if the half-life of hydrogen-3 is 11.8 y, after two half-lives the radioactivity of a sample will be reduced to one-half of the original amount.
Answers
FALSE.During alpha decay, instability, heavy nuclei (usually Z>83) drop their number of protons Z by 2 and their mass amount A by 4 by the emission of an alpha particle, which is a helium nuclei (He2+42).
What should hydrogen 3 be represented by?The molecular symbol for tritium, which is the sole radioactive isotope1 of hydrogen, is H-3, sometimes known as 3H or just T.Tritium undergoes radioactive decay as a result of being radioactive.
How can you tell whether a decay is alpha or beta?Beta decay produces a new element that has one more protons and one less neutron while alpha decay produces a new element with some less protons and some less neutrons.
To know more about radioactivity visit:
https://brainly.com/question/1770619
#SPJ4
Related Questions
Compound X has a molar mass of 180.15 g.mol^-1 and the following composition
Element mass %
Carbon 40.00%
Hydrogen 6.71%
Oxygen 53.29%
Write the molecular formula of X.
Answers
Answer:
Explanation:
here you will use the POEM:
% to mass, mass to mole, divide by small, multiply til whole (if needed)
2) find the molecular formula by using the formula
n = molecular weight or molar mass of true compound/empirical weight x 100
Assume 100 g of Compound X to make % equal mass:
% to mass, then mass to mole, now divide by small
40.00 g C x 1 mole/12.01 gC = 3.33 mole C /3.33 = 1
6.71 g H x 1mole/1.01 g H = 6.64 mole H /3.33 = 2
53.29g O x 1 mole/16.00 g O = 3.33 mole O /3.33 =1
because we end up with the whole numbers, we do not need to use the 4th step.
so the empirical formula is C1H2O1
now to figure out what the molecular formula is we use:
n = multiple = mw/ew = m
we need to find the empirical weight:
C1H2O1 = 1x12.01 + 2x1.01 + 1 x 16 = 30.03 g/mol
molecular formula = (empirical formula)n
n = 180.15/30.03 = 6
so the answer is (C1H2O2)6 = C6H12O6
The compound X has a molar mass of\(180.15 g.mol^-^1\) and consists of 40.00% carbon, 6.71% hydrogen, and 53.29% oxygen.
To determine the molecular formula of compound X, we need to find the empirical formula and then determine the factor by which the empirical formula should be multiplied to obtain the molecular formula.
First, we calculate the number of moles of each element present in 100g of compound X.
The mass of carbon in 100g of X is 40.00g, which corresponds to 40.00/12.01 ≈ 3.33 moles of carbon.
The mass of hydrogen in 100g of X is 6.71g, which corresponds to 6.71/1.01 ≈ 6.64 moles of hydrogen.
The mass of oxygen in 100g of X is 53.29g, which corresponds to 53.29/16.00 ≈ 3.33 moles of oxygen.
Next, we find the simplest whole-number ratio of the elements. Dividing the number of moles by the smallest number of moles (3.33 moles) gives us a ratio of approximately 1:2:1 for carbon, hydrogen, and oxygen, respectively.
Therefore, the empirical formula of compound X is \(CH_2O\).
Finally, to find the molecular formula, we need to determine the factor by which the empirical formula should be multiplied to obtain the molar mass of \(180.15 g.mol^-^1\). The molar mass of \(CH_2O\) is 12.01 + 1.01 * 2 + 16.00 = \(30.03 g.mol^-^1\).
Dividing the molar mass of the compound (\(180.15 g.mol^-^1\)) by the molar mass of the empirical formula (\(30.03 g.mol^-^1\)), we find that the factor is approximately 6.
Therefore, the molecular formula of compound X is \(C_6H_1_2O_6\).
Learn more about empirical formula here:
https://brainly.com/question/32125056
#SPJ11
For the balanced equation shown below, how many moles of O2 b
produced by 0.4379 moles of C6H5F?
C6H5F + 402 – 600 + 2H2O + HF
Answers
what mass of nh4cl should you add to 2.30 l of a 0.238 m solution of nh3 to obtain a buffer with a ph of 9.02?
Answers
Buffer solution is obtained by hendersons hasselbalch equation.
Buffer solution is water based solution which consists of a mixture containing a weak acid and a conjugate base of the weak acid or a weak base and conjugate acid of a weak base.it is a mixture of weak acid and a base.
The pH of the buffer solution is determined by the expression of the henderson hasselbalch equation.
pH=pKa + log[(A-)/(HA)]
Where, pKa =dissociation constant
A- = concentration of the conjugate base
[HA]= concentration of the acid
The buffer solution of ammonium chloride contains a weak base ammonia and a conjugate base ammonium.
Here, a buffer solution contains 0.238 m of ammonia. The volume of the solution is 2.30l and the ph of the buffer solution is 9.02 .By putting all the values we will solve the expression.
To learn more about hendersons hasselbalch equation please visit:
https://brainly.com/question/13423434
#SPJ4
How many moles of NH3 are produced when 0.60 mol of nitrogen reacts with hydrogen in N2 + 3H2 ----> 2NH3
Answers
Answer:
0.80 mol
Explanation:
n(H)/n(N) = 1/3
n(N) = 3×0.60
n(N) = 1.8 mol
n(N) in excess = 1.80-0.60 = 1.20 mol
n(N)/n(NH3) = 3/2
n(NH3) = 2/3×1.20 = 0.80 mol
a student uses a glue stick with an area of 4 cm3, putting
a pressure of 0.5 N/cm2 on her book. Calculate the force
she puts on the glue stick.
Answers
Answer:
So F=2N
Hope this helps.
Explanation:
P= F/A, where P is pressure, F is force, A is area.
So
P=F/A
0.5N/cm2 = F/4cm2 <--(do cross
2N=F multiplication,
4×0.5)
( And pls check on the unit of area u wrote, it should be (4cm2), not (4cm3) Unit of area is cm2.)
While visiting his uncle's farm, Derek learned that horses and donkeys are two different species. Based on this
information, what can Derek infer about horses and donkeys?
Horses and donkeys cannot survive in the same
environment.
Horses and donkeys produce fertile offspring.
Horses and donkeys are members of the same
population.
Horses and donkeys are members of different
populations.
Answers
While visiting his uncle's farm, Derek learned that horses and donkeys are two different species. Based on this information, Derek can infer that horses and donkeys are members of different populations.
Since horses and donkeys are different species, they belong to different populations. A population refers to a group of individuals of the same species that live in the same area and can interbreed. While horses and donkeys can mate, their offspring, known as mules, are usually infertile.
This means that mules cannot produce offspring of their own, which indicates that horses and donkeys are not members of the same population. In contrast, if they were members of the same population, they would be able to produce fertile offspring. Therefore, Derek can infer that horses and donkeys are members of different populations.
Learn more about mules here:
https://brainly.com/question/15852289
#SPJ11
. A strong acid, HCl, is titrated with a strong base, NaOH. Write the net ionic equation for the reaction. Do not include spectator ions in the equation.
Answers

Match the atoms to their type of bond.Gold (Au) andgold (Au)2TonicNitrogen (N) andoxygen (0)2CovalentChlorine (cl) andlithium (Li)2Metallicw

Answers
Ionic bonds are a type of bond formed with the attraction between oppositely charged ions to form a chemical compound, this type of bond will have a transfer of electrons, from the positively charged ion (cation) to the negatively charged ion (anion). Since this type of bonding heavily depend on the electronegativity of the elements, we will have compounds formed with metals, located more to the left in the periodic table, mostly group 1 and 2, and with nonmetals, more to the right in the periodic table, one example of this type of bond is Chlorine (nonmetal anion) and Lithium (metal cation).
Covalent bonds are a type of bond formed with the sharing of electrons between two elements with no big difference in their electronegativity, usually, we will see nonmetals in this type of bond, since their difference in electronegativity is not as relevant as the difference between metals and nonmetals, one example of this type of chemical bond is Nitrogen and Oxygen, 2 nonmetals that can bond together.
Metallic bond, as the name suggest, is a type of bond that will strictly involve metals and not nonmetals, this type of bond has many properties but the low electronegativity in general is one of these properties, one example for this bond is Gold and Gold
Therefore the answers are:
Gold and Gold = metallic
Nitrogen and Oxygen = covalent
Chlorine and Lithium = ionic
You need 150g of pure lithium for an experiment you're doing. You have 675g of lithium oxide (Li2O). Can you extract all the lithium you need from the amount of compound you have? Show your reasoning.
Answers
Answer: Yes we can extract all the lithium from the amount of compound we have.
Explanation:
To calculate the moles :
\(moles=\frac{\text {given mass}}{\text {Molar mass}}\)
\(\text{Moles of} Li=\frac{150g}{7g/mol}=21.4moles\)
\(\text{Moles of} Li_2O=\frac{675g}{30g/mol}=22.5moles\)
\(2Li_2O\rightarrow 4Li+O_2\)
According to stoichiometry :
2 moles of \(Li_2O\) produce 4 moles of \(Li\)
Thus 22.5 moles of \(Li_2O\) will produce=\(\frac{4}{2}\times 22.5=45moles\) of Li
As 21.4 moles is a lesser quantity than 45 moles, thus it can be produced
Yes, you can extract all the lithium you need from the compound
We'll begin by calculating the mass of lithium, Li in 1 mole of Li₂O. This can be obtained as described below:
1 mole of Li₂O = (2×7) + 16
1 mole of Li₂O = 14 + 16 = 30 g
SUMMARY
30 g of Li₂O contains 14 g of Li.
With the above information in mind, we can determine the mass of Li in 675 g of lithium oxide, Li₂O. This can be obtained as follow:30 g of Li₂O contains 14 g of Li.
Therefore,
675 g of Li₂O Will contain = (675 × 14)/30 = 315 g of Li.
From the calculation made above, we can see that there are 315 g of Li in 675 g of Li₂O. Thus, we can conclude that 150 g of Li can be extracted from the compound (i.e 675 g of Li₂O)Learn more: https://brainly.com/question/15381525
green plants use light from the sun to drive photosynthesis, a chemical reaction in which liquid water and carbon dioxide gas form aqueous glucose and oxygen gas. calculate the moles of glucose produced by the reaction of of carbon dioxide. be sure your answer has a unit symbol, if necessary, and round it to significant digits.
Answers
The majority of the energy required for life on Earth is produced and maintained by photosynthesis, which is also substantially responsible for producing and maintaining the oxygen content of the atmosphere.
6CO₂ + 6H₂O = 6O₂ + C₆H₁₂O₆
6 moles of water are required for 1 mole of glucose.
Therefore, 0.9 x 6 = 5.4 moles of water are needed for every mole of glucose.
5.4 x 18 = 97.2 g
Plants and other living things employ a process called photosynthesis to transform light energy into chemical energy that can then be released through cellular respiration to power the organism's activities. Carbohydrate molecules like sugars and starches, which are created from carbon dioxide and water, contain some of this chemical energy.
Want to know more about photosynthesis visit the link which is given below;
https://brainly.com/question/29764662
#SPJ4
Which do you think will cool the fastest?
sand
water
air
metal
Answers
a 100 gram sample of iron ore was found to contain 44g of iron. How many grams of iron are in a 450 gram sample of the ore?
Answers
Answer:
There are 198 grams of iron in a 450-gram sample of the ore.
Explanation:
From the given information:
Given that:
100-gram sample of iron ore is found in 44g of iron.
Thus. the grams of iron that can be found in a 450-gram sample of the iron ore can be computed as follows:
100g of iron ore = 44g of iron
450 gram of iron ore = x g of iron
x(g) of iron = ( 44g of iron × 450 gram of iron ore) / 100g of iron ore
x(g) of iron = 198 grams of iron
Thus, there are 198 grams of iron in a 450-gram sample of the ore.
During the increase of the gas temperature 2.5 times, the boiling point increased by 40%. How many times did the gas pressure change?
Answers
I will help you
Let's say we change the volume of a gas under isothermal conditions, and we want to find the resulting pressure. Then, the equation of Boyle's law states that: p₂ = p₁ × V₁ / V₂ or p₂ / p₁ = V₁ / V₂. As we can see, the ratio of the final and initial pressure is the inverse of the ratio for volumes.
Dimensional analysis can only be used for time, distance, mass, speed, volume, and chemical quantities.
Group of answer choices
True
False
Answers
Dimensional analysis can only be used for time, distance, mass, speed, volume, and chemical quantities: False.
What is dimensional analysis?A dimensional analysis can be defined as an analysis of the relationships that exist between different physical quantities by identifying their fundamental (base) quantities, especially for the purpose of inferences.
Dimensional analysis is also referred to as unit-factor method or factor-label method and it is typically used to convert a different type of unit to another.
Generally, dimensional analysis can only be used for the following physical quantities:
TimeDistanceMassVolumeSpeedIn conclusion, a dimensional analysis cannot be used for chemical quantities such as a mole i.e it has no dimensional formula.
Read more on dimensional analysis here: https://brainly.com/question/24514347
The specific heat of lead is 0. 11 j/g∙⁰c. How much heat is given off by lead with a mass of 85 g to decrease its temperature from 200 ⁰c to 10 ⁰c?.
Answers
The amount of heat given off by lead is Q = - 1776.5J
Given,
Mass of the lead = 85g
Decreased temperature = 200 ⁰c to 10 ⁰c
Specific heat capacity of lead= 0. 11 j/g ⁰c
where T1=200degrees Celsius and T2=10 degree Celsius.
Q=MCΔT
ΔT=T2-T1
ΔT=10-200 = -190°C
Q = Change in energy
M = mass of a substance
C= Specific heat capacity
Q = (85g) * (0.11J/g °c) * (-190°C)= -1776.5J
Q=-1776.5J
Specific heat capacity:
A material's specific heat capacity, also known as massive heat capacity, is determined using thermodynamics as the specific heat of an unit of the compound scaled by the the sample's mass.
The specific heat capacity of a substance is defined as the number of heat (J) received per unit of mass (kg) when its temperature increases 1 K (or 1 °C), and its units are J/(kg K) or J/(kg °C).
Learn more about specific heat capacity here:
https://brainly.com/question/16952828
#SPJ4
Your team is assigned the Musky Mix. Your unknown has a boiling range of 145-148 oC. You take an IR of your compound and see a carbonyl peak at 1717 cm -1. What is the most likely identity of your unknown
Answers
Based on the given information, the most likely identity of your unknown compound is option D. 2-heptanone.
The boiling range of 145-148 °C corresponds well with the boiling point of 2-heptanone, which is approximately 147 °C. Additionally, the presence of a carbonyl peak at 1717 cm⁻¹ in the IR spectrum is indicative of a ketone functional group, which is present in 2-heptanone.
The other options can be ruled out based on their boiling points and functional groups:
A. Mesityl oxide has a boiling point of around 132 °C and contains both carbonyl and alkene groups, not matching the given boiling range or the single carbonyl peak.
B. Methyl trichloroacetate has a boiling point of approximately 171 °C and contains an ester functional group, which typically shows a carbonyl peak around 1735-1750 cm⁻¹, not matching the given boiling range or carbonyl peak.
C. Ethyl butanoate has a boiling point of about 99 °C and also contains an ester functional group, again not matching the given boiling range or carbonyl peak.
Thus, 2-heptanone (D) is the most likely identity of your unknown compound due to its matching boiling range and the presence of a ketone functional group. Therefore the correct option D
The Question was Incomplete, Find the full content below :
Your team is assigned the Musky Mix. Your unknown has a boiling range of 145-148 oC. You take an IR of your compound and see a carbonyl peak at 1717 cm-1. What is the most likely identity of your unknown? A. mesityl oxide B. methyl trichloroacetate C. ethyl butanoate D. 2-heptanone
Know more about 2-heptanone here:
https://brainly.com/question/17516102
#SPJ11
how is it d?explain please?! i do not understand.please dont guess
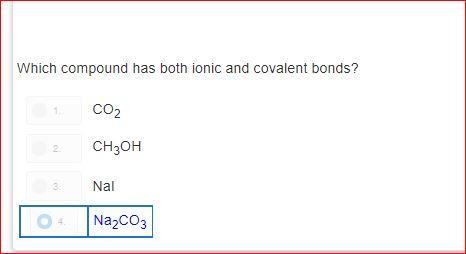
Answers
Answer:
As you can see sodium = ionic and covalent bonds aka NA 2
and CO 3 also has those two bonds the other answers dont have both numbers (bonds) Na2 and CO3 is the only answer choice that has these bonds in simpler terms ( only answer with 2 numbers)
Explanation:
a block of lead has dimensions of 4.50 cm by 5.20 cm by 6.00 cm
Answers
A block of lead has dimensions of 4.50 cm by 5.20 cm by 6.00 cm, the density of lead is mathematically given as
x= 11.30 g/cm^3
This is further explained below.
What is the density of lead?Generally, in order to determine the lead's specific gravity; To begin, we would use the formula to find out how much space the block of lead takes up;
Volume= length * width * height
After plugging the values into the formula, we get the following:
Volume = 4.50 * 5.20 * 6.00
Volume = 140.4 cm^3
The next thing that we do is determine the density of lead;
After plugging the values into the formula, we get the following:
X = 11.30 g/cm^3
In conclusion, the density of lead is 11.30 g/cm^3
Read more about density
https://brainly.com/question/15164682
#SPJ1
CQ
A block of lead has dimensions of 4.50 cm by 5.20 cm by 6.00 cm. The block weighs 1587 g. From this information, calculate the density of lead.
How many unshared (non-bonded) pairs of
electrons are in NF3?
1. thirteen
2. three
3. two
4. no pairs
5. four
6. ten
7. twenty
8. one
Answers
8. One pair.
because nitrogen has 5 valence electrons, and fluorine has 7, but if there's 3 fluorine atoms, nitrogen shares 1 to each F atom to get it to 8, meaning nitrogen will have a single pair left over since there's no more F atoms to share them with.
____ energy is the energy a roller coaster stores while moving to the top of a hill.
A. G-Force
B.Potential
C. Stirred
D. Kinetic
Answers
Answer:
B
Explanation:
trust me im 100% correct
Green plants need light in order to survive Structures in the leaves absorb light, which in turn, helps plants make their
own tood
Under which color of light will plants be least likely to make food?
red
blue
orange
green
Answers
Answer:
The color of light in which plants are less likely to produce food is green.
Explanation:
Photosynthesis is the process by which plants take advantage of sunlight to convert it into chemical energy, necessary to manufacture their nutrients, their growth and development.
Plants do not absorb all the colors of the light spectrum, since due to their pigments they absorb colors with specific wavelengths. Green plants do not absorb the color green, but reflect it —due to the chlorophyll pigment— which gives the green color to those leaves.
For this reason, the light that plants take least advantage of to produce food is green light.
Other options:
The colors blue, orange and red can be absorbed by the plant, to a lesser or greater degree.
Answer:
it would be green
Explanation:
If 26 grams of N2 are present, how many moles of NF3 will be produced?
Answers
The balanced chemical equation for the reaction between N2 and F2 to produce NF3 is N2(g) + 3F2(g) → 2NF3(g). The molar mass of N2 is 28.02 g/mol. Therefore, 26 g of N2 is equal to 0.93 moles of N2.
What is the ratio of moles of NH3 to moles of N2?The preceding balanced equation clearly shows that 2 moles of ammonia are created for every 1 mole of nitrogen. This indicates that the reaction's N2 to NH3 mole ratio is 1 to 2.
What number of moles of NH3 will be produced?There are 14 moles of NH3 produced. The balanced chemical equation must first be written. Then use the unitary approach to fix the issue. Now, the mass of ammonia is determined using the following formula.
to know more about moles here:
brainly.com/question/26416088
#SPJ1
Linking amino acid monomers together to form a polymer requires the formation of which type of bond?.
Answers
Polypeptides : Peptides are tiny chains of amino acids connected by the peptide bonds.
Function of polypeptides : Polypeptides aid make up proteins by bonding many amino acids conjointly.
Amino acids are organic components that comprises both amino and carboxylic acid functional groups.
Sequence of amino acids are known as polypeptides. The covalent bonds that hold on to amino acids conjointly are known as peptide bonds.
Types of amino acids : nonpolar, polar, negatively charged, and positively charged.
According to question
Linking amino acid monomers together to form a polymer requires the formation of peptide bonds.
To know more about Polypeptides here :
https://brainly.com/question/28270191?referrer=searchResults
#SPJ4
Solvent: Will give brainliest

Answers
Answer:
Solvent is 1.00 liter of water.
Answer:
It would be the water i think rlly srry if im wrong thats just what i got
Explanation:
because a solvent would be the one dissolving
Part A
Write balanced molecular equation for the reaction between nitric acid and calcium hydroxide.
Part B
Enter a net ionic equation for the reaction between nitric acid and calcium hydroxide.
Answers
Part A: The balanced molecular equation for the reaction between nitric acid (HNO3) and calcium hydroxide (Ca(OH)2) is 2 HNO3(aq) + Ca(OH)2(aq) → Ca(NO3)2(aq) + 2 H2O(l).
Part B: The net ionic equation for the reaction between nitric acid and calcium hydroxide is 2 H+(aq) + 2 OH-(aq) → 2 H2O(l).
Part A:
To write the balanced molecular equation, we first need to identify the formulas of the reactants and products. Nitric acid is represented by HNO3, and calcium hydroxide is represented by Ca(OH)2.
The balanced molecular equation is obtained by ensuring that the number of atoms of each element is the same on both sides of the equation. In this case, we have 2 hydrogen (H) atoms, 1 nitrogen (N) atom, 3 oxygen (O) atoms, 1 calcium (Ca) atom, and 2 hydroxide (OH) ions.
2 HNO3(aq) + Ca(OH)2(aq) → Ca(NO3)2(aq) + 2 H2O(l)
Part B:
The net ionic equation represents the reaction without including the spectator ions. In this case, the spectator ions are the calcium (Ca2+) and nitrate (NO3-) ions.
When nitric acid and calcium hydroxide react, the hydrogen ions (H+) from nitric acid combine with the hydroxide ions (OH-) from calcium hydroxide to form water molecules.
The net ionic equation for the reaction is:
2 H+(aq) + 2 OH-(aq) → 2 H2O(l)
The net ionic equation focuses on the species directly involved in the reaction, omitting the spectator ions that do not participate in the chemical changes.
To learn more about balanced molecular equation, refer:
brainly.com/question/1889840
#SPJ11
Which of the following is a pure substance?
Chlorine B. salt solution C. bronze D. salad dressing
What is the boiling point of pure water?
About 1000 C B. exactly 1000 C C. it varies D. slightly above 1000 C
Which is an example of a chemical change?
Water evaporating B. butter melting C. match burning D. powder being made
Which of these indicate a chemical change?
Color change B. temperature change C. gas-forming D. all of the above
Which of these is an impure substance?
Tap water B. gold C. carbon dioxide D. boron
Answers
Answer:
1. B
2. 100 C
3. C
4. D
5. D
Explanation:
Silver iodide powder has been used as an antiseptic and as an agent to seed clouds for rain. Silver iodide is 45.9% silver by mass. If you separate a 50.0-g sample of silver iodide into its elements, silver and iodine, how much silver would you have?
Answers
Answer:
22.95g
Explanation:
Given parameters:
Percentage by mass of silver in silver iodide = 45.9%
Mass of silver iodide given = 50g
Unknown:
Mass of silver in silver iodide = ?
Solution:
The formula of the compound is;
Silver iodide = AgI
Since the percentage by mass of silver in the compound is 45.9%;
Mass of silver = percentage by mass of silver x mass of AgI
Mass of silver = \(\frac{45.9}{100}\) x 50 = 22.95g
9. The percent by mass of carbon in
HC₂H₂O₂ is equal to
Answers
The percent by mass of carbon in HC₂H₂O₂ is approximately 39.97%.
To determine the percent by mass of carbon in HC₂H₂O₂ (acetic acid), we need to calculate the mass of carbon relative to the total molar mass of the compound.
The molar mass of acetic acid (HC₂H₂O₂) can be calculated by summing the molar masses of its individual elements:
Molar mass (HC₂H₂O₂) = 1 × molar mass (H) + 2 × molar mass (C) + 2 × molar mass (H) + 2 × molar mass (O)
Molar mass (HC₂H₂O₂) = 1 × 1.00794 g/mol + 2 × 12.0107 g/mol + 2 × 1.00794 g/mol + 2 × 15.999 g/mol
Molar mass (HC₂H₂O₂) ≈ 60.052 g/mol
The molar mass of carbon in acetic acid is 2 × 12.0107 g/mol, which is the contribution of the two carbon atoms in the compound.
Now, we can calculate the percent by mass of carbon in acetic acid using the following equation:
Percent by mass = (Mass of carbon / Total mass of HC₂H₂O₂) × 100%
Percent by mass = (2 × 12.0107 g/mol / 60.052 g/mol) × 100%
Percent by mass ≈ 39.97%
Therefore, the percent by mass of carbon in HC₂H₂O₂ is approximately 39.97%.
It is important to note that the calculation assumes the molecular formula of HC₂H₂O₂ represents one molecule of acetic acid. In a given sample, there could be multiple molecules of acetic acid present, and the percent by mass of carbon would remain the same.
For more such questions on percent by mass visit:
https://brainly.com/question/26150306
#SPJ8
Reactions are considered energetically favorable when the bond energy _______ from the formation of products is greater than the energy _______ to break the bonds in reactants.
Answers
Reactions are considered energetically favorable when the bond energy obtained from the formation of products is greater than the energy required to break the bonds in reactants.
What is the bond energy?We know that the bond energy has to do with the energy of the reactants and the products in a reaction. It has to do with the energy that can be given out or even taken in in a chemical reaction.
We know that the bond energy would depend on the bond energy of the reactants and the products. We know that the bond that is contained in the reactants is broken just as the bonds that are contained in the products are being formed.
Thus we say that a reaction is favorable when more energy is obtained than the energy that we need to break the bond.
Learn more about bond energy:https://brainly.com/question/26141360
#SPJ1
Answer:
released, consumed
Explanation:
Got this one right on the test.

water is added to a 8.23 g sample of tacl5. the only products are 5.71g of a solid containing only tantalum, chlorine and oxygen and 3.35 g of a gas which is 97.2% chlorine and the remainder is hydrogen. (a) determine the empirical formula of the gas. (b) what fraction of the chlorine of the original compound is in the solid? (c) determine the empirical formula for the solid produced. (d) write a balanced equation for the reaction between tantalum pentachloride and water
Answers
The empirical formula is the simplest formula for a compound which is defined as the ratio of subscripts of smallest possible whole number of the elements present in the formula. It is also known as the simplest formula.
write a balanced equation for the reaction between tantalum pentachloride and water?
Tantalum Pentachloride is used as the chlorinating agent of the organic compound, chemical intermediates, and preparation as tantalum.TaCl5 is used in the preparation of catalyst for the polycyclotrimerizations of alkenediynes, chloro-aryloxide compounds and for the plasma-enhanced atomic layer deposition of tantalum nitride films. This product is involved in the preparation of tantalum(V) oxychloride.Tantalum oxide (Ta2O5) is one of the most important transition metal oxides because of its extraordinary physical and chemical properties, including high dielectric and refractive coefficients and excellent photoelectric performance.To learn more about chlorine refers to:
https://brainly.com/question/29794366
#SPJ4