if an ionic bond forms between an atom of al and an atom of n, how many valance electrons are transferred? 3 4 1 2
Answers
In the formation of an ionic bond between Al and N, 3 valence electrons are transferred from the aluminum atom to the nitrogen atom.
When an ionic bond forms between an atom of Al (aluminum) and an atom of N (nitrogen), the transfer of electrons occurs to achieve a stable electron configuration.
Aluminum (Al) has 3 valence electrons in its outermost energy level, while nitrogen (N) has 5 valence electrons.
To achieve a stable electron configuration, aluminum tends to lose 3 electrons to attain a full outer shell, becoming a positively charged ion, Al³⁺.
On the other hand, nitrogen tends to gain 3 electrons to complete its outer shell, becoming a negatively charged ion, N3⁻.
Learn more about valence electrons at: https://brainly.com/question/371590
#SPJ11
Related Questions
How many miles of silver will be generated if 1.30 miles of zinc is placed into silver nitrate solution
Answers
2.60 moles.
Zn (s) + 2 Ag+ (aq) --> 2 Ag (s) + Zn2+ (aq), each mole of zinc gives out two moles of silver. Therefore, 1.30 moles of zinc will displace 2.60 moles of silver. ( 1.30 × 2)
More about silver nitrate:
An inorganic substance with the chemical formula AgNO3 is silver nitrate. This salt serves as a flexible base for numerous different silver compounds, including those employed in photography. Compared to halides, it is far less light-sensitive. Lunar caustic was previously the name given to it since early alchemists referred to silver as luna and connected it to the moon.
Learn more about silver here:
https://brainly.com/question/8071605
#SPJ4
What kind of eclipse is this
Total solar eclipse
Lunar
Annular
Partial lunar eclipse

Answers
If salt (5.99x10-6 mol) is dissolved in 1.50x 10-2 l of water which expression to find solution
Answers
In the given problem, 5.99 × 10⁻⁶ mol of salt is dissolved in 1.50 × 10⁻² l of water. We can use the following expression to find the solution:$$M = \frac{n}{V}$$Where, M = molarity, n = number of moles, and V = volume of solution.
In order to solve this problem, we have to substitute the given values in the above formula, which will be as follows:$$M = \frac{5.99 \times 10^{-6} \:mol}{1.50 \times 10^{-2} \:L}$$This can be simplified as:$$M = 0.000399333...$$Therefore, the molarity of the solution is 0.000399333... mol/L.
When a solute is dissolved in a solvent, it forms a solution. The concentration of a solution can be expressed in terms of its molarity, which is defined as the number of moles of solute present in one liter of solution. The formula for calculating molarity is given by:M = n/VWhere, M = molarityn = number of molesV = volume of solutionIn the given problem, 5.99 × 10⁻⁶ mol of salt is dissolved in 1.50 × 10⁻² l of water. We can use the above formula to calculate the molarity of the solution. Substituting the given values in the formula, we get:M = (5.99 × 10⁻⁶ mol)/(1.50 × 10⁻² L)On simplifying, we get:M = 0.000399333... mol/LTherefore, the molarity of the solution is 0.000399333... mol/L.
To know more about solution visit:
https://brainly.com/question/15757469
#SPJ11
Mg + 2AgNO3 --> Mg(NO3)2 + 2Ag
How many grams of magnesium are needed to make 350 grams of silver?
Answers
Answer:
First, we need to determine the molar ratio between magnesium (Mg) and silver (Ag) in the balanced chemical equation:
1 mol Mg : 2 mol Ag
This means that for every one mole of magnesium that reacts, two moles of silver are produced.
Next, we need to calculate the number of moles of silver that can be produced from 350 grams of silver:
mass of silver = 350 g
molar mass of silver = 107.87 g/mol
moles of silver = mass of silver / molar mass of silver
moles of silver = 350 g / 107.87 g/mol
moles of silver = 3.24 mol Ag
Now, we can use the mole ratio to determine the number of moles of magnesium required to produce 3.24 moles of silver:
1 mol Mg : 2 mol Ag
moles of Mg = moles of Ag / 2
moles of Mg = 3.24 mol Ag / 2
moles of Mg = 1.62 mol Mg
Finally, we can use the molar mass of magnesium to convert the number of moles to grams:
molar mass of Mg = 24.31 g/mol
mass of Mg = moles of Mg x molar mass of Mg
mass of Mg = 1.62 mol x 24.31 g/mol
mass of Mg = 39.3 g
Therefore, approximately 39.3 grams of magnesium are needed to produce 350 grams of silver.
Explanation:
Stoichiometry, which involves balancing the equation and using the molar mass of each substance, must be used to calculate how many grams of magnesium are required to make 350 grams of silver.
Firstly, balance the chemical equation:
Mg + 2AgNO₃ → Mg(NO₃)₂ + 2Ag
A mole of magnesium interacts with two moles of silver nitrate to form a mole of magnesium nitrate and two moles of silver, according to this equation. We can deduce from the balanced equation that the magnesium-to-silver ratio is 1:2.
Following that, we must determine the molar mass of silver:
Silver(Ag): 107.87g/mol
The requisite magnesium can then be calculated using the formula below:
Grams of Magnesium (Mg) = (molar mass of Ag x grams of Ag) / (2 x molar mass of Mg)
Grams of Magnesium (Mg) = (107.87 g/mol x 350 g) / (2 x 24.31 g/mol)
Grams of Magnesium (Mg) = 303.38 g
Thus, 350 grams of silver can be made from 303.38 grams of magnesium.
Learn about Stoichiometric Calculations:
https://brainly.com/question/29841194
Which best describes a semiconductor?
a.)Semiconductors are more conductive than a nonmetal and less conductive than a metal.
b.)Semiconductors are more conductive than a nonmetal and less conductive than a metalloid.
c.)Semiconductors have a high electrical conductivity.
d.)Semiconductors have a no electrical conductivity.
Answers
Answer:
a.)Semiconductors are more conductive than a nonmetal and less conductive than a metal.
Explanation:
Answer: A is your answer
Explanation: yor welcome =]=]=]=]
I have to turn this in please answer it!!

Answers
Answer:
Topaz I do believe
Hope This Helps!
the structures of d-gulose and d-psicose are shown above. what test could be used to distinguish between solutions of these two carbohydrates? explain your answer by predicting the results of the test for each sugar.
Answers
a small amount of Tollens' reagent (ammoniacal silver nitrate) is added to the sugar solution and the mixture is heated. If a reducing sugar is present, it will reduce the silver ions in the Tollens' reagent to metallic silver, which will form a silver mirror on the inside of the test tube.
Based on the structures of D-gulose and D-psicose, it can be predicted that both sugars will give a positive result in the Tollens' test because they both have an aldehyde group that can act as a reducing agent. However, the intensity of the reaction may differ for each sugar.
D-gulose has an aldehyde group at carbon 1, which is in the linear form of the sugar, while D-psicose has an aldehyde group at carbon 2. Since D-gulose can easily convert to its linear form, it is expected to give a stronger positive result in the Tollens' test compared to D-psicose, which may show a weaker positive result due to the steric hindrance of the bulky ketone group at carbon 3.
In summary, the Tollens' test can be used to distinguish between solutions of D-gulose and D-psicose by observing the intensity of the silver mirror formed. D-gulose is expected to give a stronger positive result due to its ability to convert to the linear form, while D-psicose may show a weaker positive result due to steric hindrance.
Learn more about aldehyde here:
https://brainly.com/question/30478354
#SPJ11
which of the following oxides would have the highest melting point? co li2o b2o3 n2o5 so2
Answers
Among the given options, B2O3 (boron trioxide) would likely have the highest melting point
Among the given oxides, the oxide with the highest melting point is B2O3 (boron trioxide).
Boron trioxide (B2O3) forms a network of covalent bonds where each boron atom is bonded to three oxygen atoms. This network structure gives B2O3 high melting and boiling points. Boron trioxide has a melting point of around 450°C (842°F).
The other oxides mentioned have lower melting points in comparison:
CO (carbon monoxide) is a gas at room temperature and does not have a defined melting point.
Li2O (lithium oxide) has a melting point of about 1,430°C (2,606°F), which is lower than B2O3.
N2O5 (dinitrogen pentoxide) is a molecular compound with a melting point of approximately -9°C (16°F).
SO2 (sulfur dioxide) is also a molecular compound and exists as a gas at room temperature. It does not have a well-defined melting point.
Therefore, among the given options, B2O3 (boron trioxide) would likely have the highest melting point
Learn more about melting point here:
https://brainly.com/question/29645520
#SPJ11
If you discover a new element, how would you know where it should go on the periodic table
Answers
Answer:
the atomic number
Explanation:
it would be in the upper corner
Inicialmente una muestra de neón tiene un volumen de 2,50 L a 15 °C. ¿Cuál es la
temperatura en °C, cuando el volumen de la muestra cambia a cada uno de los
siguientes volúmenes, ¿manteniéndose la presión constante?
a. 5,0 L b. 1250 ml c. 7,50L d. 3550 ml
Answers
naoooooooo seiiiiiiiii !!!!!!!!!!!!!!
BE FIRST TO ANSWER PLEASEEE ASAP! :D

Answers
Answer:
I want to say E or B
Explanation:
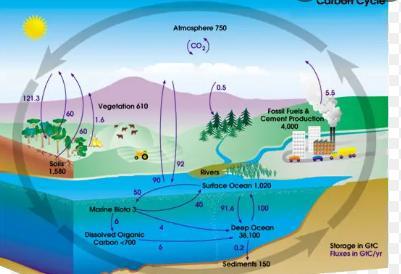
The combination of fossil fuel use and deforestation has emitted approximately 480 gigatons of carbon over the last century, but the amount of carbon in the atmosphere has only increased by approximately 190 gigatons. About 110 gigatons of this missing carbon went into which reservoir of the carbon cycle?.
Answers
About 110 gigatons of this missing carbon went into water reservoir of the carbon cycle.
What is carbon cycle?The biogeochemical cycle in which carbon is exchanged between the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth is known as the carbon cycle.
According to the question, emitted amount of carbon is 480 gigatons but the amount of carbon in the environment is approx 190 gigatons, so the remaining amount of the carbon was stoored by the oceans and other water reservoir, which may be get clear throug the attached diagram.
Hence remaining gigatons of carbon went into water reservoir.
To know more about carbon cycle, visit the below link:
https://brainly.com/question/24293689
#SPJ1
which theory explains how glacial material can be observed today near sea level at the equator, even though sea level glaciers probably never existed there
Answers
The theory that explains how glacial material can be observed today near sea level at the equator, even though sea level glaciers probably never existed there, is called glacial erratics.
Glacial erratics are large rocks that have been transported from their place of origin by glaciers and deposited far away. During the Pleistocene era, glaciers covered much of the earth, including areas near the equator. As these glaciers retreated, they left behind glacial erratics that can still be observed today. These rocks provide evidence of the extent of past glaciation and help scientists understand the history of the earth's climate.
The theory that explains the presence of glacial material near sea level at the equator is Plate Tectonics. This theory describes Earth's crust as being composed of large, moving plates that interact with one another. Over millions of years, these plates have shifted continents, leading to the distribution of glacial material in various locations, including equatorial regions. Glaciers likely formed at higher latitudes and altitudes during past ice ages, and as the plates moved, the glacial deposits were carried along, eventually reaching their current positions near sea level at the equator. This process demonstrates the dynamic nature of Earth's surface and its geological history.
To know about glaciers :
https://brainly.com/question/19709729
#SPJ11
monica has a bracelet made of sterling silver. sterling silver is a solid solution made of copper and silver. what type of solid solution is formed
Answers
The reaction R of the body to a dose M of medication is often represented by the general function R(M)=M^2(C/2−M/3where C is a constant. If the reaction is a change in blood pressure, R is measured in millimeters of mercury (mmHg). If the reaction is a change in temperature, Ris measured in degrees Fahrenheit ("F). The rate of change dR/dM is defined to
be the body's sensitivity to the medication. Find a formula for the sensitivity dR/dM=
Answers
A formula for the sensitivity dR/dM represents the sensitivity of the body's reaction to the medication. It shows how the reaction changes with respect to the dose of the medication, M. The term M*C represents the contribution of the constant C to the sensitivity, while the term \((2M^2)/3\) represents the contribution of the dose M itself.
To find a formula for the sensitivity, dR/dM, let's differentiate the given function R(M) with respect to M.
Step 1: Start with the function \(R(M) = M^2(C/2 - M/3).\)
Step 2: Apply the power rule of differentiation to differentiate M^2. The power rule states that if
\(f(x) = x^n, then f'(x) = n*x^(n-1). \\\)
n this case, n = 2.
\(dR/dM = 2M^(2-1)*(C/2 - M/3).\)
Simplifying, we have:
\(dR/dM = 2M*(C/2 - M/3).\)
Step 3: Distribute the 2M to each term inside the parentheses:
\(dR/dM = M*C - (2M^2)/3.\)
This formula represents the sensitivity of the body's reaction to the medication, dR/dM. It shows how the reaction changes with respect to the dose of the medication, M. The term M*C represents the contribution of the constant C to the sensitivity, while the term \((2M^2)/3\) represents the contribution of the dose M itself.
Learn more about sensitivity from this link:
https://brainly.com/question/14472410
#SPJ11
the formula for the sensitivity, or the rate of change of the reaction R with respect to the dose M, is
dR/dM = MC - M\(^2^/^3\)
How do we calculate?We calculate the derivative of the reaction function R(M) with respect to M.
the reaction function: R(M) = M²(C/2 - M/3)
We will apply the power rule and the constant multiple rule of differentiation,
dR/dM = d/dM [M²(C/2 - M/3)]
= 2M(C/2 - M/3) + M²(0 - (-1/3))
= 2M(C/2 - M/3) + M\(^2^/^3\)
dR/dM =\(MC - 2M^2^/^3 + M^2^/^3\)
= \(MC - M^2^/^3\)
Learn more about power rule at:
https://brainly.com/question/29288036
#SPJ4
Explain the nucleus, electrons, protons, and neutrons of an atom.
Answers
Answer: I learned about this in school
Explanation:
Its very easy
Answer:
The nucleus contains genetic material
Protons are a type of subatomic particle with a positive charge
Neutrons bind with protons
Explanation:
To be honest i dont really know much
Part A Given the following decomposition reaction, calculate the moles of water produced from 3.07 mol of H2O2. 2 H2O2(1) 42 H2O(l) + O2(g) Express your answer with the appropriate units. TH, = Value Units Submit Request Answer
Answers
The moles of water produced from 3.07 mol of H2O2 are 64.47 moles. Answer: 64.47
Part A Given the following decomposition reaction, calculate the moles of water produced from 3.07 mol of
H2O2.2 H2O2(1) → 42 H2O(l) + O2(g)
Molar ratio between H2O2 and
H2O is 2:42 or 1:21.
Therefore, moles of water produced from 3.07 mol of H2O2 is:
Moles of water produced = Moles of H2O2 × 21/1 = 3.07 × 21 = 64.47 (approx)
Therefore, the moles of water produced from 3.07 mol of H2O2 are 64.47 moles.
Answer: 64.47
To know more about moles visit:
https://brainly.com/question/15209553
#SPJ11
Expressing the answer with the appropriate units, we have:
3.07 moles of water.
Moles of water: Moles of a substance are a measure of the amount of that substance present. It is a unit used in chemistry to quantify the number of particles (atoms, molecules, or ions) in a sample.
The balanced equation for the decomposition reaction of hydrogen peroxide \(\rm(H_2O_2)\) is: \(\rm\[2H_2O_2(\ell) \rightarrow 2H_2O(\ell) + O_2(g)\]\)
According to the stoichiometry of the reaction, for every 2 moles of \(H_2O_2}\) consumed, 2 moles of \(H_2O\) are produced.
To calculate the moles of water \((H_2O)\) produced from 3.07 moles of \(H_2O_2\), we can set up a ratio: (3.07 moles \(\rm H_2O_2\)) x (2 moles \(\rm H_2O_\) / 2 moles \(\rm H_2O_2\)) = 3.07 moles \(\rm H_2O\)
Therefore, from 3.07 moles of \(\rm H_2O_2\), 3.07 moles of \(\rm H_2O\) are produced.
Thus, Expressing the answer with the appropriate units, we have:
3.07 moles of water.
Know more about Moles:
https://brainly.com/question/15209553
#SPJ4
please help due todays (show your to)!!!

Answers
Answer:28.411
Explanation: f1 m1+ f2 m2+ f3 m3
0.6628(28)+0.2634(29)+0.0738(30)=28.411
remember that for percentages you divide the number by 100 to get the decimal form.
If 10 moles of P4S3 was used, how many grams of P4O6 was produced? Leave up to 3 decimal places when possible.
Answers
If 10 moles of P4S3 were used, the mass of P4O6 produced would be 2838.8 grams.
To determine the number of grams of P4O6 produced from 10 moles of P4S3, we need to use the balanced chemical equation and the molar masses of the compounds involved.The balanced equation for the reaction between P4S3 and oxygen to produce P4O6 is:
P4S3 + 8 O2 → P4O6 + 6 SO2
From the balanced equation, we can see that the molar ratio between P4S3 and P4O6 is 1:1. This means that for every 1 mole of P4S3 consumed, 1 mole of P4O6 is produced.The molar mass of P4S3 is 220.25 g/mol, and the molar mass of P4O6 is 283.88 g/mol.
To calculate the mass of P4O6 produced, we can use the following equation:
Mass of P4O6 = Moles of P4O6 × Molar mass of P4O6
Since the molar ratio between P4S3 and P4O6 is 1:1, the number of moles of P4O6 produced is also 10 moles.
Mass of P4O6 = 10 moles × 283.88 g/mol = 2838.8 grams
for such more questions on mass
https://brainly.com/question/24191825
#SPJ8
A 0.200 m K2SO4 solution has a freezing point of −0.53°C. What is the van't Hoff factor for this solution?
Kf = 1.86°C/m
Answers
ΔTf = Kf * i * molality
where ΔTf is the change in freezing point, Kf is the freezing point depression constant (given as 1.86°C/m), i is the van't Hoff factor, and molality is the number of moles of solute per kilogram of solvent.
We are given that the freezing point depression is 0.53°C and the molality is:
molality = moles of solute / mass of solvent (in kg)
We don't have the mass of solvent, but we can assume that it is 1 kg (1000 g) since the molality is in moles per kilogram of solvent. This means that the mass of solute (K2SO4) is 0.200 * 1000 = 200 g.
The molar mass of K2SO4 is 174.26 g/mol, so the number of moles of solute is:
moles of solute = 200 g / 174.26 g/mol = 1.147 mol
Substituting these values into the freezing point depression equation, we get:
0.53 = 1.86 * i * (1.147/1)
Solving for i, we get:
i = 0.53 / (1.86 * 1.147) = 0.247
Therefore, the van't Hoff factor for this solution is approximately 0.247
How do I do molarity equations?
Answers
I hope this helped :)
Please make me the branliest! Have a good night/ good day!!
calcium metal is obtained by direct electrolysis of molten cacl2. if a metallurgical electrolysis apparatus operates at 26.30 a, how many grams of calcium metal will be produced in 36 hours?
Answers
Ca = 40 Ca2+ + 2e —->Ca, 1 mole of electrons = 96500 C, and
According to the preceding formula, 2 moles of electrons are required to make 1 mole of calcium.
96500C x 2 = 193000C
40g Calcium is produced with 193000C of electricity.
Coulombs (26.30A for 36 hours) equal Amps times Seconds (26.30A x 36 x 60 x 60 C), which equals 3408480 C.
This volume of electrical energy will result in
=3408480 / 193000 x 40g Calcium is equal to 17.66 x 40 g, or 706.4 g.
Thus, 706.4g of calcium metal will be produced in a period of 36 hours.
learn more about mole here;
https://brainly.com/question/15356425
#SPJ4
If the balance was not tared to display 0.00 g prior to adding the cube, which of the following statements are true? Select all that apply. A. The mass of the cube can be calculated. The mass displayed on the balance before the cube was added will need to be subtracted from the mass displayed on the balance with the cube. B. No calculation is needed to determine the mass of the cube. C. The mass displayed by the balance is the mass of the cube. D. The cube can be reweighed after the balance has been tared to display 0.00 g.
Answers
If the balance was not tared to display 0.00 g prior to adding the cube, the mass displayed by the balance is the mass of the cube.
What is the correct procedure for measuring the mass of objects using a balance?No additional metal weights are needed for a modern analytical balance. The surface of the weighing balance is cleaned, and the reading is tared at zero, in order to calculate the object's mass. The item is uniformly distributed across the balance's surface. The scale's reading is precisely recorded.
Taring ensures accurate measurements and readings.
Learn more about measuring mass at: https://brainly.com/question/1349677
#SPJ1
What is the number of moles of atoms in 9.03 x 10 24 atoms?
Answers
Answer:
15
Explanation:
An Atom that was neutral has now gained 3 electrons. Do you think this Atom will become a cation or an anion?Why?(In at least 2 sentences.)
Claim
Evidence
Reasoning
Answers
Answer: It will become an anion. Anions are negatively charged molecules. So if the molecule is charged by electrons and electrons have a negative charge, it must form an anion.
Explanation:
True or false
Equal forces acting on one object in opposite directions are called balanced forces
Answers
Answer:
True
Explanation:
Forces, equal in magnitude and opposite in direction, and cancel each other out are called Balanced Forces.
Balanced Forces DO NOT cause any change in the velocity of the object, but the Object may correspond to a change in shape or size.
Example :
Blasting a balloon by compressing it tightly with both hands, roughly with an equal force, is a practical example of Balanced Forces. In this case, the balloon before the burst stays at a state of rest and same after the burst. But the burst or the compression, causes a change in the shape of the balloon.
naturally occurring elements exists as a mixture of_______ and the percentages of each in any given element is constant.
Answers
Naturally occurring elements exist as a mixture of isotopes, and the percentages of each isotope in any given element are constant.
Isotopes are variants of an element that have the same number of protons in the nucleus but different numbers of neutrons. Since the number of protons determines the element's atomic number, isotopes of the same element have the same atomic number but different atomic masses.
For example, carbon-12, carbon-13, and carbon-14 are three isotopes of carbon, with atomic masses of 12, 13, and 14, respectively. The relative abundance of each isotope varies from element to element, but the percentages of each isotope in any given element are constant and can be used to identify the element.
To know more about Isotopes here
https://brainly.com/question/21536220
#SPJ4
why does water boil at less than 100 drgrees celsius in boulder colorado
Answers
Explanation:
Because boiling point of water is not 100 degrees Celsius but it depends on atmospheric pressure. Liquid boils at temperature when partial pressure of liquid becomes equal to atmospheric pressure.
Define corrosion and explain the different basis of tendency or resistance of different
metals to corrosion
Answers
Corrosion is the process of chemical or electrochemical attack of materials by their external environment.
The tendency/resistance of different metals to corrosion largely depends on the composition of the metal. Iron and steel corrode in the presence of oxygen due to oxidation, while some metals such as chromium, nickel and molybdenum provide a protective layer of oxide film on the surface of the metal which is then resistant to corrosion. Other metals such as aluminum and zinc form sacrificial oxide layers which corrode and protect the metal from becoming completely damaged.
What volume of DI water, in mL, must you use to dissolve 30.0 g of NaOH in order to make a 1.25 M solution? mL (round to whole number)
Answers
Answer:
600mL
Explanation:
Molarity of a solution (M) = number of moles (n) ÷ volume (V)
number of moles = mass/molar mass
Molar mass of NaOH = 23 + 16 + 1
= 40g/mol
mole = 30/40
n of NaOH = 0.75mol
Using Molarity = n/V
V = number of moles ÷ molarity
V = 0.75 ÷ 1.25
V = 0.6L
In milliliters (mL), the volume of NaOH will be 0.6 × 1000
= 600mL