If there is no oxygen, the citric acid cycle cannot function because ___
Answers
If there is no oxygen, the citric acid cycle cannot function because the electron transport chain, which relies on oxygen as the final electron acceptor, would be unable to proceed.
Oxygen's role in the electron transport chain is crucial for maintaining the necessary electrochemical gradient that drives ATP production through oxidative phosphorylation. Without oxygen, the electron transport chain would halt, and NADH and FADH2 would not be regenerated back to their oxidized forms, NAD+ and FAD. Since the citric acid cycle (also known as the Krebs cycle or TCA cycle) depends on the availability of NAD+ and FAD to accept electrons from various intermediate compounds, the cycle would be unable to continue. The production of ATP through substrate-level phosphorylation in the citric acid cycle would also be affected.
In the absence of oxygen, cells may resort to alternative metabolic pathways such as fermentation to produce ATP. However, these processes are less efficient and generate significantly less ATP compared to the combination of the citric acid cycle and oxidative phosphorylation. This is why organisms that rely primarily on aerobic respiration, including humans, would face severe energy deficiencies if oxygen becomes unavailable. If there is no oxygen, the citric acid cycle cannot function because the electron transport chain, which relies on oxygen as the final electron acceptor, would be unable to proceed.
Learn more about electron transport chain here:
https://brainly.com/question/6969404
#SPJ11
Related Questions
3.0 mol Na reacts with 1.4 mol
F2 according to the equation below:
2Na+ F₂ → 2NaF
How many moles of NaF form
from 3.0 mol Na?
Answers
The number of moles of NaF that will be produced from 3moles of Na is 3 moles.
How to calculate number of moles?Stoichiometry is the study and calculation of quantitative (measurable) relationships of the reactants and products in chemical reactions (chemical equations).
According to this question, sodium reacts with fluorine as follows:
2Na+ F₂ → 2NaF
Based on the above equation, 2 moles of Na will produce 2 moles of NaF
This means that 3 moles of Na will produce 3 moles of NaF.
Learn more about stoichiometry at: https://brainly.com/question/9743981
#SPJ1
GIVE REASON:-
Ammeter is connected in series and voltmeter is connected in parallel to the electric load in an electric circuit .
Answers
Answer:
An ammeter is a device that is used to measures the amount of current flowing in a circuit and a voltmeter measure electric potential difference in an electric circuit between two points.
An Ammeter is connected in series because of its low resistance capacity and it will be connected in parallel it can get damaged due to high current. A voltmeter is connected in parallel in an electric circuit because the potential difference remains the same when connected in parallel.
can
someone please help

Answers
Answer: protein bar,it gives alot of energy and wont upset your stomach!!
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
write the balanced net ionic equation for the reaction when copper(ii) sulfate and ammonium hydroxide are mixed in aqueous solution. if no reaction occurs, write only nr.
Answers
The net ionic equation for the reaction between copper(II) sulfate and ammonium hydroxide depends on whether a reaction occurs.
If a reaction occurs, the balanced net ionic equation will be provided. Otherwise, if no reaction occurs, the notation "nr" will be used to indicate no reaction.When copper(II) sulfate (CuSO4) and ammonium hydroxide (NH4OH) are mixed in aqueous solution, they may undergo a precipitation reaction if a reaction occurs.
In this case, the copper(II) ion (Cu2+) from copper(II) sulfate reacts with the hydroxide ion (OH-) from ammonium hydroxide to form a precipitate of copper(II) hydroxide (Cu(OH)2).The balanced net ionic equation for the reaction, assuming a precipitation occurs, is:
Cu2+ (aq) + 2 OH- (aq) → Cu(OH)2 (s)
On the other hand, if no reaction occurs, it means that there are no significant chemical changes taking place when the two solutions are mixed. In this case, the notation "nr" (no reaction) would be used to indicate that no reaction occurs.
It is important to note that the precise conditions, concentrations, and stoichiometric ratios of the reactants can influence whether a reaction occurs or not. Conducting the actual experiment and observing the formation or lack of formation of a precipitate would provide definitive evidence of whether a reaction takes place.
To learn more about ionic.
Click here:brainly.com/question/30373783
#SPJ11
Which property of matter is conserved in chemical reactions and shown by balanced equations?
Answers
The property of matter that is conserved in chemical reactions and shown by balanced equations is known as the Law of Conservation of Mass. According to this law, mass can neither be created nor destroyed in a chemical reaction; it can only be transformed from one form to another.For instance, when two substances are combined, they react and form a new substance.
The products that are formed contain the same number of atoms as the reactants, but in different configurations. To keep track of the number of atoms on either side of the equation, we use coefficients, which indicate the number of molecules or atoms of each substance in the reaction. However, when a chemical equation is written, it must adhere to the law of conservation of mass.The law of conservation of mass is critical in chemical reactions because it ensures that the amount of reactants that go into a reaction equals the amount of products that come out of it. This means that the total mass of reactants must equal the total mass of the products. As a result, the balanced chemical equation must reflect this law.For example, consider the reaction between hydrogen gas and oxygen gas, which forms water. The balanced chemical equation is as follows:2H2 + O2 → 2H2OIn this reaction, two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water. The coefficients in the balanced chemical equation indicate that two molecules of hydrogen and one molecule of oxygen combine to form two molecules of water, obeying the law of conservation of mass.In conclusion, the Law of Conservation of Mass is a fundamental principle in chemistry that is used to balance chemical equations. It is critical in chemical reactions because it ensures that the total mass of reactants equals the total mass of products, allowing scientists to accurately predict the outcome of a chemical reaction.For such more question on chemical reaction
https://brainly.com/question/11231920
#SPJ8
click in the answer box to activate the palette. menthol is a flavoring agent extracted from peppermint oil. it contains c, h, and o. in one combustion analysis, 10.00 mg of the substance yields 11.53 mg h2o and 28.16 mg co2. what is the empirical formula of menthol? add subscripts to complete the empirical formula.
Answers
The empirical formula is C10H20O.
Molar mass of water is 18.02 g/mol. Molar mass of carbon dioxide is 44.01 g/mol. Molar mass of hydrogen is 1.01 g/mol. Molar mass of carbon is 12.01 g/mol. Molar mass of oxygen is 16 g/mol.
nH = 11.53/18.02 × 2 = 1.28mmol
nC = 28.16/44.01 × 1 = 0.640mmol
mH = 1.28 × 1.01 = 1.292 mg
mC = 0.64 × 12.01 = 7.678mg
mo = 10 - 1.292 - 7.678 = 1.029mg
no = 1.029/16 = 0.064mmol
Divide each moles by 0.064 mmol:
nC = 0.64 / 0.064 = 10
nH = 1.28 / 0.064 = 20
no = 0.064 / 0.064 = 1
The empirical formula is C10H20O.
Learn more about Empirical formula here:
https://brainly.com/question/14044066
#SPJ4
"compounds that have the same molecular formula but possess different physical and chemical properties are called isomers and this phenomenon is known as isomerism."
Answers
The statement is correct. Compounds that have the same molecular formula but possess different physical and chemical properties are indeed called isomers, and this phenomenon is known as isomerism.
Isomerism refers to the existence of two or more compounds that have the same molecular formula but differ in the arrangement or connectivity of atoms in their structures. Isomers can exhibit variations in properties such as boiling point, melting point, density, solubility, reactivity, and biological activity.
There are two main types of isomerism: structural isomerism and stereoisomerism.
Structural Isomerism: In structural isomerism, the isomers differ in the connectivity of atoms within the molecule. They have different structural arrangements or bonding patterns.
Examples of structural isomers include chain isomerism, functional group isomerism, position isomerism, and ring-chain isomerism. Structural isomers often exhibit distinct physical and chemical properties due to their different arrangements of atoms and functional groups.
Stereoisomerism: Stereoisomers have the same structural formula, but their spatial arrangement differs. This type of isomerism arises due to the presence of stereocenters or double bonds that restrict rotation. Stereoisomers can be further divided into two subcategories:
a. Geometric Isomerism (Cis-Trans Isomerism): Geometric isomers have different spatial arrangements around a double bond or a ring. The isomers can exist as cis-isomers (similar groups on the same side) or trans-isomers (similar groups on opposite sides) and exhibit different physical and chemical properties.
b. Optical Isomerism (Enantiomerism): Optical isomers, also known as enantiomers, are non-superimposable mirror images of each other. They have the same connectivity of atoms but differ in their three-dimensional arrangement. Enantiomers rotate plane-polarized light in opposite directions and exhibit different biological activities and interactions with chiral environments.
For more such question on Compounds visit:
https://brainly.com/question/29108029
#SPJ8
Note: The complete question is:
"compounds that have the same molecular formula but possess different physical and chemical properties are called isomers and this phenomenon is known as isomerism." check the given statement is correct or not?
One isotope has a mass of 96.780 amu. The second isotope has a percent abundance of 41.7%. What is the mass of the second isotope
Answers
The mass of the second isotope is 3.54 amu.
The atomic mass of any element is the sum of masses of all its isotopes that are present in nature multiplied by their abundances, expressed as a percentage. Therefore, we can write:
Atomic mass = (% abundance of isotope 1 × mass of isotope 1) + (% abundance of isotope 2 × mass of isotope 2)
Let m be the mass of the second isotope.
Hence, Atomic mass = (100 - 41.7)% × 96.780 + 41.7% × m = 58.3% × 96.780 + 41.7% × m= 56.42 + 0.417m
This is the expression for the atomic mass of the element.
Now, the atomic mass of any element is usually given to us, but we need to find the mass of the second isotope. We can equate the expression we just found to the given atomic mass and solve for m.
Atomic mass = 57.9 = 56.42 + 0.417mm = (57.9 - 56.42)/0.417 = 3.54 × 1 amu
m = 3.54 amu
Therefore, the mass of the second isotope is 3.54 amu.
Learn more about isotope: https://brainly.com/question/3187640
#SPJ11
10. Circle the letter of each sentence that is true about how ocean currents influence climates.
a. Ocean currents influence many marine climates.
b. Only warm ocean currents influence climates.
c. The North Atlantic Drift gives Ireland a warm climate for its latitude.
d. The California Current gives the West Coast a warm climate for its latitude.
Answers
Answer:
a. Ocean currents influence many marine climates.
c. The North Atlantic Drift gives Ireland a warm climate for its latitude.
a & c
brainliest pls
carbon-14 has a half-life of 5720 years and this is a first-order reaction. if a piece of wood has converted 11.5% of the carbon-14, then how old is it?
Answers
The age of a sample can be estimated using the carbon-14 dating method based on the decay of carbon-14 to nitrogen-14.
The half-life of carbon-14 is 5720 years, meaning that half of the original amount of carbon-14 in a sample will decay to nitrogen-14 in 5720 years.
If a piece of wood has converted 11.5% of the carbon-14, then the age of the sample can be estimated by solving for the amount of time it took for the decay to occur. The rate of decay can be described by the first-order reaction equation, which states that the amount of radioactive isotope remaining is proportional to e^(-kt), where k is the decay constant and t is the time.
Solving for t, the age of the sample, can be calculated using the half-life formula, ln(2)/k = t. The age of the sample can then be estimated by multiplying the calculated time by the half-life of carbon-14. However, this method assumes that the initial amount of carbon-14 in the sample is known, which is often not the case. In practice, more complex models are used to estimate the age of samples using carbon-14 dating.
Learn more about carbon-14:
brainly.com/question/4206267
#SPJ4
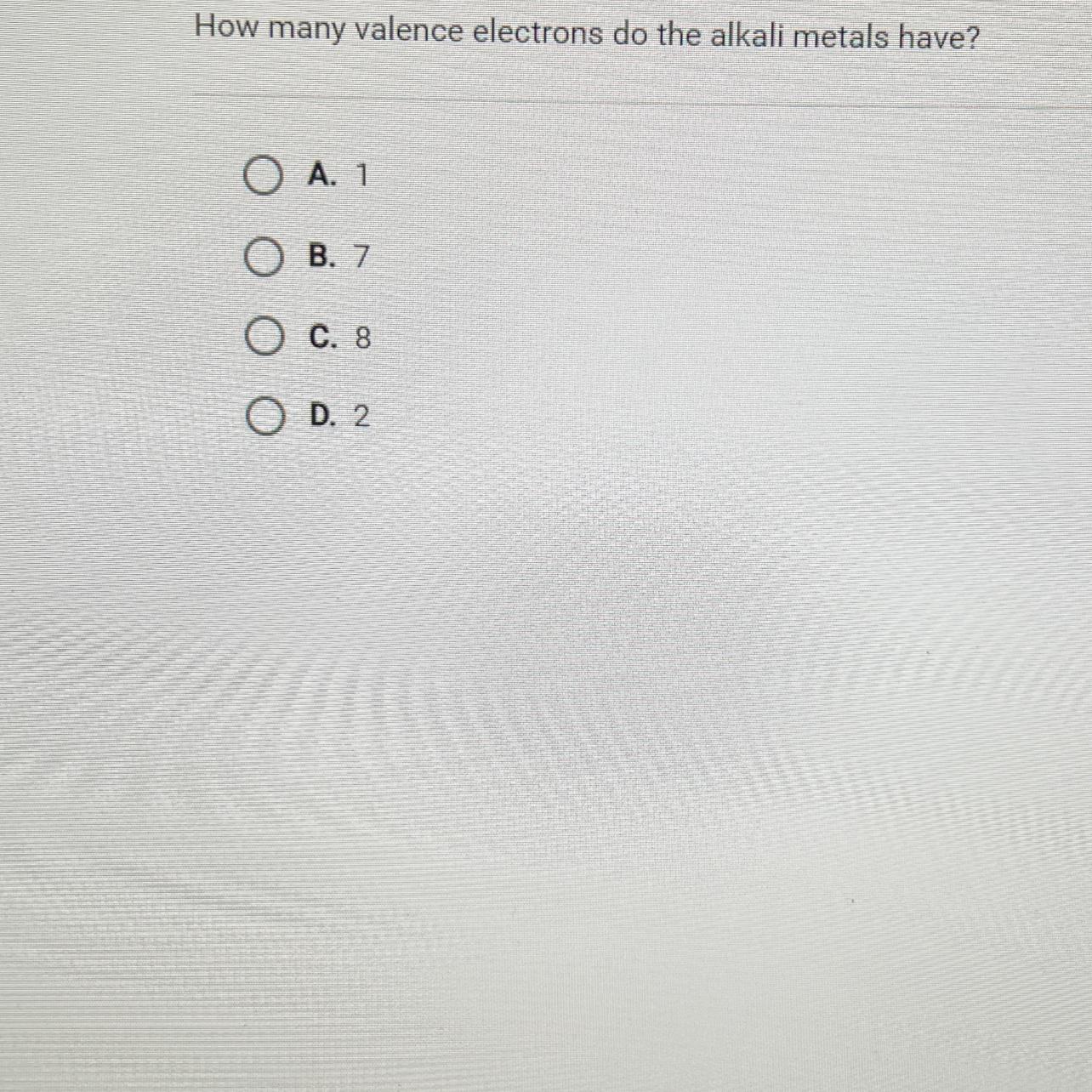
Question 10 of 10How many valence electrons do the alkali metals have?O A. 1B. 7O C. 8O D. 2SUBMIT
Answers
Alkali metals are the group A1 of the periodic table (left most column) and that group has 1 valance electron. Therefore the answer is A. 1
Write copper (I) chlorate in a chemical formula.
Answers
The chemical formula for copper (I) chlorate is CuClO3. This is a compound of copper in the oxidation state +1 and chlorate, which is an ion with the formula ClO3^- and a charge of -1
Copper(I) chlorate is formed when copper(I) ions combine with chlorate ions. The copper(I) ion has a +1 charge, while the chlorate ion has a -1 charge. To balance the charges, one copper(I) ion combines with one chlorate ion. The chlorate ion is represented by the chemical formula ClO3 with a -1 charge. The copper(I) ion is represented by Cu with a +1 charge. Therefore, the chemical formula for copper(I) chlorate is CuClO3.
To know more about chemical formulas : brainly.com/question/32018188
#SPJ11
what should you expect to observe when the ingredients in an antacid react with hcl to produce h2co3
Answers
When antacid reacts with HCl to produce H2CO3 it produce fizzy gas react due to a double displacement reaction.
Table salt and carbonic acid are produced in a two fold displacement process when hydrochloric acid and antacid are combined. Additionally, due to the instability of carbonic acid, it will decompose into water and carbon dioxide, emitting a "fizzy" gas.
Strong acid hydrochloric acid is denoted by the chemical formula HCl. The primary ingredient in antacids is sodium bicarbonate, sometimes known as baking soda and has the chemical formula NaHCO3.
Antacid undergoes a chemical reaction when it reacts as a carbonate with powerful acids like hydrochloric acid to create compounds with a different chemical makeup from the beginning components. Similar reactions will be produced by other carbonates, such as calcium carbonate, which makes up the majority of Tums.
For more information on double displacement reaction kindly visit to
https://brainly.com/question/29307794
#SPJ4
To the Mayans, what was the utmost important thing to do?
Answers
hello my name is bob free give me the free give me th efree points
Explanation:
If calcium were to loose a proton from it's nucleus through extraordinary chemical processes it would...
Answers
If calcium were to lose a proton from its nucleus, it would result in the formation of a new element with one less proton.
Calcium has 20 protons, which determines its atomic number and identity as an element. When an element loses a proton, its atomic number decreases by one, leading to the formation of a new element.
In this case, calcium losing a proton would result in an element with 19 protons, which corresponds to the element potassium (symbol K). Therefore, if calcium were to undergo extraordinary chemical processes that cause it to lose a proton, it would transform into the element potassium.
This hypothetical scenario highlights the fundamental connection between the number of protons and the identity of an element within the periodic table.
To know more about the Calcium refer here,
https://brainly.com/question/10850977#
#SPJ11
Complete question :
If calcium were to lose a proton from its nucleus through extraordinary chemical processes, what element would it become?
A flask that weighs 450 g is filled with 145 ml of benzene. The weight of the flask and benzene is found to be 754 g. From this information, calculate the density of the benzene.
Answers
Answer:
Density, \(d=2.09\ g/cm^3\)
Explanation:
Given that,
Mass of a flask is 450 g
Volume of benzene added to the flask is 145 mL or 145 cm³
The weight of the flask and benzene is found to be 754 g.
We need to find the density of the benzene.
Weight of benzene added = total weight of flask and benzene-mass of flask
m = 754 g - 450 g
m = 304 g
Density = mass/volume
So,
\(d=\dfrac{304\ g}{145\ cm^3}\\\\d=2.09\ g/cm^3\)
So, the density of the benzene is \(2.09\ g/cm^3\).
What is the atomic number of an atom?
Question 3 options:
the number of protons
the number protons and neutrons
the number of neutrons
the number of electrons and neutrons
Answers
Answer: The number of protons
Explanation:
Please I need help ASAP!

Answers
The amount of heat energy released when 12.0 grams of NaOH dissolves in water is -133.53 KJ
How do i determine the heat energy released?First, we shall determine the number of mole in 12 grams of NaOH. Details below:
Mass of NaOH = 12 grams Molar mass of NaOH = 40 g/mol Mole of NaOH =?Mole = mass / molar mass
Mole of NaOH = 12 / 40
Mole of NaOH = 0.3 mole
Finally, we shall determine the heat energy released. Details below:
NaOH(aq) -> Na⁺(aq) + OH⁻(aq) ΔH = -445.1 KJ
From the balanced equation above,
When 1 mole of NaOH were dissolved, -445.1 KJ of heat energy were released.
Therefore,
When 0.3 mole of NaOH is dissolve, = (0.3 × -445.1) / 1 = -133.53 KJ of heat energy is released.
Thus, we can conclude that the heat energy released is -133.53 KJ
Learn more about heat energy:
https://brainly.com/question/16398667
#SPJ1
How many moles are 454 grams of iron
Answers
Answer:
8.1293534120006496
Explanation:
hope this helps
Help plzzzz
Which of the following best describes the difference between a scientific theory and theories from other bodies of knowledge?
A.) Scientific theories must be based on experimental evidence, whereas theories from other bodies of knowledge are not always based on data.
"S
B.) Scientific theories are always true, whereas theories from other bodies of knowledge are usually proven to be false.
C.) scientific theories are unproven assumptions, whereas theories from other bodies of knowledge must be based on experimental evidence.
D.) Scientific theories are usually proven to be false, whereas theories from other bodies of knowledge are always true.
Answers
Answer:
option b is correct
..............
Answer:
it C not D btw
Explanation:
1 Which of the following is an example of periodicity?
a. eating breakfast
c. writing a letter b. hitting a home run d. sneezing
Answers
Answer:
A:UNDERSTANDING CONCEPTS PART A 13) Which of the following is an example of periodicity? A) eating breakfast
A gymnast whose weight is 5 10 N hangs from the middle of a bar supported by two vertical strands of rope. What is the tension in each strand?
A. 765 N
B. 0 N
C. 1020 N
D. 510 N
E. 255 N
Answers
Answer:
I think the answer is(D. 510 n)
in h-nmr spectroscopy, which factor determines the shape of the spectra?
a. The number of protons in the macromolecule
b. The energy of a proton’s α spin state
c. The transition between a proton’s α and β spin states
d. The energy of a proton’s β spin state
Answers
The shape of the spectra in h-nmr spectroscopy depends on how many protons are present in the macromolecule.
A potent method for identifying and measuring the components of complicated mixes is nuclear magnetic resonance (NMR) spectroscopy. Each atom's nucleus with a non-zero spin quantum number has an associated magnetic moment and angular momentum.
The NMR signal is created by the suitable frequency of electromagnetic radiation being absorbed. The nuclei go from the lower energy to the higher energy spin states as a result of energy absorption.
You may determine how many various settings the hydrogen atoms are in by counting the number of peaks. You can determine the ratio of hydrogen atom counts in each of these environments by comparing the areas under the peaks.
To know about spectroscopy
https://brainly.com/question/30256292
#SPJ4
No one citizen would be able to possess more than 500 iugera of public land (ager publicus) that was acquired during wars. Any excess land would be confiscated to the state and redistributed to the poor and homeless in small plots of about 30 iugera per family.
Answers
Answer:
The answer is "Tiberius Gracchus agrarian reform"
Explanation:
The Lex Sempronia Agraria law suggested by Tiberius Gracchus. This law might rearrange the power of the comer speed of the fluid or even the state, indicating land annexed in original state battles. They attempt to legislate besides agricultural restructuring as well as his early death by both the Senator, and immediately continues to move for a rural transformation plan, in specific by citing Sextian-Licinian law, 240 years old, restrict the amount of land a single person would still own.
How many molecules are in 450.0 grams of aluminum fluoride (AIF3)?
Answers
Answer:
5.3586262014272155
Explanation:
You have to go from grams --> moles --> molecules. You can find the equation online.
First you have to find the molar mass of AlF3 which is 83.98 g/mol.
To convert it to moles you simply
450.0 grams × \(\frac{1 mol}{83.98 grams}\) = 5.358 mol
To convert mol to molecules: (multiply by Avogadros # = 6.022*10^23)
5.358 mol × \(\frac{6.022*10^2^3}{1mol}\) = \(3.23 *10^{24}\)
calculate the isoionic and isoelectric ph of 0.028320.02832 m valine. enter your answers to the hundredths place.
Answers
Answer:
isoelectronic pH = 6.003
Explanation:
isoelectronic pH = (pKa1+pKa2)/2
A scientist read an article in a magazine about how air pollution can cause children to develop asthma. Since the article did not come from a peer-reviewed magazine, he doubted the claim, instead believing that children living in a smog-filled city would not have an increased chance of developing asthma. He wanted to test his own idea, so he formed a hypothesis and planned out an experiment. The scientist decided to compare 10th graders from three schools in smog-filled cities to 10th graders from three schools in smog-free rural areas. He found out how many of these students had asthma and recorded his data in the table below. After looking at the results, he was amazed at what a big risk factor living in a smog-filled city was after all. (20 points)
Answers
The results show that 10th graders from smog-filled cities had a significantly higher prevalence of asthma than their counterparts in smog-free rural areas.
Relationship between Air Pollution and Asthma.Asthma is a chronic respiratory condition characterized by inflammation and narrowing of the airways, which can cause difficulty breathing, coughing, wheezing, and shortness of breath. It affects the bronchial tubes, which are the air passages that carry air in and out of the lungs.
The experiment that the scientist designed involved comparing the prevalence of asthma among 10th graders from three schools in smog-filled cities with that of 10th graders from three schools in smog-free rural areas.
Based on these results, the scientist can reject his initial hypothesis that air pollution does not increase the risk of developing asthma in children.
The data supports the claim made in the non-peer-reviewed magazine article that air pollution can cause children to develop asthma. However, it is important to note that this experiment only shows an association between air pollution and asthma and does not establish a causal relationship. Further research would be needed to establish causality and to investigate the mechanisms by which air pollution may cause asthma.
Learn more about air pollution here https://brainly.com/question/17023720
#SPJ1
b. What is the atomic mass of the element in period 5, group 14?
Answers
Answer:
118.71 u
Explanation:
Tin. Tin is a chemical element with the symbol Sn (for Latin: stannum) and atomic number 50. It is a main-group metal in group 14 of the periodic table.
Source: https://en.m.wikipedia.org/wiki/Period_5_element#:~:text=Tin,-Main%20article%3A%20Tin&text=Tin%20is%20a%20chemical%20element,14%20of%20the%20periodic%20table.
You have 1.2 moles of NH 4 NO 3 in a 6 M solution. How many liters is that?
A) 0.5 L
B) 0.72 M
C) 0.72 L
D) 2 L
Answers
Answer:
Volume = 0.2 L
Explanation:
Given data:
Number of moles of NH₄NO₃ = 1.2 mol
Molarity of solution = 6 M
Volume of solution = ?
Solution:
Formula:
Molarity = number of moles / volume of solution
by putting values,
6 M = 1.2 mol / Volume
Volume = 1.2 mol / 6 mol/L
Volume = 0.2 L