In a distillation process which liquid component of the mixture passes through the condenser first
a. true
b. false
Answers
TRUE, In a distillation process the one with the lower boiling point liquid component of the mixture passes through the condenser first
Simple distillation, as opposed to fractional distillation, is used to separate constituents in combinations with extremely different boiling temperatures. Fractional distillation is used for mixes comprising compounds with equivalent boiling points. Equipment for simple distillation consists of a distillation flask connected to a condenser. In contrast, the apparatus used for fractional distillation is the same as that for simple distillation with the exception of the installation of a second fractionating column between the condenser and the distillation flask. Fractional distillation employs a challenging apparatus called a fractionating column. A flask to contain the mixture, a condenser, and a flask to gather the purified components are the only components needed for the setup. Fractional distillation is used in the refinement of crude oil.
Learn more about distillation here:
https://brainly.com/question/29037176
#SPJ4
Related Questions
What happens when a piece of Mg ribbon is burnt? Name the
new substance formed. Write a balanced chemical equation.
Answers
Answer:
When a Magnesium Ribbon is burnt, a powdery substance called magnesium oxide is formed.
Explanation:
There has obviously been a chemical change because several chemical properties of the magnesium have been modified: the color, the texture and the mass.
The increase in mass is due to the fact that oxygen from the air has combined with the magnesium to make magnesium oxide, MgO.
The chemical equation, Mg + O2 MgO shows this reaction but it needs to be balanced to make 2Mg + O2 2MgO.
Using stoichiometry, we can convert this eqation into an equation with moles:
2 mol Mg + 1 mol O2 2 mol MgO.
Next, we convert to grams using atomic masses obtained from the periodic table:
48g Mg + 32g O2 80g MgO
Lastly, we determine the same thing in the proportions we used. In other words, we used only 0.15g of Mg (not 48g) so everything needs to be divided by 320. So 80 / 320 = 0.25 g. If we burn 0.15 g of Mg, we obtain 0.25 g of MgO.
Hope this helps!!!
This is my first answer.
examine the chemical structure for mannitol below. do you think this molecule will be soluble in water? why?
Answers
Yes, I believe that mannitol will be soluble in water.
Mannitol is a sugar alcohol with a chemical formula of C6H14O6. Its chemical structure consists of a six-carbon ring with hydroxyl (-OH) groups attached to each carbon atom. These hydroxyl groups give mannitol its polar nature, meaning it has a partial positive and partial negative charge on different ends of the molecule. Water is also a polar molecule due to its bent shape and the presence of polar covalent bonds between its atoms.
When mannitol is added to water, the polar nature of the molecule allows it to form hydrogen bonds with the water molecules. These hydrogen bonds help to break apart the ionic and hydrogen bonds within mannitol, allowing it to dissolve and become evenly dispersed throughout the water. Therefore, mannitol is soluble in water due to its polar nature and ability to form hydrogen bonds with water molecules.
To know more about molecule, visit;
https://brainly.com/question/475709
#SPJ11
Acetylene, C2H2, burns according to the following reaction: C2H2 5O2 --> 4CO2 2H2O. Suppose 1.20 g of C2H2 is mixed with 3.50 g of O2 in a closed, steel container, and the mixture is ignited. What substances will be found in the mixture left when the burning is complete
Answers
C2H2 will be left when the burning is complete.
The equation of the reaction is; 2C2H2 + 5O2 --> 4CO2 + 2H2O
The number of moles of C2H2 reacting is = 1.20 g/26 g/mol = 0.046 moles
The number of moles of O2 is = 3.50 g/32 g/mol = 0.109 moles
Since;
2 mole of C2H2 reacts with 5 moles of O2
x moles of C2H2 reacts with 0.109 moles of O2
x = 2 mole × 0.109 moles/5 moles
x = 0.044 moles of C2H2.
It then follows that C2H2 is the reactant in excess so C2H2 will be left when the burning is complete.
Learn more: https://brainly.com/question/9743981?
Molarity of Kool Aid solutions can be calculated by comparing the concentrations of Kool Aid powder and sugar added to a given volume of water. The molar mass of Kool Aid will be the same as that of sugar for our purpose. The molecular formula for sugar is C12H22O11- Your objective for this lab will be to calculate the molarity of Kool Aid desired based on package directions. You will then be provided two concentrated Kool Aid solutions. You will use dilution calculations to determine the amount of water and concentrated solution you will need in order to prepare 65 mL of the desired molarity.
Calculate the molarity of Kool Aid desired based on the following information from the package directions.
1 package Kool Aid powder = 4. 25 grams 1 cup sugar = 192. 00 grams
2. 00 quarts of water (1. 06 quarts = 1 liter)
Answers
The amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
To calculate the molarity of Kool Aid desired, we need to determine the number of moles of Kool Aid powder and sugar in the package. Since the molecular formula for sugar is C12H22O11, we can calculate its molar mass as follows:
Molar mass of C12H22O11 = (12 * 12.01) + (22 * 1.01) + (11 * 16.00)
= 144.12 + 22.22 + 176.00
= 342.34 g/mol
Given that the package contains 4.25 grams of Kool Aid powder, we can calculate the number of moles of Kool Aid powder using its molar mass:
Number of moles of Kool Aid powder = Mass / Molar mass
= 4.25 g / 342.34 g/mol
≈ 0.0124 mol
Similarly, for the sugar, which has a molar mass of 342.34 g/mol, we can calculate the number of moles of sugar using its mass:
Number of moles of sugar = Mass / Molar mass
= 192.00 g / 342.34 g/mol
≈ 0.5612 mol
Now, to calculate the molarity of the desired Kool Aid solution, we need to determine the volume of water. Given that 1.06 quarts is equal to 1 liter, and we have 2.00 quarts of water, we can convert it to liters as follows:
Volume of water = 2.00 quarts * (1.06 liters / 1 quart)
= 2.12 liters
To find the molarity, we use the formula:
Molarity (M) = Number of moles / Volume (in liters)
Molarity of Kool Aid desired = (0.0124 mol + 0.5612 mol) / 2.12 L
≈ 0.286 M
To prepare 65 mL of the desired molarity, we can use dilution calculations. We need to determine the volume of concentrated solution and the volume of water needed.
Let's assume the concentration of the concentrated Kool Aid solution is C M. Using the dilution formula:
(C1)(V1) = (C2)(V2)where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Given that C1 = C M and V1 = V mL, and we want to prepare a final volume of 65 mL (V2 = 65 mL) with a final concentration of 0.286 M (C2 = 0.286 M), we can rearrange the formula to solve for the volume of the concentrated solution:
(C M)(V mL) = (0.286 M)(65 mL)
V mL = (0.286 M)(65 mL) / C M
So, the amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
For more such questions on concentrated visit:
https://brainly.com/question/28564792
#SPJ8
A group of students found metamorphic rocks near a campsite. The presence of metamorphic rock is most likely evidence of that the rock formed from .
Answers
Answer:
Igneous or sedimentary rocks.
Explanation:
The presence of metamorphic rock is most likely evidence of that the rock formed from igneous or sedimentary rocks. The igneous rock converted into metamorphic rocks when the rock experience intense heat and pressure within the Earth's crust. These metamorphic rocks are crystalline and often have a “squashed” texture. So due to high temperature and pressure, the metamorphic rock is formed from igneous rock.
When an atom gives up or accepts an extra electron, so that the number of protons no longer is equal to the number of electrons, the result is O an isotope O a neutron .O an ion O a mineral
Previous question
Ne
Answers
An Ion.
An Ion has the same number of protons but loses or gains electrons, which make it either positively or negatively charged.
An ion that had lost electrons, because they need a full outer shell and perhaps they had too many electrons, and given to another atom becomes positively charged. This is because electrons are negatively charged, losing them makes the whole atom positive. Since there are now more positive protons than negative electrons.
But why don't atoms have a charge?
Because the number of electrons and protons are the same so, they cancel each other out and have a neutral charge.
So, if an atom accepts another electrons, maybe to fill in an outer shell to make it positive. It will become negatively charged as it now has more electrons than protons.

Why do humans feel so attached in relationships? What is the reason?
Answers
Answer:
We attach when we are convinced that we are incapable of healing ourselves or giving ourselves what we really want and need.
Explanation:
Hope you get right
the colligative molality of seawater is approximately 1.10 m. calculate the vapor pressure of sea water at 20 °c. the vapor pressure of pure water at 20 °c is 17.54 torr.
Answers
the colligative molality of seawater is approximately 1.10 m, the vapor pressure of sea water at 20 °c is 16.412 torr, the vapor pressure of pure water at 20 °c is 17.54 torr.
What is vapour pressure?Vapour pressure rises with temperature and is a measurement of a substance's propensity to transform into a gaseous or vapour state. The boiling point of a liquid is the temperature at which the pressure imposed by its surroundings equals the vapour pressure present at the liquid's surface. A liquid's thermodynamic propensity to evaporate is indicated by the equilibrium vapour pressure.
Given that-
Vapor pressure of pure water (P₀) = 17.54 torr
Molality (m) = 1.10 m
Let, vapour pressure of sea water = P
As we know,
m = [(P₀ - P) / P₀] × (1000 / M(solute))
1.10 = [(17.54 - P) / 17.54] × (1000 / 58.44)
P = 16.412 torr [considering NaCl in sea water]
Hence, vapour pressure of sea-water is: 16.412 torr
To know more about colligative molality refer to:
https://brainly.com/question/14611438
#SPJ1
Consuela lives on the east coast of Florida. She notices a neap tide occurs twice a
month. Neap tides occur when the Sun, the Moon, and Earth are in specific positions.
Sun
mm
Moon
4
Moon
Moon
Earth
Not to Scale
Moon
Which two of the four Moon positions shown above will cause nean tides 2

Answers
Answer: 2 and 4
Explanation:
An orbital is the space occupied by a pair of electrons.
true or false??
Answers
Answer:
True
Explanation:
An orbital is is the space occupied by a pair of electrons. The maximum number of electrons in an orbital is 2.
The maximum number of electrons in in the orbitals are two.
For s-sublevel with one orbital we have two electrons
p-sublevel with three orbitals we have six electrons
d - sublevel with five orbitals we have ten electrons
f - sublevel with seven orbitals we have fourteen electrons
Each orbital can take a maximum of two electrons.
how many liters are in 1 mole of oxygen gas?
Answers
Answer:
22.4L
Explanation:
Glad to help :)
Am I correct?? Help please

Answers
Answer:
I would say you are correct.
Explanation:
Answer:
I believe it'll be A (even though you chose it already).
Explanation:
A 1.732 g sample of iron is heated in air. It oxidizes to form a product with a mass of 2.205 g.
What is the empirical formula of the product?
Answers
Answer:
A 1.732 g sample of iron is heated in air. It oxidizes to form a product with a mass of 2.205 g. What is the empirical formula of the product? FeO 4. Caffeine is made of 49.48% carbon, 5.19% hydrogen, 28.85% nitrogen, and 16.48% oxygen by mass.
Explanation:
The empirical formula of the product formed by the reaction of iron and oxygen is FeO.
Let's consider the following generic chemical equation:
Fe + O₂ ⇄ FexOy
According to the law of conservation of mass, the sum of the masses of the reactants is equal to the sum of the masses of the products. The mass of O₂ that reacted, and remains in the oxide is:
\(mFe + mO_2 = mFexOy\\mO_2 = mFexOy - mFe = 2.205 g - 1.732 g = 0.473 g\)
The oxide has 1.732 g of Fe and 0.473 g of oxygen and a total mass of 2.205 g. To determine the empirical formula, we need to calculate the percent composition.
\(\%Fe = \frac{mFe}{mFexOy} \times 100 \% = \frac{1.732 g}{2.205 g} \times 100 \% = 78.55\%\)
\(\%O = \frac{mO}{mFexOy} \times 100 \% = \frac{0.473 g}{2.205 g} \times 100 \% = 21.45\%\)
Now, we will divide each percentage by the atomic mass of the element.
\(Fe: 78.55/55.85 = 1.406\\O: 21.45/16.00 = 1.340\)
Finally, we divide both numbers by the smallest one.
\(Fe: 1.406/1.340 \approx 1 \\O: 1.340/1.340 = 1\)
The empirical formula of the product is FeO.
You can learn more about empirical formulas here: https://brainly.com/question/1363167

The d orbital starts in the 4th row, or 4th energy level. However, what energy level (period number) does d actually start with?
Answers
The d-orbital starts in the third energy level (n = 3) of an atom.
Each energy level can contain one or more sublevels, including s, p, d, and f sublevels. The first energy level (n = 1) has one s orbital and can hold a maximum of 2 electrons. The second energy level (n = 2) has one s orbital and three p orbitals, allowing for a maximum of 8 electrons. The third energy level (n = 3) has one s orbital, three p orbitals, and five d orbitals, accommodating a maximum of 18 electrons.
The d-orbitals are found in the third energy level, corresponding to the third period of the periodic table. Therefore, the period number for the energy level where the d-orbital starts is 3.
The filling order of orbitals follows the pattern: 1s, 2s, 2p, 3s, 3p, 4s, 3d, and so on. The d-orbitals start filling after the p-orbitals in the third energy level. The electron configuration for the third energy level is written as 3s^2 3p^6 3d^1-10, depending on the element.
For example, the electron configuration of iron (Fe) is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^6 4s^2. This configuration indicates that the d-orbitals of iron are half-filled with 5 electrons.
Learn more about energy level
https://brainly.com/question/30546209
#SPJ11
Why are noble gases called "safe" gases?
Answers
Answer:
Noble gases are called "safe" gases because they are inert.
General Formulas and Concepts:
Reading a Periodic TableChemical Properties of Noble GasesExplanation:
Because Noble Gases have filled their outer shell electrons fully, they tend not to form compounds with other elements or react with others to produce another compound.
Take Ne (neon) for instance. It's electron configuration is 1s²2s²2p⁶. The outer most shell is the 2p sublevel, and according quantum numbers, the 2p sublevel only has 3 spaces for 2 electrons each that it can hold. Therefore, Ne has a full outer shell and no electron spots to create a bond with other elements.
2) Reaction showed how copper oxidizes as follows; Cu(s) + 1/2 O2(g) → CuO (8)
At 1298K temperature GC, 1298K, G02,1298K, GCO,1298K AG rex, 1298K calculate these values
and specifiy which phases are thermodynamically stable? ΔG0 = - 162200+ 69.24T J (298K-1356K)
Answers
At 1298K temperature, the reaction ΔG0 value is calculated to be -100,329 J. The thermodynamically stable phases are Cu(s) and CuO.
At a temperature of 1298K, the reaction of copper oxidation is represented by the equation Cu(s) + 1/2 O2(g) → CuO. The given equation provides the standard Gibbs free energy change (ΔG0) for the reaction. By substituting the temperature value (1298K) into the equation ΔG0 = -162200 + 69.24T J (298K-1356K), we can calculate the ΔG0 value.
Plugging in the values, we get ΔG0 = -162200 + 69.24 * 1298 J = -100,329 J. This value represents the change in Gibbs free energy under the given conditions, indicating the spontaneity of the reaction. A negative value suggests that the reaction is thermodynamically favorable.
Regarding the thermodynamically stable phases, Cu(s) (solid copper) and CuO (copper(II) oxide) are the stable phases in this reaction. The symbol "(s)" denotes the solid phase, and "(g)" represents the gaseous phase. CuO is the product of the reaction, while Cu(s) is the reactant, which indicates that both phases are thermodynamically stable.
Learn more about Thermodynamically
brainly.com/question/1368306
#SPJ11
Geef de systematische namen van SiF2
Answers
The question is: Give the systematic name of \(SiF_{2}\).
Answer: The systematic name of \(SiF_{2}\) is silicon difluoride.
Explanation:
The given compound has chemical formula \(SiF_{2}\). It shows that there is one atom of silicon and two atoms of fluorine are present.
So, the number "two" will be represented by the prefix "di" while naming this compound.
Hence, systematic name of this compound is silicon difluoride.
Thus, we can conclude that systematic name of \(SiF_{2}\) is silicon difluoride.
I’m the _____ state,volume and shape are both definite definite means that those characters and changeable in the _____ common volume and shape or both.
Answers
Answer:
I'm the constant, volume and shape are both definite. Constant means that those characters are not changeable in the same common volume and shape or both.
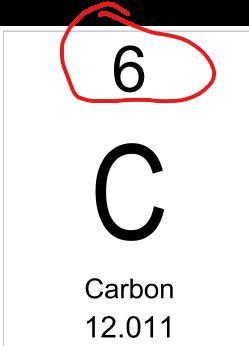
What number helps us identify the element?
Answers
Answer:
The Atomic Number
Explanation:
The atomic number is the number of protons of an atom in its nucleus.
Each element has a unique atomic number.
(view screenshot too, to find where it is in element table)
How do you measure the wavelength of a longitudinal wave?
a. the distance from a rarefaction to a compression
b. the length of a rarefaction
c. the length of a compression
d. the length of one compression and rarefaction
Answers
The right response is D. the length of one compression and rarefaction.
What is wavelength?A wave's wavelength is the separation between two adjacent locations that are in phase with one another. It is the actual distance covered by a single wave cycle.
Hence, one compression and one rarefaction are identical in length to one wavelength.
In a longitudinal wave, the medium's particles vibrate perpendicular to the wave's direction of propagation. In the medium, this causes areas of compression and rarefaction. One wavelength is equal to the space between any two compressions or rarefactions that follow one another.
You can mark a spot on a medium and measure the distance between it and the following point that reaches the same phase of a longitudinal wave to determine its wavelength (either a compression or a rarefaction). To get a more precise measurement, you can then repeat this process numerous times and take an average.
To know more about wavelength, visit:
https://brainly.com/question/12924624
#SPJ1
Are the oldest rocks in the canyon found near the bottom
Answers
Answer: yes they are
Explanation:
because its so hot and the heat just stays at the bottom
32.30 mL of 0.220 M H2SO4(aq) are titrated with 46.30 ml of a NaOH solution. What is the molarity of the NaOH(aq)? Show your work. 32,3004,220m) = (46.30 mL/CM). 46-30 m 46.30 mL
Answers
The molarity of the NaOH(aq) solution is 0.156 M.
The balanced equation for the reaction between sulfuric acid and sodium hydroxide is H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l). From the balanced equation, it can be deduced that the mole ratio of NaOH to H₂SO₄ is 2:1. Hence, the moles of H₂SO₄ reacted = Molarity of H₂SO₄ × Volume of H₂SO₄ used in liters = 0.220 M × 0.03230 L = 0.007106 moles.
Using mole ratio, the moles of NaOH reacted = 2 × 0.007106 = 0.01421 moles. Molarity of NaOH = Moles of NaOH / Volume of NaOH used in liters= 0.01421 moles / 0.04630 L = 0.306 M or 0.156 M (rounded off to three significant figures). Thus, the molarity of the NaOH(aq) is 0.156 M.
Learn more about molarity here:
https://brainly.com/question/2817451
#SPJ11
The pH of a solution is 3.17, what is the (H3O+)Group of answer choices6.8 X 10 minus 4 Molar3.5 X 10 minus 6 Molar3.5 X 10 plus 6 Molar6.8 X 10 plus 4 Molar
Answers
we are given the pH of the solution as 3.17 are we are required to find the [H₃O+]
we know that :
pH = - log[H₃O+]
-pH = log[H₃O+]
10⁻ᵖᴴ = 10ˡᵒᵍ[ᴴ₃ᴼ⁺]
10⁻ᵖᴴ = [H₃O+]
therefore:
10⁻³.¹⁷ = [H₃O+]
6.8x10⁻⁴ M = [H₃O+]
therefore the [H₃O+] is 6.8x10 minus 4 Molar
832 J of energy is used to raise the temperature of an unknown metal from 65oC to 71oC. If the specific heat of the metal is 0. 466 J/g*C, what is the mass of the metal sample? g (five sig figs)
Answers
The formula for calculating the amount of energy required to raise the temperature of a substance is:
q = m * c * ΔT
where q is the amount of energy, m is the mass of the substance, c is the specific heat, and ΔT is the change in temperature.
We can rearrange this formula to solve for the mass of the metal:
m = q / (c * ΔT)
Substituting the given values, we get:
m = 832 J / (0.466 J/g*C * (71oC - 65oC))
m = 832 J / (0.466 J/g*C * 6oC)
m = 832 J / 2.796 J/g
m = 297.1387678 g
Rounding to five significant figures, the mass of the metal sample is 297.14 g.
To know more about substance refer here
https://brainly.com/question/13320535#
#SPJ11
a) explain why caesium is more reactive than potassium.
b) Write the formula of
i) caesium nitrate
ii) caesium sulphate
Answers
Answer:
Explanation:
(a) Firstly, caesium abd potassium are both in Group 1 of the periodic table. Group 1 metals (also called alkali metals) are the most reactive metals of the periodic table. Caesium is more reactive than Potassium because it has a higher electropositivity than Potassium. Electropositivity is the tendency of a metal to donate electron(s) to form a cation. Electropositivity increases down the group; this is because it is easier for atoms to loose electrons on the outermost shell that are far away from the central nucleus as against atoms whose outermost electrons are closer to the central nucleus. Thus, the more "bulky" an atom is, the farther it's outermost electrons (valence electrons) get from the central nucleus and the easier it is to lose the outermost electron(s). And the easier it is for the valence electron(s) to be removed, the more reactive the atom would be and vice-versa.
Caesium is more reactive than potassium because it is more bulky than potassium, with an atomic number of 55, while potassium has an atomic number of 19.
NOTE: The closer an electron is to the nucleus, the more difficult it is to be removed from it's shell.
(b) i. Formula for Caesium Nitrate:
Symbol for Caesium is Cs and Nitrate is NO₃⁻.
Cs⁺ + NO₃⁻ ↔ CsNO₃
Formula for Caesium Nitrate is CsNO₃
ii. Formula for Caesium sulphate
Symbol for caesium is Cs and Sulphate is SO₄²⁻
Cs⁺ + SO₄²⁻ ↔ Cs₂SO₄
Formula for Caesium sulphate is Cs₂SO₄
NOTE: When writing the formulae, the charges would be exchanged to form the subscript as seen on the product sides above.
If a substance has a mass of 0.00235 grams and you need the mass in kilograms, will the number appear to become smaller or larger?
Answers
Answer: shorter
Explanation:
what species is present in the supernatant of the complete reaction between lead (ii) nitrate and potassium iodide
Answers
The \(NO_{3} ^{-}\) species is present in the supernatant of the complete reaction between lead (ii) nitrate and potassium iodide.
The clear liquid that remains above the solid residue after centrifugation, precipitation, crystallization, or settling is known as the supernatant. The species typically has a lower density and is precipitate-free. In order to more quickly and fully cause the precipitate (or "pellet") to gather on the bottom of the tube, centrifugation modifies the effective gravitational force acting on the tube or bottle.
The term "supernatant" is used to describe the residual solution. A variety of the cytokines found in supernatants are powerful angiogenesis modulators. Additionally, they have microvesicular bodies that carry RNA transcripts. Therefore, \(NO_{3} ^{-}\) species is present in the supernatant of the complete reaction between lead (ii) nitrate and potassium iodide.
Learn more about supernatant here:
https://brainly.com/question/15518699
#SPJ4
If the same large amount of heat is added to a 250 g piece of aluminum and a 150 g piece of aluminum, what will happen?
Answers
please vote me brainliest i really need it for i can do my work
____33. When an atom undergoes gamma decay, the atomic number of the element
a. remains the same.
b. decreases by one.
c. increases by one.
d. increases by two.
____34. Radioactive materials change into new _______________ during radioactive decay.
a. compounds
b. atoms of the same material
c. elements
d. neutrons
____35. Nuclear fission is the:
a. Production of uranium.
b. Splitting of protons by bombarding them with neutrons.
c. Splitting of atoms by bombarding them with neutrons.
d. Result of neutrons smashing into each other.
____36. During fission:
a. Electrons are released and cause more fission.
b. Protons are released and cause more fission.
c. Uranium is produced.
d. Neutrons are released and cause more fission.
____37. Separating U-235 from U-238 was difficult because:
a. There was so much more U-235 than U-238
b. There was so much more U-238 than U-235.
c. A chain reaction would result.
d. They are chemically identical.
Part IV: True/False
____ 38. The mass of the nucleus is small compared to the mass of the atom.
____ 39. Alpha particles would be harder to turn than Beta particles.
____ 40. Positive charge was spread all throughout the atom in the Rutherford Model.
____ 41. The Manhattan Project was located in New York for most of its duration.
____ 42. The cathode rays produced by Thomson were identical for all filaments.
____ 43. It is possible to know where an electron is and what path it is on.
____ 44. X-rays are produced when fast moving electrons strike phosphor coatings.
Answers
Answer: Because only energy is emitted during gamma decay, the number of protons remains the same. Therefore, an atom does not become a different element during this type of decay. Q: The Figure below shows how helium-3 (He-3) decays by emitting a gamma particle.
Answer:
yes
Explanation:
33c 32 c 33d 34a 35 b 36a 37 d
2 NH3 + 3 CuO →3 Cu + N₂ + 3 H₂O
In the above equation, how many grams of N₂ can be made when 4.3 moles of CuO are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer
as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your
answer incorrect.:
Element Molar Mass
Hydrogen 1
Nitrogen 14
Copper 63.5
Oxygen 16
Answers
40.1g of nitrogen gas is produced.
The equation given is
2 NH₃ + 3 CuO →3 Cu + N₂ + 3 H₂O
This equation is already balanced.
When 3 moles of CuO are consumed, 1 mole of nitrogen gas is produced.
We get 1 mole of nitrogen from 3 moles of copper oxide.
We need to find the number of moles of nitrogen gas produced when 4.3 moles of copper oxide are consumed.
4.3/3 x 1 = 1.433 mols
1.433 mols of nitrogen gas are producedThe molar mass of nitrogen gas is 14+14 = 28gThe amount of nitrogen gas produced in grams is 28x1.433 = 40.1g40.1g of nitrogen gas can be made when 4.3 moles of CuO are consumed.
Learn more about molarity here:
https://brainly.com/question/24305514
#SPJ10