Imagine designing an experiment in which the presence of a gas is determined by simply listening to the gas with your ear. The human ear can detect pressures as low as 2 x 10^-5 N*m^-2. Assuming that the eardrum has an area of roughly 1 mm^2, what is the minimum collisional rate that can be detected by ear? Assume that the gas of interest is N2 at 298 K.
Answers
Answer:
Explanation:
Pressure = Force/Area
so,
Force =Pressure x Area
Force =(2x 10⁻⁵ )N/M² x (1 x (10⁻³)² M²
Force = 2 x 10⁻¹¹N
as we know,
Force= mass x acceleration ( F=m.a)
a = F/m
a =(2 x 10⁻¹¹N)/28
g since 1 N=1.kg.m.s⁻²
a=(2 x 10-11kg.m.s⁻² )/(28 x 10⁻³kg)
a = 5.6 x 10-7 m.s⁻²
thus minimum collision rate that can be detected is 5.6 x 10-7 m.s⁻²Related Questions
for the following reaction determine the standard entropy for b(g) if the standard entropy change of the chemical reaction
Answers
The standard entropy change for a reaction can be calculated using the sum of the standard entropies of the products minus the sum of the standard entropies of the reactants.
The entropy of a substance is a measure of its thermal energy, with greater entropy indicating a greater degree of disorder or randomness in the distribution of thermal energy. In a chemical reaction, the standard entropy change refers to the change in entropy that occurs when a reaction takes place under standard conditions, typically defined as a temperature of 25°C and a pressure of 1 atm.
To calculate the standard entropy change for a reaction, the standard entropies of the reactants and products must be determined and compared. The sum of the standard entropies of the products minus the sum of the standard entropies of the reactants is equal to the standard entropy change for the reaction.
Here is an example of a reaction with the calculation of its standard entropy change for b(g) species:
2 H2(g) + O2(g) -> 2 H2O(g)The standard entropies of H2, O2 and H2O can be looked up in a thermodynamics table and are, respectively, 130.7 J/mol K, 205.0 J/mol K and 188.8 J/mol K.
Hence:
ΔS° reaction = ΔS° products - ΔS° reactants= (2 * 188.8 J/mol K) - (130.7 J/mol K + 205.0 J/mol K)= 2 * 188.8 J/mol K - 335.7 J/mol K= 377.6 J/mol KSo the standard entropy change for this reaction is 377.6 J/mol K, indicating that the entropy of the system increases during this reaction.
Learn more about standard entropy here: brainly.com/question/29485021
#SPJ4
Which of the following is not a valid conversion factor?

Answers
It should be 1000m Instead of 1 meter/ 10 millimeter since 1m=1000mm
Answer:
d
Explanation:
It would require ? Liters of water to dissolve 36 grams of the substance.

Answers
The correct answer is 3 liters
Explanation:
If a substance has a solubility of \(12 \frac{grams}{liter}\), this means in 1 liter, the grams that can be dissolved are 12 grams. Now, considering Justin and Ellie need to dissolve 36 grams to calculate the number of liters just divide the total of grams into 12 as each liter dissolves only 12 grams. The process is shown below:
36 grams (the amount that will be dissolved) ÷ 12 (grames dissolved per liter) = 3 liters (liters to dissolved 36 grams)
Answer:
It would be 3 liters
Explanation:
Check the boxes listed with compounds. (More than one box)

Answers
At what state is bromine at 100 degree
Answers
Answer:
a liquid
Explanation:
HELP NEEDED!!!!
The volume of a gas is 760 ml. The temperature changes from 20 oC to 40 oC. What will be the new volume?
A. 1520 ml
B. 1220 ml
C. 812 ml
D. 612 ml
Answers
Calculate the empirical formula for each of the following substances. (Express answer as a chemical formula) 1) 2.90 g of Ag and 0.125 g of N 2) 2.22 g of Na and 1.55 g of S 3) 2.11 g of Na, 0.0900 g of H, 2.94 g of S, and 5.86 g of O 4) 1.84 g of K, 0.657 g of N, and 2.25 g of O
Answers
Answer:
1) Ag3N
2)Na2S
3)NaHSO4
4) KNO3
Explanation:
We divide each mass by the element's relative atomic mass
1) 2.90/108-Ag, 0.125/14-N
0.027-Ag, 0.0089-N
Divide by the lowest ratio
0.027/0.0089-Ag, 0.0089/0.0089 N
3-Ag, 1-N
Empirical formula- Ag3N
2)2.22/23-Na, 1.55/32-S
0.097-Na, 0.048-S
Divide by the lowest ratio
0.097/0.048-Na, 0.048/0.048-S
2-Na, 1-S
Empirical formula- Na2S
3) 2.11/23-Na, 0.0900/1-H, 2.94/32-S,5.86/16-O
0.09-Na, 0.09-H, 0.09-S,0.366-O
Divide by the lowest ratio
0.09/0.09-Na, 0.09/0.09-H, 0.09/0.09-S, 0.366/0.09-O
1-Na, 1-H, 1-S, 4-O
Empirical formula- NaHSO4
4)1.84/39, 0.657/14-N, 2.25/16-O
0.047-K, 0.047-N, 0.14-O
Divide through by the lowest ratio
0.047/0.047-K, 0.047/0.047-N, 0.14/0.047-O
1-K, 1-N, O-3
Empirical formula- KNO3
How many grams of C5Hg would you need to measure out to have 0.172 mol?
Answers
We would need to measure out approximately 44.84 grams of C5Hg to have 0.172 mol.
To determine the grams of C5Hg needed to have 0.172 mol, we need to use the molar mass of C5Hg and the given amount in moles. The molar mass of a compound represents the mass of one mole of that compound.
The molar mass of C5Hg can be calculated by summing the atomic masses of all the atoms in the compound:
C5Hg: (5 * atomic mass of C) + (1 * atomic mass of Hg)
Using the atomic masses from the periodic table:
Atomic mass of C = 12.01 g/mol
Atomic mass of Hg = 200.59 g/mol
Molar mass of C5Hg = (5 * 12.01 g/mol) + (1 * 200.59 g/mol) = 60.05 g/mol + 200.59 g/mol = 260.64 g/mol
Now, we can calculate the grams of C5Hg needed for 0.172 mol using the mole-to-mass conversion:
Grams of C5Hg = Moles of C5Hg * Molar mass of C5Hg
Grams of C5Hg = 0.172 mol * 260.64 g/mol = 44.84 grams (rounded to two decimal places)
For more such question on mol. visit :
https://brainly.com/question/24191825
#SPJ8
A gas occupying 50.0 ml volume in a confined space at 20.0 dc at 50.0 kpa is heated to 40.0 dc. What is the pressure exerted by the gas in the container?
Answers
Answer:The pressure exerted by the gas is 100kPa
Explanation:Let's apply the Charles Gay Lussac law, to solve the question.
At constant volume, the pressure varies proportionally with the temperature.
P initial / T° initial = P final / T° final
50kPa / 20°C = P final / 40°C
Temperature has increased the double, so the pressure will be increased, the double too.
100 kPa
If you need more help go to this link https://brainly.com/question/14378507
In order to find the density of an object, Maria is trying to measure its volume. However, the object does not fit in the
tool she is using. To solve this problem, Maria decides to break apart the object.
Will Maria be able to find density following this method? Why or why not?
O Yes, if she measures the volume and mass of all the pieces of the object, she should be able to calculate density
O Yes, if she measures the volume and mass of one of the pieces of the object, she should be able to calculate
density.
O No, once the object is broken apart and the shape has been altered, it is not possible to calculate the volume of
the whole object to find density.
O No, once the object is broken apart and the shape has been altered, it is not possible to calculate the mass of the
object to find density.
Answers
Answer: She needs to divide the mass by the volume to find the density of the object.
Explanation:
Please help. Thank you so much

Answers
Enthalpy change (H) and entropy change (S) are 11.7 103 Jmol-1 and 105 Jmol-1K-1, respectively, for a reaction at 25 °C.
What is the change in the free basic energy at 25 °C?The absolute entropies of a reactants and their products are S°(N2H4) (= half of this period J/(mol•K), S°(N2) = 191.6 J/(mol•K), or S°(H2) = 130.7 J/(mol•K) at 25°C, where the standard enthalpy change (H°) is 50.6 kJ/mol.
What is the calomel electrode's reduction potential at 25 C?E0 is known as 0.268 V at standard potential at 25°C, despite a slight variation in the computed value above. Similar to a silver-silver chloride electrode, the electrode potential is dependent on the chloride ion concentration.
To know more about entropy visit:
https://brainly.com/question/24278877
#SPJ1
which describes how light wave interact with wood?
A. absorption and reflection
B. diffraction and transmission
C. reflection and refraction
D. transmission and refraction
Answers
BAnswer:
Explanation:
Answer:
A.) Asorption on Edge
Explanation:
The rotational spectrum of 79BrºF shows a series of equidistant lines spaced 0-714 33 cm - apart. Calculate the rotational constant B, and hence the moment of inertia and bond length of the molecule. Determine the wavenumber of the J = 9+= 10 transition, and find which transition gives rise to the most intense spectral line at room temperature (say 300 K).
and calculate the number of revolutions per second which the Brf molecule undergoes when in (a) the J = 0 state, (b) the J = 1 state, and (c) the J = 10 state. Hint: Use E = {lwin conjunction with Eqs (2.10) and (2.13), but remember that here w is in radians per second.[its Q season 2 from fundamentals of molcular spectruscopy . banwell.c.n]
Answers
In the J = 0 state, the BrF molecule does not undergo any revolutions per second. In the J = 1 state, it undergoes approximately 0.498 revolutions per second, and in the J = 10 state, it undergoes approximately 15.71 revolutions per second.
To calculate the rotational constant B, we can use the formula:
B = 1 / (2 * π * Δν)
Where:
B = rotational constant
Δν = spacing between consecutive lines in the rotational spectrum
Given that the spacing between consecutive lines is 0.71433 cm^(-1), we can substitute this value into the formula:
B = 1 / (2 * π * 0.71433 cm^(-1))
B ≈ 0.079 cm^(-1)
The moment of inertia (I) of the molecule can be calculated using the formula:
I = h / (8 * π^2 * B)
Where:
h = Planck's constant
Given that the value of Planck's constant (h) is approximately 6.626 x 10^(-34) J·s, we can substitute the values into the formula:
I = (6.626 x 10^(-34) J·s) / (8 * π^2 * 0.079 cm^(-1))
I ≈ 2.11 x 10^(-46) kg·m^2
The bond length (r) of the molecule can be determined using the formula:
r = sqrt((h / (4 * π^2 * μ * B)) - r_e^2)
Where:
μ = reduced mass of the molecule
r_e = equilibrium bond length
To calculate the wavenumber (ν) of the J = 9+ to J = 10 transition, we can use the formula:
ν = 2 * B * (J + 1)
Substituting J = 9 into the formula, we get:
ν = 2 * 0.079 cm^(-1) * (9 + 1)
ν ≈ 1.58 cm^(-1)
To determine the most intense spectral line at room temperature (300 K), we can use the Boltzmann distribution law. The intensity (I) of a spectral line is proportional to the population of the corresponding rotational level:
I ∝ exp(-E / (k * T))
Where:
E = energy difference between the levels
k = Boltzmann constant
T = temperature in Kelvin
At room temperature (300 K), the population distribution decreases rapidly with increasing energy difference. Therefore, the transition with the lowest energy difference will have the most intense spectral line. In this case, the transition from J = 0 to J = 1 will have the most intense spectral line.
To calculate the number of revolutions per second, we can use the formula:
ω = 2 * π * B * J
Where:
ω = angular frequency (in radians per second)
J = rotational quantum number
For J = 0:
ω = 2 * π * 0.079 cm^(-1) * 0 = 0 rad/s
For J = 1:
ω = 2 * π * 0.079 cm^(-1) * 1 ≈ 0.498 rad/s
For J = 10:
ω = 2 * π * 0.079 cm^(-1) * 10 ≈ 15.71 rad/s
For more such questiosn on BrF molecule visit;
https://brainly.com/question/30624940
#SPJ8
The specific rotation of (R) carvone is (+) 61°. The optical rotation of a sample of a mixture of R &S carvone is measured as (-) 23°. Which enantiomer is in excess and what is its enantiomeric excess? What are the percentages of (R) - & (S) - carvone in the sample
Answers
Answer:
See explanation
Explanation:
% optical purity = specific rotation of mixture/specific rotation of pure enantiomer * 100/1
specific rotation of mixture = 23°
specific rotation of pure enantiomer = 61°
Hence;
% optical purity = 23/61 * 100 = 38 %
More abundant enantiomer = 100% - 38 % = 62%
Hence the pure (S) carvone is (-) 62° is the more abundant enantiomer.
Enantiomeric excess = 62 - 50/50 * 100 = 24%
Hence
(R) - carvone = 38 %
(S) - carvone = 62%
Answer:
Explanation:
ee= -23/-61 × 100= 38%
S + R = 100%
S - R = 38%
Solve simultaneously;
S= 69% ( excess)
R= 100-69= 31%
If 20.0 g NaOH reacts with 30.0 g of H2SO4 how much Na2SO4 is produced?
Given: 2NaOH(aq) + H2SO4(aq) | Na2SO4(aq) + 2H2O(l)
Answers
Answer:
10g
Explanation:
find the limiting agent first
using mole ratio
2 moles of NAOH reacts with 2 moles of H2SO4
then 20g of NAOH require 20g of H2SO4
USING mole ratio on the limiting agent and product
2moles of NAOH produces 1 mole of NA2SO4
THEN
20g of NAOH will produce X
THEN solve for X
X =10g
PLEASE HELP!!!!!!!!!!
A teaspoon of salt, NaCl has a mass of about 5.0 g. How many formula units are in a teaspoon of salt?
SHOW WORK PLS!!!!
Answers
The molar mass of NaCl is 58.44 g/mol, which means that one mole of NaCl contains 6.022 x 10^23 formula units (Avogadro's number).
To determine the number of formula units in a teaspoon of salt, we need to first determine how many moles of NaCl are present in 5.0 g of salt. This can be done using the following formula:
moles = mass / molar mass
moles = 5.0 g / 58.44 g/mol = 0.0854 mol
Next, we can use Avogadro's number to convert moles of NaCl to formula units:
formula units = moles x Avogadro's number
formula units = 0.0854 mol x 6.022 x 10^23 formula units/mol = 5.14 x 10^22 formula units
Therefore, there are approximately 5.14 x 10^22 formula units of NaCl in a teaspoon of salt.
Epinephrine is a hormone secreted into the bloodstream in times of danger and stress. It is 59% Carbon, 7.1% Hydrogen, 26.2% Oxygen and 7.7% Nitrogen by mass. Determine the empirical formula. The molar mass is 148 g/mol.
Answers
The empirical formula for epinephrine is C₉H₁₃NO₃.
What is the empirical formula?The empirical formula can be described as the simplest ratio of the number of elements present in a compound. The molecular formula can be defined as a multiple of the empirical formula of a compound.
The empirical formula provides the ratio of the number of atoms and the percentage composition directly gives its empirical formula.
Given, the amount of Carbon in the Epinephrine = 59 % = 0.59 g
the amount of Hydrogen in the Epinephrine = 7.1 % = 0.07 g
the amount of Oxygen in the Epinephrine = 26.2 % = 0.262 g
the amount of Nitrogen in the Epinephrine = 7.7 % = 0.077 g
The number of moles of Carbon = 59/12 = 4.91
The number of moles of Hydrogen = 7.1/1 = 7.1
The number of moles of Oxygen = 26.2/16 = 1.64
The number of moles of Nitrogen = 7.7/14 = 0.55
The ratio of C : H : O : N can be represented as 4.91 : 7.1 : 1.64 : 0.55
The simplest ratio is 9 : 13 : 3 : 1
Therefore, the empirical formula of Epinephrine is C₉H₁₃NO₃.
Learn more about the empirical formula, here:
brainly.com/question/14044066
#SPJ1
how does cohesion affect the evaporation rate of water
Answers
Answer:
Evaporation occurs because among the molecules near the surface of the liquid there are always some with enough heat energy to overcome the cohesion of their neighbors and escape. At higher temperatures the number of energetic molecules is greater, and evaporation is more rapid.
Will you answer this for me ?

Answers
Answer:
Explanation:
balance
2 C2H6 + 7 O2 --> 4 CO2 + 6 H2O
given 360 g H20 (g)
required =586.67 g CO2
360 g H20 x (1mole/18 g H20) X (4 mole CO2/6 moles H20) X (44g CO2/1mole) =586.67 g CO2
9.0 mol Al reacts with 6.0 mol O2 to form Al2O3 according to the reaction below. how many moles of al2o3 form from 9.0 mol Al

Answers
Answer: 4.5 moles
Explanation:
To understand how to solve this problem, you must understand the ratios written in this chemical equation.
The equation shows that 4 moles of Al forms 2 moles Al₂O₃. This creates the ratio 2:4 or \(\frac{2}{4}\)
To solve, you can set the two ratios to each other and cross multiply.
\(\frac{2}{4} = \frac{x}{9}\)
18 = 4x
x = 4.5 mol Al₂O₃
*both \(\frac{2}{4}\) and \(\frac{4.5}{9}\) can be simplified as \(\frac{1}{2}\), which verifies your answer*
Compare and contrast thermal energy and heat
Answers
Explanation:
The difference between heat and thermal energy is that thermal energy is not in the process of being transferred; it is not in transit, but remains as part of the internal energy of the system; heat, on the other hand, is energy in transit, i.e. energy in the process of being transferred from a hotter system.
the object in question 1 has a mass of 21.535g. what metal is the cylinder most likely made of? why? pls help asap!!

Answers
Answer:
Aluminium
Explanation:
From the question given above, the following data were obtained:
Diameter (d) = 1.25 cm
Height (h) = 6.48 cm
Volume (V) of cylindrical=.?
Mass (m) of cylindrical object = 21.535 g
Density of the cylindrical object =..?
Next, we shall determine the radius of the cylindrical object. This is illustrated below:
Diameter (d) = 1.25 cm
Radius (r) =?
r = d/2
r = 1.25/2
Radius (r) = 0.625 cm
Next, we shall determine the volume of the cylindrical object. This can be obtained as follow:
Height (h) = 6.48 cm
Radius (r) = 0.625 cm
Pi (π) = 3.14
Volume (V) of cylindrical=.?
V = πr²h
V = 3.14 × 0.625² × 6.48
V = 7.948 cm³
Recall:
1 cm³ = 1 mL
Therefore
7.948 cm³ = 7.948 mL
Thus, the volume of the cylindrical object is 7.948 mL
Finally, we shall determine the metal in which the cylindrical object is made up of by calculating the density of the cylindrical object. This can be obtained as follow:
Mass (m) of cylindrical object = 21.535 g
Volume of the cylindrical object = 7.948 mL
Density of cylindrical object =?
Density = mass /volume
Density = 21.535 / 7.948
Density of cylindrical object = 2.7 g/mL
Comparing the density of the cylindrical object (i.e 2.7 g/mL) with those given in the table in the question, the cylindrical object is made of aluminium since they have the same density.
Trying to explain why a cactus needs little water to survive is an example of
a.
a prediction.
b.
drawing a conclusion.
c.
scientific inquiry.
d.
classification.
Answers
It’s a or b i think it’s b
a ball is pushed from a stop and rolls 6 m in 2 s. Student A says the average speed of the ball is 3 m/s. Student B says the average speed of the boll is 1.5 m/s. which student is correct and explain your answer
Answers
The average speed of an object is the ratio of the distance to the time. Hence, the speed of the ball rolling 6 m in 2 seconds is 3 m/s. Therefore, student A is correct.
What is average speed?Speed is a physical quantity measuring the distance covered by an object within unit time. Speed is a scalar quantity. The rate of speed is called velocity. The unit of both quantities in common is m/s.
If an object covers more distance within small time, the object is said to have a greater speed. The speed of the object is the ratio of its change in distance to the change in time.
Given that, distance covered by the ball = 6 m
time taken = 2 seconds
speed = distance/ time
speed = 6 m / 2 s= 3 m/s
Therefor, the speed of the ball is 3 m/s and student A is correct.
Find more on speed:
brainly.com/question/7359669
#SPJ1
Need help, thank you so much! This is not a timed or graded test or assessment

Answers
Answer
Based on the descriptions given about the type of bond, the following statements are true:
This is an ionic bond
A compound having this bond could be NaCl.
This would be a hard, brittle compound.
Why KHPo4 ignore effective as a buffer but kh2po4 is not
Answers
KH2PO4 is a more suitable choice as a buffer because it has a greater buffering capacity due to the presence of the weak acid and its conjugate base.
KHPo4 is not considered an effective buffer compared to KH2PO4 due to its limited buffering capacity. The effectiveness of a buffer is determined by the concentration and dissociation properties of its conjugate acid-base pair.
KH2PO4 is a salt composed of the weak acid H2PO4- and its conjugate base HPO4^2-. In an aqueous solution, KH2PO4 can dissociate to release H+ ions from the H2PO4- component, which acts as a weak acid, and the HPO4^2- component can accept H+ ions, acting as a weak base. This allows KH2PO4 to effectively resist changes in pH when small amounts of acid or base are added to the solution.
On the other hand, KHPo4 consists of the strong acid H3PO4 and the weak base HPO4^2-. H3PO4 fully dissociates in water, providing a large concentration of H+ ions, making it difficult for the HPO4^2- to effectively act as a base and maintain pH stability.
Therefore, KH2PO4 is a more suitable choice as a buffer because it has a greater buffering capacity due to the presence of the weak acid and its conjugate base.
For more question on conjugate
https://brainly.com/question/14684465
#SPJ8
a lab group completely reacted to a 0.5 f piece of aluminum in excess of CuCl2. instead of rinsing off the copper, the group weighed it and obtained 1.50 g Cu. Calculate the percent yield of copper in their reaction. write the balanced chemical equation.
Answers
1. Percent yield = (Actual yield/Theoretical yield) x 100
Actual yield = 1.50 g Cu
Theoretical yield = (0.5 f Al) x (1 mol Al/27.0 g Al) x (1 mol Cu/1 mol Al) x (63.5 g Cu/1 mol Cu) = 1.35 g Cu
Percent yield = (1.50 g Cu/1.35 g Cu) x 100 = 111.1%
2. Balanced chemical equation: 2 Al + 3 CuCl2 → 2 AlCl3 + 3 Cu
Answer: 2Al + 3CuCl2 → 2AlCl3 + 3Cu, Percent yield is 84.9%
Explanation: Find moles of aluminum using molar mass then x by coefficient ratio of cu/al. Then divide 1.5 by that value and x 100.
need help on this one question. And explain
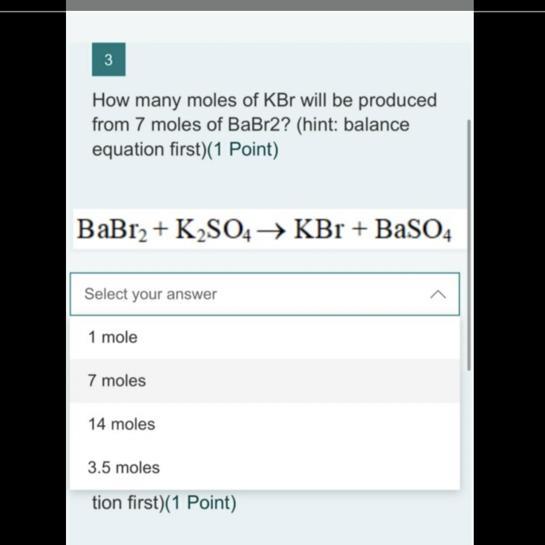
Answers
Answer:
14 moles
Explanation:
This is balanced equation:
BaBr2 + K2SO4 => 2KBr + BaSO4
1 mole of BaBr2 will produce 2 moles of KBr
=> 7 moles of BaBr2 will produce 7x2 = 14 moles of KBr
yourown definition of molecular
polarity
Answers
Answer:
Polarity is caused by the difference in electronegativity (EN) of a compound. For example, water, H2O, is polar because oxygen's EN is much higher than hydrogens. Therefore, the electrons spend more time around the oxygen and less time around the hydrogen causing a slightly positive pole at the hydrogens and a slightly negative at the oxygen. Most polar molecules are not symmetrical (like water). Non-polar molecules like CH4 (methane) do not have poles.
Hope that helps
Write the balanced half-reaction that occurs at the anode in a lead-acid (storage) battery during discharge. Phases are optional. anode half-reaction: Write the balanced half-reaction that occurs at the cathode in a lead-acid (storage) battery during discharge. Phases are optional. cathode half-reaction: Write the balanced overall cell reaction that occurs in the lead-acid (storage) battery during discharge. Phases are optional. overall cell reaction:
Answers
Answer: Anode: \(Pb+SO_4^{2-}\rightarrow PbSO_4+2e^-\)
Cathode: \(PbO_2+4H^++SO_4^{2-}+2e^-\rightarrow PbSO_4+2H_2O\)
Overall cell reaction : \(Pb+2SO_4^{2-}+PbO_2+4H^+\rightarrow 2PbSO_4+2H_2O\)
Explanation:
Lead storage battery is a secondary cell used in automobiles and invertors. The anode is made up of lead and undergoes oxidation during discharging and cathode is made up of lead oxide and acts as cathode during discharging. The electrolyte used is dilute .
Lead storage battery acts as electrochemical cell while discharging.
Discharging reaction for Anode:
Discharging reaction for Cathode: \(PbO_2+4H^++SO_4^{2-}+2e^-\rightarrow PbSO_4+2H_2O\)
Overall cell reaction : \(Pb+2SO_4^{2-}+PbO_2+4H^+\rightarrow 2PbSO_4+2H_2O\)
A lead storage battery is an energy storage device. At the anode lead and sulfate ion reacts to produce Lead(II) sulfate and release two electrons.
What are anode and cathode?In secondary cells or the recharge-discharge cells during recharge, the positive electrode is the anode, while during discharge cathode is the positive electrode.
The anode (lead) of the cell undergoes an oxidation reaction during the discharge, whereas the lead oxide or the cathode undergoes reduction.
The discharging reaction at the anode of the cell is given as,
\(\rm Pb + SO_{4}^{2-} \rightarrow PbSO_{4} + 2e^{-}\)
The discharging reaction at the cathode of the cell is given as,
\(\rm PbO_{2} + 4H^{+} + SO_{4}^{2-} + 2 e^{-} \rightarrow PbSO_{4} + 2H_{2}O\)
The overall cell reaction is given as,
\(\rm Pb + 2SO_{4}^{2-} + PbO_{2} + 4H^{+} \rightarrow 2PbSO_{4} + 2H_{2}O\)
Therefore, the gain and loss of electrons are represented at the cathode and the anode of the cell.
Learn more about discharge battery here:
https://brainly.com/question/8341588