If a compound begins with a metal, it most likely is a _______ compound
metallic
molecular
covalent
ionic
Answers
Answer:
A
Explanation:
If a compound begins with metal, it is most likely to be a metallic compound. Thus, option A is correct.
The metallic compound has been formed when the metal has been bonded with a metal or more than 1 metal by the ionic or covalent interaction.
The ionic interactions result in between the metal and non-metal or between two metals, and the covalent interactions have been found in the nonmetals.
The molecular compound has been the one that has been formed a molecule. All the compounds are molecular compounds.
Thus, if it starts with metal, it has been most likely to form a metallic compound. Thus, option A is correct.
For more information about the compounds with metal, refer to the link:
https://brainly.com/question/18665430
Related Questions
An organism's genetic material is called its
Answers
Answer:
Genome
Explanation:
draw lewis structures for the fulminate ion including possible resonance forms.
Answers
The Lewis structure of the fulminate ion (CNO-) and possible resonance forms are shown below:
Explanation: The atomic numbers of carbon, nitrogen, and oxygen are 6, 7, and 8, respectively. As a result, carbon has 4 valence electrons, nitrogen has 5, and oxygen has 6 valence electrons. The overall number of valence electrons is calculated by adding up the valence electrons of each atom in the molecule.
Lewis Structures of Fulminate Ion
Step 1:Calculation of the total valence electrons available
The total valence electrons of carbon, nitrogen, and oxygen can be calculated as follows:
Carbon: 4 valence electrons
Nitrogen: 5 valence electrons
Oxygen: 6 valence electrons
Total valence electrons: 4 + 5 + 3(6) + 1 (charge) = 26
Step 2: Determination of the central atom and determination of the structure type The atom with the lowest electronegativity value is usually the central atom. Carbon is the central atom because it has the lowest electronegativity value. Fulminate ion (CNO-) is a trigonal planar structure.
Step 3: Drawing the structure The skeletal structure of the fulminate ion (CNO-) is shown below. The negative charge is assigned to the nitrogen atom, and three single bonds are formed between the carbon, nitrogen, and oxygen atoms.
Step 4: Filling in the lone pairs In a Lewis structure, atoms want to have a full valence shell. Therefore, nitrogen and oxygen atoms each have one lone pair of electrons
Step 5: Formal charges The formal charges are calculated using the formula:
FC = (number of valence electrons on the neutral atom) - (number of lone pair electrons) - (number of bonds).Formal charges on nitrogen
= 5 - 4 - 2 = -1
Formal charges on carbon = 4 - 0 - 3 = +1
Formal charges on oxygen = 6 - 2 - 1 = +3
The sum of the formal charges on the molecule should equal the total charge on the molecule. The sum of the formal charges of the fulminate ion is -1 +1 +3 = +1, which equals the total charge on the ion
.Step 6: Resonance structureThe fulminate ion (CNO) has two possible resonance structures.
The first resonance structure occurs when the double bond is located between the nitrogen atom and the oxygen atom and the negative charge is located on the nitrogen atom.
The second resonance structure has the double bond located between the carbon and nitrogen atoms, and the negative charge is on the oxygen atom.
To know more about atomic numbers, visit:
https://brainly.com/question/16858932
#SPJ11
What is the charge of a beta particle?
Answers
Answer:
Beta particles have a charge of minus 1.
Explanation:
Hope this helps!
Answer:
MARK AS BRAINLIEST ANSWER
PLZ FOLLOW ME
Explanation:
Beta particles have a mass which is half of one thousandth of the mass of a proton and carry either a single negative (electron) or positive (positron) charge. As they have a small mass and can be released with high energy, they can reach relativistic speeds (close to the speed of light)
HOPE IT HELPS YOU
Please help me on numbers 1-4!!

Answers
a derived unit is a unit derived by fundamental units of lengths ,mass and time
so measurements
help me please im in 4th grade and online is frustrating

Answers
Answer:
A
Explanation:
The answer is A) because the A container has tightly packed molecules but not as tight as C so that means A is liquid, and C is a solid which makes B a gas
Answer:
The answer would be A
Explanation:
because in the first picture Liquid could be in many shapes like If I put water in my water bottle it would be the same shape as the water bottle.
In the third picture, the solid molecules will always be so close to each other like they will be stick to each other.
In the second picture, It will be separate always, as it cant stick to each other.
I hope I helped you!!!!
:)
What is the density of a sample of lead that has a mass of 4.85 kg and a density of 280 cm ^3?
Answers
Answer:
The answer is 0.017 kg/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass = 4.85 kg
volume = 280 cm³
We have
\(density = \frac{4.85}{280} \\ = 0.01732...\)
We have the final answer as
0.017 kg/cm³Hope this helps you
in step 6 of the citric acid cycle when succinate is converted to fumarate, hydrogen atoms are transferred to fad. the ____________ is catalyzed by a dehydrogenase enzyme.
Answers
The cycle of citric acid Hydrogen atoms are transported to FAD during the conversion of succinate to fumarate. This process is catalyzed by a succinate dehydrogenase enzyme.
During the process of succinate dehydrogenation, succinate is oxidized to fumarate by losing electrons, which are transferred to FAD, reducing it to FADH2. This transfer of electrons from succinate to FAD is an oxidation-reduction reaction, also known as redox reaction. The energy released during this reaction is harnessed to generate ATP, which is an important energy currency for the cell. Succinate dehydrogenase is an enzyme complex that contains multiple subunits and cofactors, including flavin adenine dinucleotide (FAD) and iron-sulfur clusters. It is located in the inner mitochondrial membrane, which allows it to transfer electrons to the electron transport chain, leading to the production of ATP. It's important to note that the citric acid cycle is a crucial metabolic pathway that takes place in the mitochondria of eukaryotic cells, and it is also known as the TCA cycle or Krebs cycle. The cycle is a series of chemical reactions that converts acetyl-CoA, derived from carbohydrates, fats, and proteins, into carbon dioxide and water, releasing energy in the form of ATP, hydrogen atoms other high-energy molecules.
To know more about hydrogen atoms please refer: https://brainly.com/question/29130026
#SPJ4
What is the primary reason why elements form compounds?
Answers
Answer:
To gain stability
Explanation:
If the outermost shell is not completely filled with electrons, the element has one of the three options: gaining electrons, losing electrons or sharing electrons. By gaining or losing electrons, ionic compounds are produced. Sharing of electrons results in the formation of covalent compounds.
carbon-dating was performed on an artefact that was found to have 25% of the original carbon-14 remaining in it
Answers
When is a rock considered an ore?
Check all that apply.
-when it occurs in sufficient amounts to be used to build roads and buildings
-only when it contains lead
-when it contains at least one metallic mineral in sufficient amounts to be extracted profitably
-only when it contains iron
-when it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably
Answers
The correct statements are that a rock is considered an ore when it contains at least one metallic mineral in sufficient amounts to be extracted profitably and when it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably.
When it contains at least one metallic mineral in sufficient amounts to be extracted profitably.
When it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably.
Step-by-step explanation:
When it occurs in sufficient amounts to be used to build roads and buildings: This statement is incorrect. Rocks that are used for construction purposes, such as limestone or sandstone, are not considered ores unless they also contain economically valuable minerals.
Only when it contains lead: This statement is incorrect. Ores are not limited to containing only lead. Ores can contain various metallic minerals, not just lead.
When it contains at least one metallic mineral in sufficient amounts to be extracted profitably: This statement is correct. Ores are rocks that contain valuable metallic minerals in concentrations that make extraction economically feasible. These minerals can include gold, silver, copper, aluminum, zinc, and many others.
Only when it contains iron: This statement is incorrect. While iron ores are commonly known and widely used, ores are not limited to containing only iron. There are numerous other metallic minerals that can be extracted profitably from rocks.
When it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably: This statement is correct. Fluorite and sulfur are examples of non-metallic minerals that can be extracted profitably from rocks, and if a rock contains these minerals in sufficient quantities, it can be considered an ore.
learn more about from metallic mineral the given link:
https://brainly.com/question/89259
#SPJ11
Acetaminophen contains 4 elements. There are 20 atoms in its chemical formula. Hydrogen is the most abundant element in this compound. Which of the following chemical formulas represents acetaminophen? *
Answers
Answer:
w
Explanation:
w
Chemical formula of acetaminophen is C₈H₉NO₂, this drug is also called as paracetamol and is used as a pain killer or an analgesic.
What is chemical formula?
Chemical formula is a way of representing the number of atoms present in a compound or molecule.It is written with the help of symbols of elements. It also makes use of brackets and subscripts.
Subscripts are used to denote number of atoms of each element and brackets indicate presence of group of atoms. Chemical formula does not contain words. Chemical formula in the simplest form is called empirical formula.
It is not the same as structural formula and does not have any information regarding structure.It does not provide any information regarding structure of molecule as obtained in structural formula.
There are four types of chemical formula:
1)empirical formula
2) structural formula
3)condensed formula
4)molecular formula
Learn more about chemical formula ,here:
https://brainly.com/question/11995171
#SPJ2
1. Why is the timing of tides predictable?
A. because winds are predictable
B. because the moon’s path is predictable
C. because ocean water density is predictable
D. because the sun’s movements are predictable
Answers
Answer:
Because the moon's path is predictable
Explanation:
The moon affects the tides
.What is the difference between solutions that are "nonelectrolytes," "weak electrolytes," and"strong electrolytes?" Explain your reasoning.
Which one of the following aqueous solutions would you expect to have the smallestconductance: (a) 0.060 M NaCl, (b) 0.030 M NaCl, or (c) 0.015 M NaCl? Explain your reasoning.
Answers
Nonelectrolytes do not conduct electricity. Weak electrolytes partially dissociate into ions and have limited conductivity. Strong electrolytes completely dissociate into ions and have high conductivity. Among the given options, option (c) with 0.015 M NaCl is expected to have the smallest conductance due to its lower concentration of ions.
The terms "nonelectrolytes," "weak electrolytes," and "strong electrolytes" refer to the ability of a substance to conduct electricity when dissolved in water (or any other suitable solvent).
1. Nonelectrolytes:
Nonelectrolytes are substances that do not conduct electricity when dissolved in water. They exist as molecules in their solid or liquid form and do not dissociate into ions when dissolved. Examples of nonelectrolytes include sugar (sucrose), alcohol (ethanol), and most organic compounds. Since they do not produce ions, they cannot carry an electric current.
2. Weak electrolytes:
Weak electrolytes are substances that partially dissociate into ions when dissolved in water. They conduct electricity to a limited extent. In a solution of a weak electrolyte, only a small fraction of the molecules dissociate into ions, while the majority remain in molecular form. Weak acids and weak bases are common examples of weak electrolytes. An example is acetic acid (CH₃COOH), which partially dissociates into hydrogen ions (H+) and acetate ions (CH₃COO⁻) in water.
3. Strong electrolytes:
Strong electrolytes are substances that completely dissociate into ions when dissolved in water. They are excellent conductors of electricity. In a solution of a strong electrolyte, all or nearly all of the solute molecules break apart into ions. Strong acids (e.g., hydrochloric acid, HCl) and soluble ionic compounds (e.g., NaCl, KNO₃) are examples of strong electrolytes.
Now, considering the given options:
(a) 0.060 M NaCl
(b) 0.030 M NaCl
(c) 0.015 M NaCl
NaCl is an ionic compound, and when dissolved in water, it completely dissociates into Na+ and Cl- ions, making it a strong electrolyte. The conductivity of an electrolyte solution is directly proportional to the concentration of ions present. Therefore, the solution with the highest concentration of ions will have the highest conductance.
Among the given options, option (a) with a higher concentration of NaCl (0.060 M) is expected to have the highest conductance. Option (b) has a lower concentration (0.030 M), and option (c) has the lowest concentration (0.015 M). Hence, option (c) with the lowest concentration of NaCl is expected to have the smallest conductance.
To learn more about Nonelectrolytes refer here:
https://brainly.com/question/15194255#
#SPJ11
If Earth is in between the sun and the Moon in both Image 1 and Image 2, why do you think a lunar eclipse is only happening in Image 2?
Answers
Answer: well I don’t have either picture but here goes anyway. It could be that the suns rotation matched up with the moon being 400 miles away from it and 400 times bigger it could be in image 1 the sun is only at a partial eclipse. With no images kinda had to wing it but I hope it helps :D
A lunar eclipse is an event that occurs when the sun, moon, and earth are aligned. It is happening in image 2 as Earth is obstructing the sun’s light.
What is a lunar eclipse?In lunar eclipse Earth completely covers the surface of the moon which makes the surface look red and dark. It happens only when the Moon is opposite the sun in the sky.
It is not seen in the first image as the earth is incapable of totally blocking the sunlight and hence does not result in a lunar eclipse. In the second image, the Earth has covered the whole surface of the moon and hence is blocking the sunlight.
Therefore, a lunar eclipse is seen only in image 2.
Learn more about the lunar eclipse here:
https://brainly.com/question/4198384
the water in a beaker has a volume of 50 millimeters, is this an extensive property?
Answers
No, the volume of water in a beaker is not an extensive property.
Extensive properties are those that depend on the amount or size of the substance being measured. In other words, they are properties that change with the quantity of the substance. Examples of extensive properties include mass, volume, and total energy.
In the given scenario, the volume of water in the beaker is 50 milliliters. This volume remains the same regardless of the quantity of water present. Whether it's 50 milliliters or 500 milliliters, the volume measurement does not change. Therefore, the volume of water in the beaker is an example of an intensive property.
Intensive properties are independent of the amount or size of the substance. They are characteristics that remain constant regardless of the quantity of the substance. Examples of intensive properties include temperature, density, and color.
It's important to note that the distinction between extensive and intensive properties depends on the specific property being considered. While volume is typically an extensive property for a bulk substance, in the case of a fixed volume of water in a beaker, it becomes an intensive property.
In summary, the volume of water in a beaker is not an extensive property but rather an intensive property because it does not change with the quantity of the substance.
For more such questions on extensive property visit:
https://brainly.com/question/13055036
#SPJ8
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
Which statements best describe the first stage of cellular respiration? Check all that apply.
The stage happens in cytoplasm.
The stage happens in mitochondria.
Oxygen combines with small molecules.
Glucose is broken down.
Energy is released.
( 20 points) HELP ME QUICK IM TIMED
Answers
Answer:b
Explanation:
Answer:
The correct answer is The stages happens in the cytoplasm.
Explanation:
Explanation:Cellular respiration helps in the complete breakdown of carbohydrates basiclly glucose to form carbon dioxide,water and ATP molecules as energy source.Aerobic respiration consist of 3 principle steps
The first step is called glycolysis which deals with the catabolism of glucose into pyruvate by 10 enzymes catalyzed reactions.
Glycolysis occurs in the cytoplasm as many of the glycolytic enzymes are present in the cytoplasm of the cell in which the glycolysis is occuring.
An isotope of an element has a different number of _____.
neutrons
protons
electrons
Answers
I believe that the answer is
A) Neutrons
I hope this helps you ^-^
Answer:
neutrons
Explanation:
Regina has a shelf that is eight feet off the ground. When she was younger she used the shelf for storage, and her mom would help her use a ladder to reach things on it occasionally. Now she wants to use the shelf to hold her textbooks that she needs to use every day. She needs to be able to do this by herself without using the ladder. Regina needs to design a tool to help her reach the books ont he shelf easily and safely. She wants to use materials she finds around the house to make the tool.
Describe how Regina's needs for the bookshelf changed over time.
Answers
Answer:
It changed do to storage into a actual shelf
Explanation:
where is the answer BOZO
4. How many molecules are in 0.150 mol NaCl?
Answers

1 mole is equal to 6.023 × 10 ²³ molecules. 9.033 × 10²² molecules are in 0.150 mol NaCl.
What do you mean by mole ?The term mole is defined as the amount of substance of a system which contains as many elementary entities.
1 mole is equal to 6.023 × 10 ²³ molecules.
One mole of any substance is equal to 6.023 × 10²³ units of that substance such as atoms, molecules, or ions. The number 6.023 × 10²³ is called as Avogadro's number or Avogadro's constant.
It is given that,
Number of moles (n) = 0.15
We need to find the number of particles in 0.15 mol of NaCl.
Let N are the number of particles i.e.
Number of particles = number of moles × Avagadro's number
N = n × NA
N = 0.150 × 6.023 × 10²³
= 9.033 × 10²²
Thus, 9.033 × 10²² molecules are in 0.150 mol NaCl.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ2
how many particles are in 13.2 moles of N2O4
Answers
To determine the number of particles in 13.2 moles of N2O4, we need to use Avogadro's number, which states that there are approximately 6.022 × 10^23 particles (atoms, molecules, or formula units) in one mole of any substance.
N2O4 has a molar mass of 92.02 g/mol.
First, let's calculate the number of moles of N2O4 in 13.2 moles:
Number of moles of N2O4 = 13.2 moles
Next, we can use the molar mass of N2O4 to convert moles to grams:
Mass of N2O4 = Number of moles × Molar mass
Mass of N2O4 = 13.2 moles × 92.02 g/mol
Now, we can convert the mass of N2O4 to the number of particles using Avogadro's number:
Number of particles = Mass of N2O4 / Molar mass × Avogadro's number
Number of particles = (13.2 moles × 92.02 g/mol) / (92.02 g/mol) × (6.022 × 10^23 particles/mol)
Calculating this expression:
Number of particles = 13.2 × (6.022 × 10^23) = 7.954 × 10^24 particles
Therefore, there are approximately 7.954 × 10^24 particles in 13.2 moles of N2O4.
Learn more about Avogadro's number on:
https://brainly.com/question/28812626
#SPJ1
1. Which type of organic compound is represented by the structural formula shown below?
a) Ether
b) Ester
C) Alcohol
d) Aldehyde

Answers
Answer:
c. alcohol
Explanation:
Alcohol is -OH
Ether is -OR
Ester is O=C-OR
Aldehyde is O=C-H
- Only alcohol fits
Alcohol is the type of organic compound is represented by the structural formula.
What is Alcohol?This can be defined as the type of organic compound that carries at least one hydroxyl functional group (−OH) bound to a saturated carbon atom.
The hydroxyl group(OH) is attached to a Carbon atom in the diagram which depicts features of an Alcohol.
Read more about Alcohol here https://brainly.com/question/13621129
why is it so difficult to experience the northern lights here in michigan? I need to know noww
Answers
Answer:
Think about where you're located on the globe. That'll tell you your answer
Explanation:
A photon of wavelength 1,094 nm is emitted when an electron in hydrogen makes a transition to the third level. determine the level that the electron started it.
Answers
The electron started in the second energy level (n₁ = 2) before transitioning to the third level.
To determine the initial level of the electron in a hydrogen atom, we can use the Rydberg formula, which relates the wavelength of a photon emitted or absorbed during an electron transition to the energy levels in hydrogen:
1/λ = R * (1/n₁² - 1/n₂²)
Where, λ is the wavelength of the photon,
R is the Rydberg constant (approximately 1.097 x 10^7 m^-1),
n₁ is the initial energy level,
n₂ is the final energy level.
Given that, the wavelength of the emitted photon is 1,094 nm (or 1.094 x 10^-6 meters) and the electron transition occurs to the third level (n₂ = 3), we can substitute these values into the formula and solve for n₁:
1/λ = R * (1/n₁² - 1/n₂²)
1/(1.094 x 10^-6) = 1.097 x 10^7 * (1/n₁² - 1/3²)
Simplifying the equation:
1.094 x 10^6 = 1.097 x 10^7 * (1/n₁² - 1/9)
1/n₁² - 1/9 = (1.094 x 10^6) / (1.097 x 10^7)
1/n₁² - 1/9 ≈ 0.0997
1/n₁² ≈ 0.0997 + 1/9
1/n₁² ≈ 0.1997
n₁² ≈ 1 / 0.1997
n₁² ≈ 5.004
n₁ ≈ √5.004
n₁ ≈ 2.24
Therefore, the electron started in the second energy level (n₁ = 2) before transitioning to the third level.
Learn more about electron from the given link:
https://brainly.com/question/26084288
#SPJ11
When a bag containing mothballs is opened, mothballs’ molecules go through the following change.Which of the following is the correct question to ask to determine what kind of change the molecules experienced?A. How has the size of the molecules changed?B. How much has the temperature of the molecules changed?C. What is the position of the molecules relative to each other?D. Is this change in the molecules reversible by placing the mothballs back in their bag?

Answers
Mothballs are made of naphtalene. The chemical structure of naphthalene is formed by two benzene rings, which gives this substance the classification of an aromatic compound.
The active principle of naphthalene is explained by a very interesting physical characteristic: sublimation, which is defined as the direct transition from a solid to a gaseous state. Once sublimated, naphthalene appears as a toxic vapor for undesirable microorganisms.
So, when a bag of mothballs is opened, they will sublime. So the molecules will not be able to come back to the bag, because they're in the gaseous state, which is too difficult for us to "pick up" the molecules of mothballs again and place them in their bag again.
It is like picking up water vapor, it is impossible.
Answer: Alternative "D"
Can someone help me with this please

Answers
The text discusses the issue of global warming caused by the release of greenhouse gases, primarily from human activities such as burning fossil fuels and deforestation. It highlights the potential consequences of rising temperature.
What is the gist of the greenhouse effect?The greenhouse effect explains how heat is trapped at the Earth's surface by "greenhouse gases." You may think of these heat-trapping gases as a blanket keeping the Planet warmer than it otherwise would be.
What type of global warming is created by the atmosphere?Water vapour, carbon dioxide, methane, and other airborne gases all contribute to the greenhouse effect, which warms the Earth's surface and troposphere (lowest layer of the atmosphere).
To know more about temperature visit:-
https://brainly.com/question/11050215
#SPJ1
Which compound is an organic acid?
A.CH3OH
B.CH3OCH3
C.CH3COOH
D.CH3COOCH3
Answers
Answer:
C
Explanation:
CH3COOH
ethanoic acid
Answer:
C: CH3COOH
Explanation:
An organic acid (acetic acid) - a carboxylic acid has a functional group of:
R - COOH
PLEASE HELP ME ON CHEMISTRY ASAP PLS
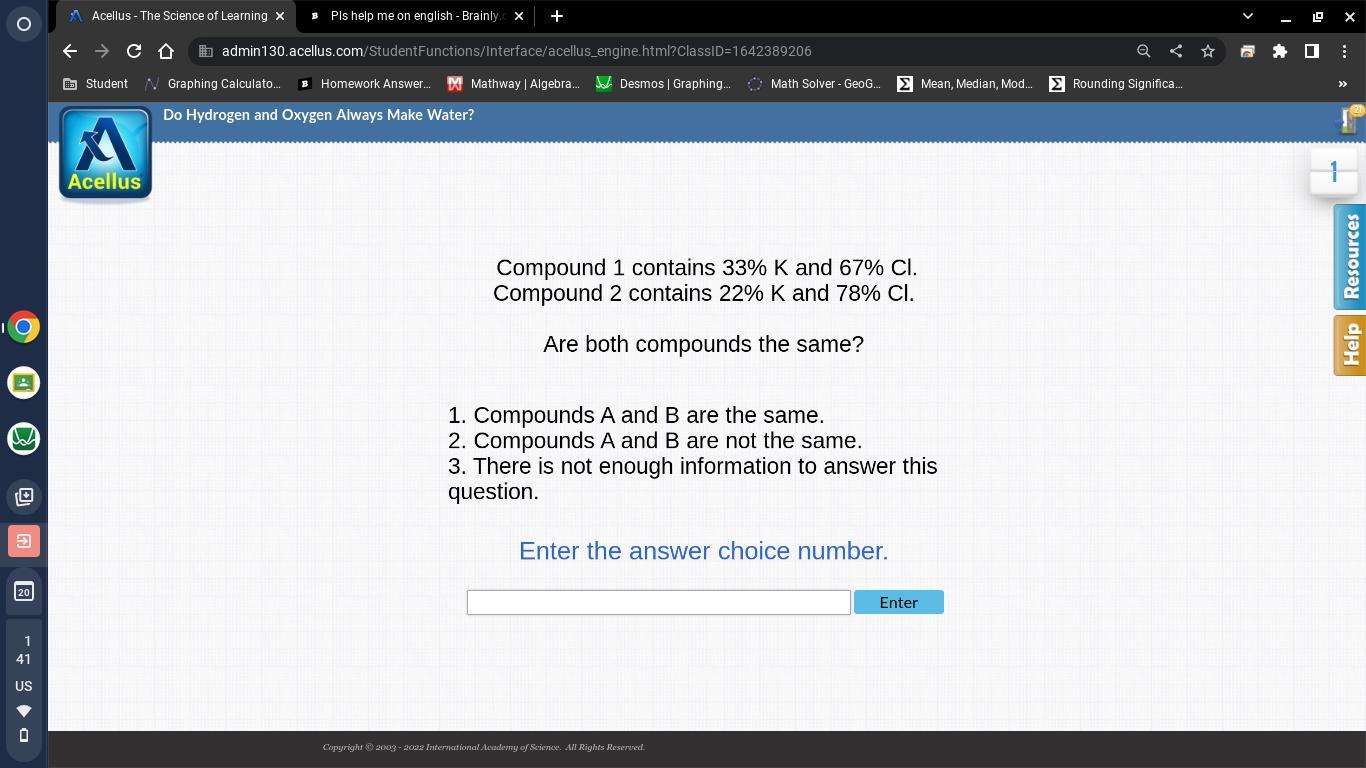
Answers
Answer:
compound identification is unknown B
Explanation:
It is not possible to determine whether the two compounds you have described are the same or not based on the information provided. The percentage of each element in a compound can be used to determine the compound's empirical formula, but it does not necessarily give information about the compound's molecular structure or identity.
For example, two compounds could have the same empirical formula (i.e., the same proportions of elements) but have different molecular structures, resulting in different chemical and physical properties. Alternatively, two compounds could have different empirical formulas but the same molecular structure, resulting in the same chemical and physical properties.
Without more information about the chemical and physical properties of the compounds, it is not possible to determine whether they are the same or not.
Put the following elements in order from smallest to largest atomic radius: Carbon, Oxygen, Tin, Strontium
Rank the following elements by increasing atomic radius: Carbon, Aluminum, Oxygen, Potassium
Answers
The order of the atomic radius is;
1) Potassium, Aluminum, Carbon, Oxygen
2) Potassium, Aluminum, Carbon, Oxygen
What is the order of the atomic radius?We know that the atomic radius has to do with the distance between one atom and another in that are not covalently combined. We know that the atomic radius is a function of the group to which the atom belongs.
We know that the atomic radius increases down the group but would tend to decrease across the period as more shells are added to the atom of the element.
Learn more about atomic radius:https://brainly.com/question/14544878
#SPJ1
what is the function of the cell membrane?
Answers
Answer:
Its the thin lining around the cell the moniters what gets in and out of the cell.
Explanation: