Answers
Adding CO to the reaction would result in formation of more products at equilibrium.
What is chemical equilibrium?Chemical equilibrium, the state in which there is no net change in the amounts of reactants and products in the course of a reversible chemical reaction. A reversible chemical reaction is one in which products that is once formed react to form the original reactants. The types of balances are:
Stable equilibrium.Unstable equilibrium.Neutral equilibrium.As additional reactants are added, the equilibrium shifts in order to produce more product. Adding more reactants shifts the equilibrium towards the products. Therefore the equilibrium shifts to the right.
To know more about chemical equilibrium, click-
https://brainly.com/question/6705807
#SPJ1
The complete question is follows:

Related Questions
How to polymers work?
Answers
Answer:
A polymer is any of a class of natural or synthetic substances composed of very large molecules, called macromolecules, which are multiples of simpler chemical units. Polymers make up many of the materials in living organisms and are the basis of many minerals.
Explanation:
Explanation:
The first man-made polymers were actually modified versions of these natural polymers. Celluloid, the stuff from which silent-movie film was made, was a plastic created from chemically modified cellulose. The first completely synthetic polymer (that is, made by people through chemical synthesis), invented in the early years of the twentieth century, was Bakelite: a plastic made by reacting phenol and formaldehyde under pressure at high temperatures.
# be careful #
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
Given the following data for water:
Heat of fusion = 334 J/g
Heat of vaporization = 2,256 J/g
Specific heat of solid = 2.09 J/g °C)
Specific heat of liquid = 4.184 J/g °C)
Specific heat of gas = 1.84 J/g °C)
Calculate how much energy is needed to change 100.0 grams of liquid water at 15.0 °C to vapor at 125.0 °C. (3 points)
Oa
O
b
44,000 J
89,400 J
104,000 J
266,000 J
Answers
1. Heating the liquid water from 15.0 °C to 100.0 °C:
q = m * Cp * ΔT
= 100.0 g * 4.184 J/g °C * (100.0 °C - 15.0 °C)
= 34,972 J
2. Vaporizing the liquid water at 100.0 °C:
q = m * Hvap
= 100.0 g * 2,256 J/g
= 225,600 J
3. Heating the water vapor from 100.0 °C to 125.0 °C:
q = m * Cp * ΔT
= 100.0 g * 1.84 J/g °C * (125.0 °C - 100.0 °C)
= 4,600 J
The total energy required is the sum of the three steps:
Q = q1 + q2 + q3
= 34,972 J + 225,600 J + 4,600 J
= 265,172 J
Therefore, the energy needed to change 100.0 grams of liquid water at 15.0 °C to vapor at 125.0 °C is approximately 265,172 J, which is closest to option (d) 266,000 J.
Ill mark you as Brainlist
Part A:
Write a molecular equation for the gas evolution reaction that occurs when you mix aqueous hydrobromic acid and aqueous potassium sulfite.
Express your answer as a chemical equation including phases.
Part B:
Write a net ionic equation for the previous reaction.
Express your answer as a chemical equation including phases
Answers
The net ionic equation for this gas evolution reaction is H+(aq) + SO32-(aq) SO2(g) + H2O, while the balanced molecular equation is 2HBr(aq) + K2SO3(aq) SO2(g) + 2H2O(l) + 2KBr(aq) (l).
What is the net ionic equation for potassium carbonate and hydrobromic acid?The reaction's chemical formula is K2CO3(aq)+2HBr(aq)2KBr(aq)+CO2(g)+H2O. (l) Strong electrolytes in the process, K2CO3, HBr, and KBr totally dissociate in water to generate their corresponding ions.
What byproduct results from the reaction of HBr and Koh?A salt (the KBr) and water will be created when the HBr and KOH interact. While balancing this equation, make careful to count both hydrogen atoms on the reactants side.
To know more about ionic equation visit:-
https://brainly.com/question/29299745
#SPJ1
Octane (C8H18) is a major component of gasoline. Which of the following would you expect to be the LEAST soluble in gasoline?1. table salt2. candle wax3. motor oil4. diesel fuel5. table sugar
Answers
The component which is least soluble in gasoline is 2.candle wax. So, correct option is 2.
The octane rating still up in the air by blending fills from just ordinary heptane and iso-octane (2,2,4-trimethylpentane, an exceptionally extended octane), and relegating hostile to thump evaluations of zero for typical heptane and 100 for unadulterated iso-octane. The counter thump rating of this combination would be equivalent to the level of iso-octane in the blend. Various isomers of octane can add to a lower or higher octane rating. For instance, n-octane (the straight chain of 8 carbon molecules with no stretching) has a - 20 (negative) Exploration Octane Rating, while unadulterated iso-octane has a RON rating of 100. A few energizes have an octane rating higher than 100, outstandingly those containing methanol or ethanol.
Candle wax exist in solid state.On dissolving candle wax in octane it will least dissolve becuase of inert reaction between octane and candle wax.
Hence,correct answer is 2.
To know more about octane,visit here:
https://brainly.com/question/21268869
#SPJ4
barrier islands are low and narrow sandy islands that form a rim offshore from a coastline. these islands protect inland shores from the surf,especially during storms. theses islands are becoming increasingly developed because people want to live by the open ocean, yet the island themselves are not permaneny. why aren't the islands permanent?
A. People developthe islands and remove sand during housing construction.
B. Offshore earthquakes cause the islands to sink below sea level.
C. The wind and the waves are constantly redistributing the sand.
D. Development companies mine the sand for use in inland construction projects.
Answers
Barrier islands are not permanent because the wind and the waves are constantly redistributing the sand.
What are barrier islands?Barrier islands are particularly flat or lumpy sand regions that are formed parallel to the mainland shore by wave and tide action. They typically appear in groups called chains, which can range in size from a few to more than a dozen islands.
They are called "barriers" because they act as a physical barrier, protecting the mainland from the effects of strong waves, storm surges, and flooding. Barrier islands are formed by a variety of processes, including longshore drift, sediment deposition, and sea level changes.
Barrier islands typically consist of sandy beaches on their seaward side and marshes, lagoons, or dunes on their landward side.
Learn about Barrier islands here https://brainly.com/question/1647030
#SPJ1
Question 2/8
From left to right across the second period of the periodic table,
Answers
Answer:
HE
Explanation:
HE is a noble gas
If AB = 5 inches and AD = 8, find BD. Round to the nearest tenth if necessary.
WILL GIVE BRAINLIEST
Answers
Answer:
6.5
Explanation:
half of 5 is 2.5, half of 8 is 4. 2.5+4=6.5
:)
Researchers investigated the influence of environmental pH on the activity of peroxidase, an enzyme that catalyzes the conversion of hydrogen peroxide to water and oxygen gas. In an experiment, the researchers added a hydrogen peroxide solution containing guaiacol to several identical test tubes and adjusted the solution in each test tube to a different pH . The researchers included the guaiacol because it caused the solutions to change color as the reactions proceeded, which the researchers relied on for measuring reaction rates. Finally, the researchers added the same amount of peroxidase to each test tube and measured the rate of each reaction at 23°C . The results of the experiment are represented in Figure 1.
Based on Figure 1, which of the following statements best predicts the effect that a change from a moderately acidic environment ( pH near 6) to a basic environment will have on peroxidase activity?
answer choices
Peroxidase activity will decrease.
Peroxidase activity will increase.
Peroxidase activity will stay the same.
Peroxidase activity will increase at first and then decrease.

Answers
From the given graph, if we change from a moderately acidic environment ( pH near 6) to a basic environment, the peroxidase activity will decrease. Therefore, option (A) is correct.
What is pH?pH can be described as the negative logarithm of hydrogen ion (H⁺) concentration. Mathematically, pH is generally inversely proportional to the concentration of hydrogen ions (H⁺).
pH = -log ([H⁺])
In chemistry, pH is a scale used to determine the acidity or basicity of an aqueous solution. Acidic solutions are calculated to have lower pH values than basic or alkaline solutions.
The pH scale possesses ranges from 0 to 14 while pH 7.0 is neutral. A low pH (about 1 or 2) is acidic and a high pH (12 or 14) is basic.
When we change the moderately acidic environment to a basic environment. We actually increase the pH value in the graph showing the peroxidase activity decreases when pH increases more than 6.
Learn more about pH value, here:
brainly.com/question/1503669
#SPJ1
My teacher laid this much out for us but I don’t know how to get the products in each one.

Answers
Answer:dang
Explanation:
How much water has to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M?
Answers
Approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
To find the amount of water that needs to be evaporatedThe relationship between the initial and final concentrations and volumes must be taken into account.
Given: Initial concentration \((C^1) = 1 M Initial volume (V^1) = 250 mL\)
\((C^2) = 3 M final concentration\)
We can use the equation:
\(C^1 * V^1 = C^2 * V^2\)
Where:
\(V^2\)is the final volume of the solution
Rearranging the equation to solve for V2:
\(V^2 = (C^1 * V^1) / C^2\)
Substituting the given values:
\(V^2 = (1 M * 250 mL) / 3 M\)
\(V^2 = 250 mL / 3\)
\(V^2\) ≈ \(83.33 mL\)
To find the amount of water that needs to be evaporated, we subtract the final volume from the initial volume:
Amount of water to be evaporated = \(V^1 - V^2\)
Amount of water to be evaporated = 250 mL - 83.33 mL
Amount of water to be evaporated ≈ 166.67 mL
Therefore, approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
Learn more about Initial concentration here: brainly.com/question/30720317
#SPJ1
What is the molarity of a solution that contains 0.180 moles KOH in 0.350 L of solution?
Answers
Answer:
molarity of solution = 0.514
Explanation:
What is the oxidation state of O in NO₂?
OA. +2
OB. -2
O C. O
OD. -4
Answers
Answer:
Let it be x
\({ \tt{ (oxidation \: state \: of \: nitrogen) + 2x = overall \: charge}} \\ { \tt{ - 4 - 2 x = 0}} \\ { \tt{2x = - 4}} \\ { \tt{x = - 2}}\)
first response gets brainliest

Answers
which is the graph of the function g(x) = f(-x)
Answers
To graph the function g(x) = f(-x), you can start with the graph of f(x) and then reflect it about the y-axis.
What is a graph of the function g(x) = f(-x)?To find the graph of the function g(x) = f(-x), we can start with the graph of the function f(x) and then reflect it about the y-axis.
If the graph of f(x) is symmetric with respect to the y-axis, meaning it is unchanged when reflected, then g(x) = f(-x) will have the same graph as f(x).
However, if the graph of f(x) is not symmetric with respect to the y-axis, then g(x) = f(-x) will be a reflection of f(x) about the y-axis.
In either case, the resulting graph of g(x) = f(-x) will be symmetric with respect to the y-axis.
Learn more about the graph of functions at: https://brainly.com/question/17089414
#SPJ1

Citric acid is one component of some soft drinks. Suppose that 8 L of solution are made from 0.24 g of citric acid, C6H8O7. What is the molarity of citric acid in the solution?
Answers
Answer:
The correct answer is 0.15 × 10⁻³ M.
Explanation:
C₆H₈O₇ is the molecular formula of citric acid. The mass of the one mole of the substance that comprise Avogadro's no of molecules is termed as the molar mass of the substance.
The molar mass of the citric acid (C₆H₈O₇) will be,
6 × atomic mass of carbon + 8 × atomic mass of hydrogen + 7 × atomic mass of oxygen = 6×12 + 8×1 + 7×16 = 192. Thus, the molar mass of citric acid is 192 g/mol.
The value of the solution given in the question is 8 L.
The mass of citric acid given is 0.24 g or 240 mg, which can also be written as 240/1000 g or 240 × 10⁻³ g.
The number of moles can be calculated by using the formula = mass / molar mass. Thus, by putting the values we get,
= 240 × 10⁻³ g / 192 g/mol
= 1.25 × 10⁻³ mol
The number of moles of the solute present in the 1 liter of the solution is termed as the molarity of the solution. The formula of molarity or M is,
= no of moles of solute / volume of solution in L
Now putting the values we get,
= 1.25 × 10⁻³ mol / 8 L
= 0.156 × 10⁻³ M
Hence, the molarity of citric acid in the given solution is 0.15 × 10⁻³ M
the chunks of fluid magma fall back to the surface to feed a lava flow, which may have a rough …?
… (type of lava) surface or a smooth …?
Answers
The chunks of fluid magma that fall back to the surface to feed a lava flow can give rise to two different types of surfaces: rough and smooth.
A rough lava surface is often associated with a type of lava called 'aa' (pronounced "ah-ah"). Aa lava flows are characterized by a jagged and clumpy texture. This occurs because the chunks of magma, known as clinkers, are fragmented and accumulate on top of the flow. As the flow advances, the clinkers are pushed forward, creating a rough and uneven surface. Aa lava flows tend to be slower-moving and can be hazardous to walk on due to their rough texture.On the other hand, a smooth lava surface is typically associated with a type of lava known as 'pahoehoe' (pronounced "pah-hoy-hoy"). Pahoehoe lava flows are characterized by a smooth, undulating surface with a rope-like or ropy texture. The fluid magma of pahoehoe lava allows it to flow more freely, resulting in a surface that appears molten and smooth. Pahoehoe flows can travel greater distances compared to aa flows and are generally easier to traverse.The type of lava surface, whether rough or smooth, depends on various factors such as the viscosity of the magma, eruption style, and the rate of cooling during the lava flow.
for such more questions on fluid
https://brainly.com/question/30543213
#SPJ8
Enter your answer in the provided box.
Answer the following questions about the fermentation of glucose (C6H12O6, molar mass 180.2 g/mol) to ethanol (C2H6O) and CO2.
C6H12O6(s) → 2 C2H6O(l) + 2 CO2(g) ΔH = −16 kcal/mol
glucose ethanol
How many kilocalories of energy are released from 40.0 g of glucose?
kcal of energy released
Report answer to TWO significant figures.
Answers
Answer:
Explanation:
40/ 180.2 x (-16 / 1 mole glucose)=-3.6 KJ
I have four questions hope you can help me


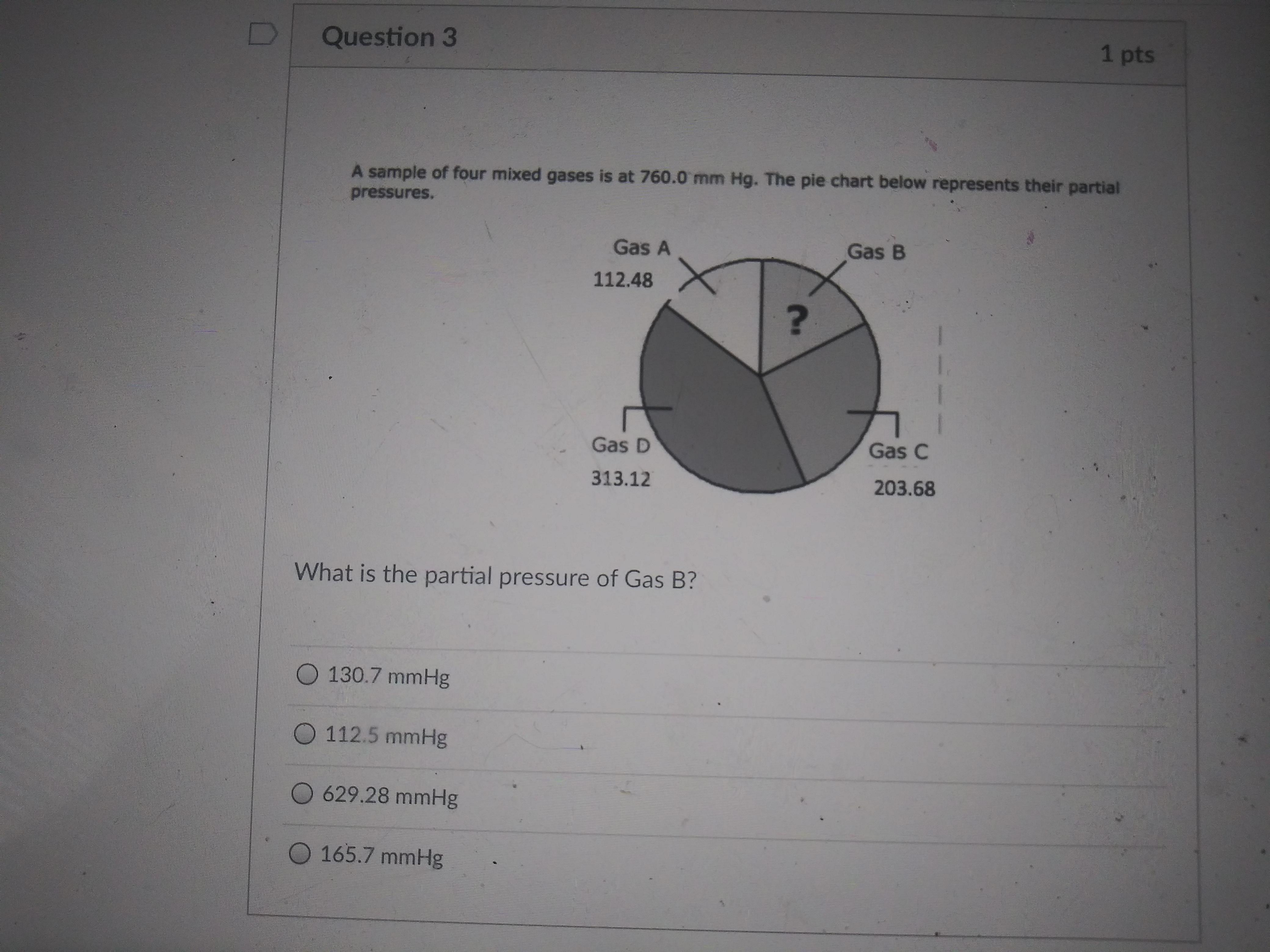

Answers
Answer:
2+2 kaszkqkq
Explanation:
Alkenes undergo an addition reaction with borane in tetrahydrofuran (THF).
For the reaction below:
((image))
Draw the structure of the major organic product.
Use the wedge/hash bond tools to indicate stereochemistry.
Use wedge and hash bonds ONLY when needed to show reaction stereochemistry.
If the reaction produces a racemic mixture, just draw one stereoisomer.
Answers
Alkyne chemistry refers to the branch of chemistry that deals with triple bonds between carbon atoms. Due to the presence of pi-electrons that are not tightly bound, alkynes undergo addition reactions.
Alkynes have a triple bond, which makes it possible to add halogens, water, and other substances to them through an addition reaction. A series of steps are used to create addition goods. The development of addition products is caused by the stability of vinylic cations. Asymmetric alkynes must adhere to Markovnikov's rule in order to conduct addition reaction. Below are a few addition reactions of alkynes that are explained: Alkenes are created when alkynes react with dihydrogen in the presence of catalysts like Pt/Pd/Ni. Alkanes are created when the produced alkenes further react with dihydrogen.
Learn more about alkynes here-
https://brainly.com/question/23508203
#SPJ4
Wich of the following does not directly affect the weather
Answers
Answer:
Tings that effect weather is basically the following:
Distance from seaAltitudeDistance to the equator or poles.MountainsJet streams,etciron chloride + sodium hydroxide
Answers
Answer:
Sodium hydroxide reacts with iron(III) chloride to produce iron(III) hydroxide and sodium chloride.
Hope this helps plz hit the crown :D
If 335 g of water at 65.5 °C loses 9750 J of heat,
what is the final temperature of the water? Liquid
water has a specific heat of 4.18 J/(g*°C).
Answers
Answer:
We can use the formula for heat lost by a substance to calculate the final temperature:
Q = m * c * ΔT
where Q is the heat lost, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the change in temperature.
In this case, we know the values of Q, m, and c, and we need to find ΔT. Rearranging the formula, we have:
ΔT = Q / (m * c)
Substituting the given values, we get:
ΔT = 9750 J / (335 g * 4.18 J/(g*°C)) ≈ 6.9 °C
Therefore, the final temperature of the water is:
65.5 °C - 6.9 °C ≈ 58.6 °C
So the final temperature of the water is approximately 58.6 °C.
The final temperature of the water is approximately 58.5°C.
To find the final temperature of the water, we first need to understand that the heat lost by the water is calculated using the formula q = mcΔT, where 'q' is the Heat Transfer, 'm' is the mass of the water, 'c' is the specific heat of the water, and 'ΔT' is the change in temperature.
First, rearrange the formula to find ΔT = q/(mc).
Then, insert the given values (q = -9750 J, m = 335 g, c = 4.18 J/g°C).
The negative sign denotes heat loss.
You will find ΔT is approximately -7°C.
This is the amount the temperature decreases.
Subtract ΔT from the initial temperature of the water (65.5°C - 7°C), to get the final temperature of approximately 58.5°C.
Learn more about Heat Transfer here:
https://brainly.com/question/34419089
#SPJ2
Enrichment Activity 2: Identify the Dependent and Independent Variables
1.In a short bondpaper,draw the tables as shown below.Read and analyze the given situations and identify the Dependent. and independent Variables.Write your answer in its corresponding column.
Independent: Dependent:
2.Read carefully the sample situations.
3.Analyze,then determine the Dependent and independent variables.
4.Write your answers under each column.
the situations are in the picture

Answers
I hope the attached answer of image helps you.
By Benjemin

How much heat is gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C? The specific heat of nickel is 0.443 J/g · °C.
Answers
Explanation:
To calculate the heat gained by nickel, we can use the formula:
q = m * c * ΔT
where q is the heat gained, m is the mass of the nickel, c is the specific heat of nickel, and ΔT is the change in temperature.
Given:
- Mass of nickel, m = 31.4 g
- Specific heat of nickel, c = 0.443 J/g · °C
- Change in temperature, ΔT = 64.2 °C - 27.2 °C = 37.0 °C
Substituting the values into the formula, we get:
q = (31.4 g) * (0.443 J/g · °C) * (37.0 °C)
Simplifying the calculation, we get:
q = 584 J
Therefore, the heat gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C is 584 J.
A calorimeter contains 145 g of water at 24.5 °C. A 23.2 g sample of MgBr₂ is added to the water in the calorimeter. After the solid has dissolved, the temperature of the water is 63.0 °C. Calculate the enthalpy of solution, in kJ/mol, for dissolving magnesium bromide in water. Assume that all heat is transferred to the water, and that the specific heat of the solution is the same as that of pure water.
Answers
First, we need to calculate the heat absorbed by the water:
q = m × c × ΔT
where q is the heat absorbed, m is the mass of water, c is the specific heat of water, and ΔT is the change in temperature.
q = 145 g × 4.184 J/g°C × (63.0°C - 24.5°C)
q = 16394.12 J
Next, we need to calculate the amount of substance of MgBr₂ that was added to the water:
n = m/MW
where n is the amount of substance, m is the mass of MgBr₂, and MW is the molecular weight of MgBr₂.
MW(MgBr₂) = 184.11 g/mol
n = 23.2 g ÷ 184.11 g/mol
n = 0.1259 mol
Finally, we can calculate the enthalpy of solution, ΔH, using the following formula:
ΔH = q ÷ n
where ΔH is the enthalpy of solution.
ΔH = 16394.12 J ÷ 0.1259 mol
ΔH = 130233.72 J/mol
Converting to kilojoules per mole (kJ/mol):
ΔH = 130.23 kJ/mol
Therefore, the enthalpy of solution for dissolving magnesium bromide in water is 130.23 kJ/mol.
The enthalpy of solution of magnesium bromide in water is calculated using the formula for heat transfer and taking into account the mass of the solvent, the specific heat of the solvent, and the change in temperature. The result is 186 kJ/mol.
Explanation:To calculate the enthalpy of solution of magnesium bromide (MgBr₂), we need to use the formula for heat transfer: Q = m * c * ΔT, where m is the mass of the solvent (water), c is the specific heat of the solvent, and ΔT is the change in temperature.
In our case, the mass of water is 145g, the specific heat of water (c) is 4.18 J/g°C, and the change in temperature (ΔT) is 63.0 °C - 24.5 °C = 38.5 °C.
Using the formula Q = m * c * ΔT, we can calculate the heat transferred to the water: Q = 145g * 4.18 J/g°C * 38.5 °C = 23432 J or 23.432 kJ. This is the heat absorbed by the water.
The enthalpy of the solution is defined as the amount of heat absorbed (or released) when one mole of a substance is dissolved in water. So, to find the enthalpy of solution, we first need to convert the grams of MgBr₂ to moles. The molar mass of MgBr₂ is 184.11 g/mol, so 23.2 g of MgBr₂ is 23.2 g / 184.11 g/mol = 0.126 mol.
Now we can calculate the enthalpy change by dividing the heat absorbed by the moles of the solute: ΔH = Q / n = 23.432 kJ / 0.126 mol = 186 kJ/mol. Therefore, the enthalpy of solution for magnesium bromide in water is 186 kJ/mol.
Learn more about Enthalpy of Solution here:https://brainly.com/question/32919331
#SPJ2
A student weighs out a 2.17 g sample of KOH, transfers it to a 300. mL volumetric flask, adds enough water to dissolve it and then adds water to the 300. mL tick mark.
What is the molarity of potassium hydroxide in the resulting solution?
Answers
The molarity of potassium hydroxide in the resulting solution is 0.129 M.
How to calculate molarity?Molarity of a substance refers to the concentration of a substance in solution, expressed as the number of moles of solute per litre of solution.
According to this question, a student weighs out a 2.17g sample of KOH, transfers it to a 300. mL volumetric flask, adds enough water to dissolve it and then adds water to the 300. mL tick mark.
No of moles of KOH = 2.17g ÷ 56.11g/mol = 0.039 moles
Molarity = 0.039 moles ÷ 0.3L = 0.129 M
Learn more about molarity at: https://brainly.com/question/31545539
#SPJ1
5.478 grams of potassium acetate and 2.143 grams of iron(III) hydroxide are added to a beaker containing 100.0 mL of water and stirred vigorously. A solid settles to the bottom of the beaker. If the water is decanted and the solid is dried, what is the maximum mass of solid that should be recovered
Answers
Solution :
\($Fe(OH)_3+3CH_3COOK \rightarrow Fe(CH_3COO)_3 + 3KOH$\)
(iron (potassium
hydroxide) acetate)
Number of moles of \($Fe(OH)_3 = \frac{\text{mass of }Fe(OH)_3}{\text{molar mass of }Fe(OH)_3}$\)
\($=\frac{2.143 \ g}{106.867 \ g/mol}$\)
= 0.02005 mol
Number of moles of \($CH_3COOK = \frac{\text{mass of }CH_3COOK}{\text{molar mass of }CH_3COOK}$\)
\($=\frac{5.478 \ g}{98.15 \ g/mol}$\)
= 0.0585 mol
Since 1 mol of \($Fe(OH)_3$\) reacts with 3 mols of \($CH_3COOK$\)
Therefore, number of moles of \($CH_3COOK$\) reacted \($=\frac{0.0585}{3 }$\) mol
= 0.0195 mol
Therefore the limiting reagent is \($CH_3COOK$\) and hence the number of moles of \($Fe(CH_3COO)_3$\) produced is 0.0195 mol
Amount of \($Fe(CH_3COO)_3$\) produced = moles x molar mass
= 0.0195 moles x 232.98 g/mol
= 4.5431 g
Chemicals in the chemical cycle are brought into plants mainly through their
A . leaves
B. stems
C. roots
this is science
Answers
Explanation: I’m not in college but as a freshman in high school in bio, I learned about this.
a blank can be seen when a substance change into a new substance
Answers
As per chemical changes, a color change can be seen when a substance change into a new substance.
Chemical changes are defined as changes which occur when a substance combines with another substance to form a complete new substance.Alternatively, when a substance breaks down or decomposes to give new substance it is also considered to be a chemical change.
There are several characteristics of chemical changes like those of change in color, change in state , change in odor and even change in composition . During chemical change there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
Learn more about chemical changes,here:
https://brainly.com/question/23693316
#SPJ1