1- are reacted with an unlimited supply of Br2, how many grams of H2O can be formed?
Answers
Answer:
Hi
Please mark brainliest

How do groundwater and soil interact?
Answers
Answer:
Groundwater and soil water can interact if the groundwater is relatively close to the soil root zone.
Explanation:
which change in the blood chemistry causes an increase in respiration?
Answers
There are several changes in blood chemistry that can lead to an increase in respiration.
One of the most significant factors is the buildup of carbon dioxide in the bloodstream. As carbon dioxide levels rise, the body's respiratory system responds by increasing the rate and depth of breathing to expel excess CO2 and maintain proper blood pH levels.
Another factor that can increase respiration is a decrease in oxygen levels in the blood. When oxygen levels drop, the body attempts to compensate by breathing faster and deeper to take in more oxygen. This response is particularly important in situations where oxygen delivery to the body's tissues is compromised, such as during exercise or at high altitudes.
Overall, respiration is closely tied to blood chemistry, with many different factors influencing how and why we breathe. By maintaining a delicate balance of gases and nutrients in the bloodstream, our bodies are able to efficiently extract oxygen and eliminate waste products, ensuring that our cells and tissues receive the oxygen they need to function properly.
To know more about blood chemistry visit:
https://brainly.com/question/30453877
#SPJ11
How many moles of caffeine, c8h10o2n4, are contained in a 100. Mg sample of caffeine? group of answer choices 0. 0085 0. 019 0. 51 0. 0028 0. 52
Answers
The number of moles of caffeine is 0.00052 mol
To calculate the number of moles of caffeine in a 100 mg sample, we need to use the formula:
moles = mass / molar massThe molar mass of caffeine (C₈H₁₀O₂N₄) is 194.19 g/mol. Converting the mass of the sample to grams (100 mg = 0.1 g), we can plug in the values and solve for moles:
moles = 0.1 g / 194.19 g/molmoles = 0.00052 molThe mole is widely used in stoichiometry calculations, which involve determining the amount of reactants needed to produce a certain amount of products or the amount of products produced from a certain amount of reactants. It is also used in the calculation of molar mass, which is the mass of one mole of a substance, and in the conversion between mass, moles, and number of entities in chemical reactions. Therefore, the number of moles of caffeine in a 100 mg sample of caffeine is 0.00052 moles.
To learn more about moles, here
https://brainly.com/question/26416088
#SPJ4
7. Circle the correct word: Electrons contribute to an atom's (mass or volume).
Answers
Answer:
i looked it up on google and i kept seeing mass if that helps, ill copy and paste it
Explanation:Electrons are much smaller in mass than protons, weighing only 9.11 × 10-28 grams, or about 1/1800 of an atomic mass unit. ... Electrons contribute greatly to the atom's charge, as each electron has a negative charge equal to the positive charge of a proton.
theoretically, if you were to use one serving of the food you chose to start a fire under a pot of water, what volume of water could you bring to a boil at 100 °c if all of the fire's energy was absorbed by the water?
Answers
The volume of water that could be brought to a boil using one serving of the chosen food depends on the energy content of the food and the heat energy required to boil the water.
To determine the volume of water that could be brought to a boil at 100 °C using one serving of a chosen food as the fuel source, we need to consider the energy content of the food and the energy required to heat the water.
First, we need to identify the chosen food and its energy content. Different foods have different energy densities, which can be measured in units such as calories or joules per serving. Let's assume the chosen food has an energy content of X calories per serving.
The energy required to raise the temperature of water can be calculated using the specific heat capacity of water. The specific heat capacity of water is approximately 4.18 joules per gram per degree Celsius (J/g°C).
To calculate the volume of water, we need to convert the energy content of the food to joules and divide it by the energy required to raise the temperature of water. Since the energy content of food is typically given in calories, we need to convert calories to joules. One calorie is equivalent to 4.18 joules.
Let's assume one serving of the chosen food provides Y calories. The energy content in joules would be Y * 4.18 joules.Now, we can calculate the volume of water using the equation:
Volume of water = Energy content of food (in joules) / (Energy required to heat water * Change in temperature)
Let's assume we want to bring the water to a boil, which requires raising the temperature from room temperature (assumed to be 25 °C) to 100 °C. Therefore, the change in temperature would be 75 °C.
Volume of water = (Y * 4.18) / (4.18 * 75)
Simplifying the equation, we find that the volume of water that could be brought to a boil using one serving of the chosen food as the fuel source is:
Volume of water = Y / 75
For more such question on actual volume visit:
https://brainly.com/question/30986210
#SPJ8
Primary succession is most likely caused by?
Answers
Answer:
volcanic eruption.
mark my answer as brainlest......
How do you think chemical change is defined?
Answers
Answer:
A chemical change happens when one chemical substance is transformed into one or more different substances,
Explanation:
Luis and spencer are camping out. each boy decides to build his own fire. spencer brought an ax to chop up a tree limb into foot-long sections for his fuel. luis does not have an ax, so he gathers small branches and breaks them into foot-long pieces. both sources of fuel have an equal mass, and luis and spencer light their fires at the same time. whose fire will last the longest? compare the rate at which each fuel source will burn, and be sure to explain your reasoning. hint: consider the surface area of each fuel source.
Answers
Luis wood will last the longest compared to spencer as his is dry wood it will burn faster and last longer whereas spencer's wood is fresh and has to dry in order to last longer.
A small piece of wood burns more quickly because when something burns, it combines with oxygen to cause combustion. Smaller pieces of wood burn more quickly than larger ones because they provide more surface area for the flame to spread across.
Smaller-diameter pieces should be divided because doing so increases the amount of wood that is exposed. More surface area means faster drying and better burning of the wood. Smaller (3-inch) pieces should be used whole.
to know more about combustion visit
https://brainly.com/question/15117038
#SPJ4
The table above shows the structural formulas and molar masses for three different compounds. Which of the following is a list of the compounds in order of increasing boiling points?
A. Butane < 1-propanol < acetone
B. Butane < acetone < 1-propanol
C. 1-propanol < acetone < butane
D. Acetone = butane < 1-propanol

Answers
Butane < acetone < 1-propanol is a list of the compounds in order of increasing boiling points.
Which substance has a greater boiling point?The intermolecular interactions between molecules in a compound play a major role in boiling point. Higher boiling temperatures are a result of greater intermolecular interactions, bigger masses, and less branching.
The highest boiling point is for HF. As we go from HF to HI, the van der Waals forces of attraction between all hydrogen halides get stronger.
Boiling point rises as the difference in electronegativity grows. Additionally, the bp grows as the molecule's size does. So the sequence is CO2, CS2, CCl4, and then H2O.
Van der Waals attraction and dipole-dipole attraction draw acetone molecules together.
To learn more about compounds refer to:
https://brainly.com/question/26487468
#SPJ1
Which term best describes this reaction? 2 carbons double bonded to each other, with CH 3 above the left C and H above the right C and below both. A lowercase n appears next to the left C. Right arrow. StartBracket across single bond to 2 carbons single bonded to each other, and C H 3 above the left C and H above the right C and below both; single bond from C across EndBracket; lowercase n beside the EndBracket. condensation polymerization addition polymerization hydrohalogenation hydration
Answers
Answer:
addition polymerization
Explanation:
In addition polymerization, the monomers are simply joined to each other to form a polymer having the same empirical formula as the monomer but of higher relative molecular mass. The monomers in addition polymerization are usually simple unsaturated molecules such as alkenes.
We can deduce the reaction to be an addition polymerization because of the the attachment of n to both the unsaturated monomer and the saturated polymer without the loss of any small molecule. If it was a condensation polymerization, there would have been an accompanying loss of a small molecule such as water.
Answer:
C.addition polymerization
Explanation:
other guy is right on edg. 2020
a compound has a molecular mass of 147 g and its empirical formula is c3h5o2. what is the molecular formula of this compound?
Answers
The molecular formula of the compound having empirical formula \(C_{3} H_{5} O_{2}\) is \(C_{6} H_{10} O_{4}\) .
The chemical formula is a way of representing information about the chemical proportions of atoms that constitute a particular chemical compound or molecule. The molecular formula is a chemical formula that shows the number of different atoms present in a molecule. It give the kind and number of atoms of each element present in a molecular compound. The molecular formula is the same as the empirical formula in many cases. Empirical formula can be determined from the percent composition of a compound. To determine the molecular formula it is necessary to know the molar mass of the compound.
146g/mole = n× {3×12.011 + 5×1.00794 + 2×16.00} g/ mole
n = 2
So the molecular formula= 2×C3H5O2
=\(C_{6}\)\(H_{10} O_{4}\)
To learn more about Molecular formula please visit:
https://brainly.com/question/15960587
#SPJ4