I’m unsure how to answer this with sig figs in mind:Use scientific notation to to express this quantity: 131. mg

Answers
Use scientific notation to to express this quantity: 131. mg
Explanation:
131. mg has 3 SF
131. mg = 1.31 * 100 mg
131. mg = 1.31 * 10^2 mg
Answer: 1.31 * 10^2 mg

Related Questions
How does vapor pressure affect intermolecular forces.
Answers
Answer:
it is directly related to the intermolecular forces present between its molecules
Which phrase describes a nuclear chain reaction?(1 point)
a reaction in which particles spontaneously emit radiation
a series of reactions in which particles from one reaction trigger the next reaction
a type of reaction which only takes place in the sun and other stars
a reaction in which small nuclei combine into a larger nucleus
Answers
Answer:
a series of reactions in which particles from one reaction trigger the next reaction
Explanation:
cause thats a chain
how many grams of phosphorus are in 50-gram sample of aluminum phosphate
Answers
There are approximately 12.7 grams of phosphorus in a 50-gram sample of aluminum phosphate.
To determine the number of grams of phosphorus in a 50-gram sample of aluminum phosphate, we need to know the molar mass and the chemical formula of aluminum phosphate.
The chemical formula for aluminum phosphate is AlPO4. It indicates that each molecule of aluminum phosphate contains one aluminum atom (Al), one phosphorus atom (P), and four oxygen atoms (O).
To calculate the molar mass of aluminum phosphate, we can add up the atomic masses of its constituent elements based on their stoichiometric ratios:
Molar mass of AlPO4 = (molar mass of Al) + (molar mass of P) + (4 * molar mass of O)
Using the periodic table, we can find the atomic masses of the elements:
Molar mass of Al = 26.98 g/mol
Molar mass of P = 30.97 g/mol
Molar mass of O = 16.00 g/mol
Now, let's calculate the molar mass of aluminum phosphate:
Molar mass of AlPO4 = (26.98 g/mol) + (30.97 g/mol) + (4 * 16.00 g/mol)
= 121.95 g/mol
The molar mass of aluminum phosphate is 121.95 g/mol.
To determine the number of grams of phosphorus in a 50-gram sample of aluminum phosphate, we need to calculate the mass fraction of phosphorus in the compound. The mass fraction is the ratio of the molar mass of phosphorus to the molar mass of aluminum phosphate.
Mass fraction of phosphorus = (molar mass of P) / (molar mass of AlPO4)
= (30.97 g/mol) / (121.95 g/mol)
≈ 0.254
Multiplying the mass fraction by the mass of the sample gives us the grams of phosphorus:
Grams of phosphorus = (mass fraction of phosphorus) * (mass of the sample)
= 0.254 * 50 g
≈ 12.7 g
For more such questions on aluminum phosphate visit:
https://brainly.com/question/15072110
#SPJ8
when a gas undergoes an isothermal process, there is
Answers
Answer:
no change in the temperature of the gas.
Explanation:
isothermal process of a gas, can result to compression of the gas or expansion of the gas at a constant temperature (ΔT = 0).
For isothermal gas expansion, work is done in reducing the pressure of the gas by increasing its volume at a constant temperature. The change in the internal energy of this process is zero (ΔU = 0).
For isothermal gas compression, work is done in decreasing the volume of the gas by increasing its pressure at a constant temperature. The change in the internal energy of this process is also zero (ΔU = 0).
Therefore, when a gas undergoes an isothermal process, there is no change in the temperature of the gas.
Can someone help me here please i need this :(
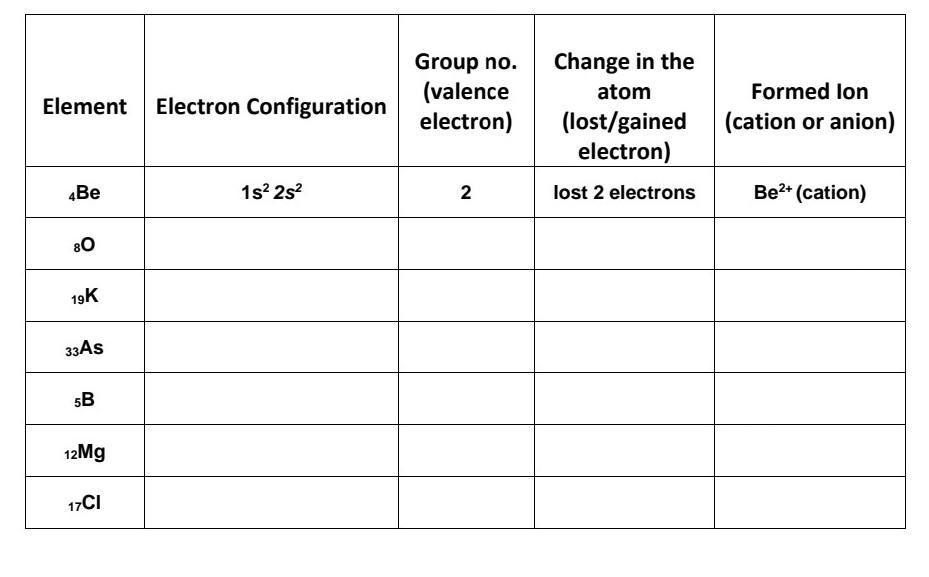
Answers
How many number of oxygen molecules are required to produce 0.22 g of CO2 according to the reaction?
c+o2=co2
Answers
n=.22/44=.005 mole of CO2
from the equation we see the relationship between the moles of co2 and O2 and we find that they have the same number of moles
So we need .005mole of O2
Multiple the number of moles with avogadro’s number to know the number of O2molecules
.005x6.022 x10^23
In an ecosystem, marsh snails feed on phytoplankton and painted turtles feed on marsh snails. Pollution has resulted in the depletion of phytoplankton. What effect would this situation likely have on the population of marsh snails and painted turtles? As phytoplankton were destroyed, the population of marsh snails would most likely . Then the population of painted turtles would mostly likely .
Answers
Answer:
The correct answers are: decrease and decrease.
Explanation:
Phytoplanktons are the producers in this ecosystem while marsh snails are the primary consumers and painted turtles are the secondary consumers. It is known that the producers affect direct or indirect to all other trophic levels and it is also known that in the food chain decrease in the producer or bottom of the chain will affect all.
The numbers of marsh snails will decrease due to its food source and as painted turtles depend on marsh snail the population of these also decrease as marsh snails are the primary food source of the painted turtles.
Answer:
1and1
Explanation:
From the following items, which is closest in size to one mole of gas at stp?
Answers
At STP, a mole of pure gas is closest in size to a marble. As a tiny, solid material, a gram of gas at the STP is considerably smaller than a marble. D is the correct response.
At Standard Pressure and Temperature, 22.4 L of any gas will be required to hold 1 mole (STP). The Ideal Gas Law and a balanced chemical equation can be used to determine the amount or mass of gas consumed or created in a chemical process. In other words, the gas that has the greatest number of molecules of a certain gas at a given temperature will occupy the largest volume.
Learn more about STP
brainly.com/question/29356493
#SPJ4
Question: From the following items, which is closest in size to one mole of gas at STP?
A) A car
B) An elephant
C) A microwave
D) A marble
Which of the following has the largest electronegativity? AI, Cu, S, or O?
Answers
Answer:
O(Oxygen)
Explanation:
Oxygen or O among the given options has the highest electronegativity of 3.5 as compared to 2.4 of sulfur. Thus, there is a lesser attraction in the bonds of Sulfur. The other two elements Al(Aluminium) and Cu are less electronegative than Sulfur and Oxygen as they are metals and possess electropositivity while the latter displays more electronegativity due to being non-metals. Hence, Oxygen is the answer.
8. (07.05 LC)
Which statement is correct about the rate of most chemical reactions? (5 points)
1.It increases when the concentration of reactants decreases.
2.It does not depend on the concentration of reactants.
3.It increases when the temperature increases.
4.It does not depend on the temperature.
Answers
Answer: 3.It increases when the temperature increases.
Explanation:
The speed of most chemical reactions increase as if the temperature of those same reactions increase as well. This is because a higher temperature allows for reactants to gain the necessary activation energy needed to react.
Enzymes are another reason. Enzymes are catalysts in reactions which reduce the activation energy needed for a reaction to occur and enzymes work best in certain higher temperatures - not too high - so this also leads to the reaction speed increasing due to temperature increases.
Answer:
It increases when the temperature increases.
Explanation:
I took the test and the other guy is right ;)
Arrange the oxoacids of bromine according to strength.
Strongest acid
Weakest acid
Answer :
a. HBrO2
b. HBrO
c. HBrO3
d. HBrO4
Answers
Oxoacids get more acidic as the number of oxidations on the halogen rises.
Perchloric acid is the most potent acid there is. As NH 4Br is an acidic salt, the solution will be acidic even though the Br ions come from a strong acid (HBr), but the NH 4 + ions come from a weak base (NH 3). Br2 is therefore regarded as a mild Lewis acid. Large acceptor atoms in soft acids have a low positive charge, are highly polarisable, and have poor electronegativity. One of the finest nucleophiles, the bromide ion is a poor base. It chooses to take on a nucleophilic role.
To find more on acidic refer here:
https://brainly.com/question/24255408
#SPJ4
People with diabetes have to monitor and restrict their sugar intake. What volume of apple juice could
a diabetic person drink, if the person's sugar allowance for that beverage was 8.0 g? Assume that the
apple juice has a sugar concentration of 8.0 % w/v.
Answers
The volume of apple juice could a diabetic person drink : 100 ml
Further explanationGiven
sugar allowance = 8 g
sugar concentration of apple juice : 8.0% w/v
Required
Volume of apple juice
Solution
The concentration of a substance can be expressed in several quantities The concentration shows the amount of solute in a unit of the amount of solvent.
% w/v = mass of solute (weight) in g / 100 ml solution
So for 8% w/v = 8 g of sugar(solute) in 100 ml of apple juice
explain difference acids and bases
Answers
Acids are substances that turn wet blue litmus paper to red while bases are substances that turn red litmus paper to blue.
Acids vs BasesAcids and bases can be differentiated using their definitions as follows:
Acids are substances that turn blue litmus paper to red while bases are substances that turn red litmus paper to blue.Acids are substances that produce hydrogen ions as their positive ions in an aqueous solution while bases are substances that produce hydroxyl ions as their negative ions in aqueous solutions.An acid is a proton donor while a base is a proton acceptor.More on acids and bases can be found here: https://brainly.com/question/15192126
#SPJ1
Acid has hydrogen ion whereas base has hydroxyl ion.
What are acids and bases?Acid is a type of chemical compound that is dissolved in water and gives a solution with Hydrogen ions more than purified water. A base is a substance that gives electrons, takes protons, or releases hydroxide (OH-) ions. Acid is also called a proton donor. While a base is also called a proton acceptor.
Acids have a sour taste whereas Bases have a bitter taste. The pH value of acid is lower than 7. Basis has a pH value higher than 7. The strength of Acid depends on the number of Hydrogen ions. The strength of the Base depends on the amount of Hydroxide ions
An acid is any hydrogen-carrying substance that is able of giving a proton that is to say a hydrogen ion to another substance. A base is a molecule or ion able to gain a hydrogen ion from an acid.
so we can conclude that the molecules which have hydrogen ions are considered acids while those molecules which have hydroxyl ions are considered bases.
Learn more about acid here: https://brainly.com/question/25148363
#SPJ1
Why is an ecosystem still healthy although there's still a lot of consumers in it?
Answers
Answer:
because it is a sign that there are more producers for the consumers.
Explanation:
An ecosystem consist of the abiotic or inorganic components, the animals[consumers], the plants[producers] and the decomposers. An ecosystem simply means the environment of organisms that are living together and the way these organisms interact with another.
The producers that is the plants are the ones manufacturing food by themselves either by photosynthesis or chemosynthesis and the consumers are the ones relying on the producers for food.
The ecosystem is still healthy even when there's still a lot of consumers in it because it shows that there are come producers that is to say the producers are more also. If this is not the case, then the ecosystem will not be healthy as the consumers will eat the producers until the producers will not be enough.
water at 20 oc is flowing in a 100-mm diameter pipe at an average velocity of 2m/s. if the diameter of the pipe is suddenly expanded to 150-mm. a) what is the new velocity? b) what is the volumetric flowrate in both pipes? c) what is the mass flowrate in pipe 1? d) what is the mass flowrate in pipe 2?
Answers
a) New velocity = 2.093 m/s
b) Volumetric flowrate in 100 mm diameter pipe= 157 liter/sec
Volumetric flowrate in 150 mm diameter pipe = 355 liter/sec
c) Mass flowrate in pipe 1= 0.0075 m²/s
Mass flowrate in pipe 2 = 0.017 m²/s
In fluid dynamics, the volume of fluid which passes per unit time is called volumetric flow. It is denoted by the symbol Q.
Given d= 100mm= 0.1m
and average velocity= 2m/s
A= π/4 *d²
= π/4 *(0.1)²
= 0.0075 m²/s
Q= A* V
= 0.0075 * 2
= 0.0157 m³/s
= 157 liter/sec
when d= 150mm= 0.15m
V = Q/A
= 0.0157/0.0075
= 2.093 m/s
A= π/4 *(0.15)²
= 0.017 m²/s
Q=A *V
=0.017* 2.093
= 0.035m³/s
= 355 liters/sec
To know more about volumetric flowrate, refer
https://brainly.com/question/13254954
#SPJ4
need help with this as soon as possible
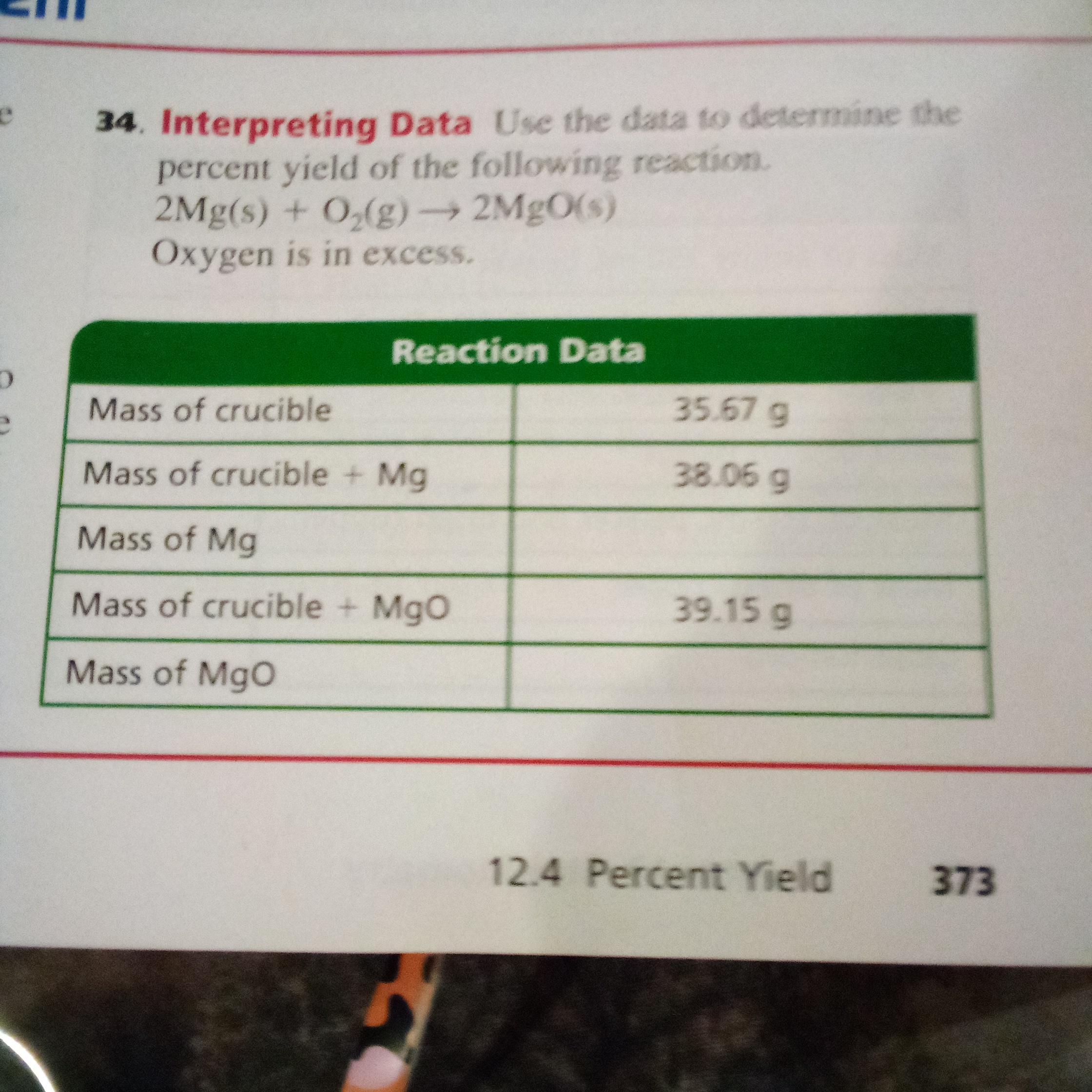
Answers
Mass of Mg is 2.39g and Mass of MgO is 3.48 g. Mg is a chemical element Magnesium.
How to calculate mass ?The average mass of an element's atoms expressed in atomic mass units is the element's atomic mass. The mass of each isotope is multiplied by its abundance to produce the atomic mass, which is a weighted average of all the isotopes of that element.
The atomic mass of an element, measured in amu, is equal to the mass in grammes of one mole of an element. This relationship between the atomic mass and the mole concept is important in chemistry.
Mass of Mg = Mass of crucible and Mg - Mass of empty crucible = 38.06g - 35.67g = 2.39g
Mass of MgO = 39.15 g - 35.67 g = 3.48 g
To learn more about atomic mass refer :
https://brainly.com/question/3187640
#SPJ1
_____ is a disaccharide important in the formation of alcoholic beverages.
Answers
Maltose is a disaccharide important in the formation of alcoholic beverages.
Maltose:
Maltose, also known as maltobiose or maltose, is a disaccharide formed from two glucose units linked by α(1 → 4) bonds. In isomeric isomaltose, the two glucose molecules are linked by an α(1 → 6) bond. Maltose is both members of the amylose homologous family, which is the major structural motif in starch. When beta-amylase breaks down starch, it removes two units of glucose at a time to form maltose. An example of this reaction can be found in germinating seeds, which is why malt is named after it. Unlike sucrose, it is a reducing sugar.
Also known as malt sugar, it is made up of two glucose molecules bonded together. It is an important disaccharide in the formation of alcoholic beverages.
Alcoholic Beverage:
Alcoholic beverages (also called alcoholic beverages, adult beverages, or beverages) are beverages that contain ethanol, a type of alcohol that acts like a drug and is made by fermenting grains, fruits, or other sources of sugar. Drinking alcoholic beverages, often referred to as "drinking", plays an important social role in many cultures. Most countries have laws regulating the production, sale and consumption of alcoholic beverages.
Regulations may require the alcohol percentage (alcohol or proof) to be displayed and warning labels to be used. Alcoholic beverages are legal in most countries around the world, although in some countries such activities are outright banned. The global liquor industry passed $1 trillion in 2018.
Learn more about Maltose:
https://brainly.com/question/19755762
#SPJ4
Answer:
Ethanol is a disaccharide important in the formation of alcoholic beverages. Ethanol is produced by yeast during fermentation when sugar is converted to alcohol. This process involves the breaking down of carbohydrates, such as glucose and fructose, into simple sugars, which are then converted into ethanol.
Capture energy from sunlight and use it to produce food for the cell; perform
photosynthesis
O cell membrane
O cell wall
O Mitochondria
O chloroplasts
Answers
Answer:
The answer is option 4.
Explanation:
Chloroplast is the place where they absorb sunlight to perform photosynthesis to produce food.
Which of the following is a covalently bonded molecule?
A) CI2
B) air
C) Ne
D) salt
Answers
Answer:
D:salt this is the correct answer
PLEASE HELP ASAPPP
A cylinder shaped water tank has a height of 212 ft (6461.76 cm), and is able
to hold 15,000 gallons of water (15,000,000 mL or 15,000,000 cm3). The
volume of a cylinder is expressed by the equation
V = tr^2h
Where V is the volume, r is the radius, and h is the height of the cylinder.
Express the radius (r) in terms of volume (V) and height (h), then determine
the radius of a cylinder water tank (in centimeters) that is filled to capacity.
Answers
Answer:
r = \(\sqrt{\frac{v}{\pi h} }\)
27.2cm
Explanation:
Given parameters:
Height of tank = 6461.76cm
Volume of water = 15000000cm³
Volume of cylinder = \(\pi\) r² h
i. Express radius in terms of volume;
we are to make radius the subject of the expression;
v = \(\pi\) r² h
divide both sides by πh
\(\frac{v}{\pi h}\) = \(\frac{\pi r^{2}h }{\pi h }\)
r² = \(\frac{v}{\pi h}\)
r = \(\sqrt{\frac{v}{\pi h} }\)
ii.
Radius of the water tank;
r = \(\sqrt{\frac{15000000}{\pi x 6461.76} }\) = 27.2cm
What is the complete ionic equation for this reaction?
2KOH(aq) + H2SO4(aq) → 2H20(1) + K2SO4(aq)
O A. 2K+ + OH + H2SO4 → OH + 2H+ + K2SO4
B. OH + 2H+ + 2H20()
C. 2KOH + H2SO4 → 2H20 + K2SO4
D. 2K+ + OH + 2H+ + SO42- → 2H20() + 2K+ + SO42-
SUBMIT
Answers
Answer:
The answer is "Option D".
Explanation:
The entire ionic equation for all the substances, which are ionic compounds but are available in an aquatic is represented in the form with ions in the full ionic equation.
In the Net ionic equation, it doesn't have the particulate matter throughout the net ionic equations throughout the equations.
In the Spectator ions, it doesn't participate in interactions mostly on reaction and the material hand. From both sides, the very same ions are present.
The evenly balanced chemical formula is,
\(2KOH (aq)+ H_2SO_4(aq) \longrightarrow 2H_2O(l) + K_2SO_4\)
It is the separate organic compound that full ion formula will match the choice D.
true or false...
electromagnetic waves can travel in empty space or space that has no particles
Answers
It is true that electromagnetic waves can travel in empty space or space that has no particles.
The correct option is True.
What are electromagnetic waves?Waves of the electromagnetic field make up electromagnetic radiation; they travel through space while carrying momentum and radiant energy. It consists of X-rays, gamma rays, microwaves, infrared, light, and radio waves.
Electromagnetic waves include radio waves, television waves, and microwaves. The only thing separating them is the wavelength. The wavelength is the distance between each wave's peak and the next.
Learn more about electromagnetic waves at: https://brainly.com/question/25847009
#SPJ1
if the gas is air with a fixed mass m of 1 kg, a pressure p of 20 atm, and a temperature t of 600 k; calculate the work done by expanding the gas in the cylinder to a pressure of 1 atm. assume the expansion to be reversible and adiabatic. perform the calculation directly by integrating compare this value of work to the change in internal energy of the gas and to the change in enthalpy of the gas. comment. if the temperature is raised to 1200 k before the expansion begins, determine the work of expansion if the initial pressure is unchanged. compare this result to the result from part a. repeat the calculations in parts a-c using argon as the working fluid. explain what gas property leads to a different result.
Answers
To calculate the work done by expanding the gas in the cylinder, we can use the formula for reversible adiabatic expansion:
Work = (p1V1 - p2V2) / (γ - 1),
where p1 and p2 are the initial and final pressures, V1 and V2 are the initial and final volumes, and γ is the specific heat ratio.
In this case, the initial pressure is 20 atm and the final pressure is 1 atm. Assuming the volume changes, we need more information to proceed with the calculation. However, we can make some comments.
The change in internal energy (ΔU) of the gas is given by the formula
ΔU = Q - W,
where Q is the heat added to the system and W is the work done on the system. Since the expansion is adiabatic (no heat exchange),
ΔU = -W.
The change in enthalpy (ΔH) of the gas is given by the formula
ΔH = ΔU + Δ(PV).
For an adiabatic process,
Δ(PV) = 0,
so ΔH = ΔU.
If the temperature is raised to 1200 K before the expansion begins, the initial conditions change. To determine the work of expansion, we need more information about the volume changes.
When repeating the calculations using argon as the working fluid, the specific heat ratio (γ) changes.
Argon has a different γ value compared to air, which leads to a different result.
To know more about reversible adiabatic visit:
https://brainly.com/question/32656653
#SPJ11
hahaha ha ha ha ha ha ha ha help me, please
Describe the different states of matter and give some examples.
1. liquid
2. solid
3. gas
there that the question and now I'm waiting for an answer .
ill mark you brainlies
Answers
Answer:
The liquid state sometimes is described simply as the state that occurs between the solid and gaseous states. Some examples of liquids are:
- Water
- Milk
- Vinegar
Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. Some examples of solids are:
- Rocks
- Brick
- Coin
What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer. Some examples of gases are:
- Helium
- Oxygen
- Neon
What element has a mass number of 28 and 15 neutrons? How do you know?
Answers
Mass number 28: Nickel.
15 neutron: Phosphorus.
The element has a mass number of 28 and 15 neutrons is Aluminium.
All elements have are made up particles sch as neutrons, protons and electrons.Inside the atom of every element contains the protons and neutrons which collectively are called The nucleon.The number of protons,P and neutrons,N give the mass nmber of the element as represented by A, So,A= P +N
In a neutral atom, the number of protons equals number of electrons.Now given an element with mass number 28 and 15 neutrons, we first find the proton number
A= P +N
28= P +15
P=28-15
P=13
The proton number of an element is also the same as its atomic number,The atomic number of an element tells us the identity of that element , therefore the element with atomic number 13 is Aluminum
Read more about mass number, protons and neutrons here: https://brainly.com/question/14140169
Which of the following is an example of a “nature vs. nurture" question?
А
What is Samantha's favorite TV show?
B
When did Samantha get that sun burn?
с
Which parent did Samantha inherit her hair color from?
D
Is Samantha's sense of humor inherited, or did she learn it?
Answers
Answer:
The answer to your question is D
Answer:
its d bro so easy
Explanation:
because it talks about nature and nuture so samantha inherited it and also learned stuff
balance this equation

Answers
Balanced equation
2Pb(NO₃)₂⇒2PbO+4NO₂+O₂
Further explanationGiven
Reaction
Pb(NO₃)₂⇒PbO+NO₂+O₂
Required
Balanced equation
Solution
Give coefficientPb(NO₃)₂⇒aPbO+bNO₂+cO₂
Make equationPb, left=1, right=a⇒a=1
N, left=2, right=b⇒b=2
O, left=6, right=a+2b+2c⇒6=1+2.2+2c⇒6=5+2c⇒1=2c⇒c=1/2
The reaction becomes :
Pb(NO₃)₂⇒PbO+2NO₂+1/2O₂ x2
2Pb(NO₃)₂⇒2PbO+4NO₂+O₂
What volume would a sample of gas occupy in LITERS at 0.985 atmospheres and a volume of 3.65 liters if the pressure were raised to 861.0 mmHg?
Answers
Answer:
3.18 L
Explanation:
Step 1: Given data
Initial pressure (P₁): 0.985 atmInitial volume (V₁): 3.65 LFinal pressure (P₂): 861.0 mmHgFinal volume (V₂): ?Step 2: Convert P₁ to mmHg
We will use the conversion factor 1 atm = 760 mmHg.
0.985 atm × 760 mmHg/1 atm = 749 mmHg
Step 3: Calculate the final volume of the gas
Assuming ideal behavior and constant temperature, we can calculate the final volume using Boyle's law.
P₁ × V₁ = P₂ × V₂
V₂ = P₁ × V₁/P₂
V₂ = 749 mmHg × 3.65 L/861.0 mmHg = 3.18 L
27. A solution has a pH of2. Which best describes the solution? (a point)
a strong acid
a strong base
a neutral solution
a weak base
Answers
why is the minimum internal temperature for poultry set higher than that for beef or fish?
Answers
The minimum internal temperature for poultry is set higher than that for beef or fish due to the risk of foodborne illness caused by bacteria such as Salmonella and Campylobacter that are commonly found in poultry.
These bacteria can be present on the surface of the poultry and can also be present in the internal tissues, such as the liver and intestines.
To kill these bacteria and reduce the risk of foodborne illness, it is recommended that poultry be cooked to an internal temperature of at least 165°F (74°C). This temperature is sufficient to kill harmful bacteria that may be present in the poultry.
In contrast, beef and fish are generally considered to be lower-risk foods in terms of bacterial contamination.
While it is still important to cook these foods to a safe temperature to ensure they are fully cooked and free from harmful bacteria, the minimum internal temperature requirements are lower than for poultry.
For example, beef is typically cooked to an internal temperature of 145°F (63°C), while fish is typically cooked to an internal temperature of 145°F to 150°F (63°C to 66°C), depending on the type of fish.
To know more about foodborne illness click here:
https://brainly.com/question/24477516#
#SPJ11