If a +1 ion has 29 protons, what element is it?
Answers
Answer: Copper (I)
Explanation:
The number of protons in an atom directly correlates to the identity of the atom, so this ion is copper. The charge relates to the balance of protons and electrons, so this ion has one more proton than it does electrons, making our final answer Copper (I). (Make sure to state the charge of transition elements! In this case our charge is 1+, so we'll put a (I) after the name!)
Related Questions
An unknown alkane, X, was found to contain 9.0g of carbon and 2.0g of hydrogen. Calculate the simplest formula for this alkane. A₁ (H) = 1 A,(C) = 12
Answers
When the mass of atoms are given, we can easily find out the simplest (Empirical) formula of the molecule.
Here, the Simplest (Empirical) formula for the given alkane is \(C_3H_8\)
What is Empirical Formula ?Empirical formula is defined as the formula of a compound which gives the simple whole number ratio of the atoms of various elements present in one molecule of compound.
For this problem, we are given the mass in grams of each element.
Begin by finding the moles of each :
No. of mole = Given weight / Atmoic Weight
So,
No. of Moles of Carbon = 9/12 = 0.75
No. of Moles of Hydrogen = 2/1 = 2
Next, derive the Carbon-to-Hydrogen molar ratio by dividing by the lesser number of moles :
C = 0.75/0.75 = 1
H = 2/0.75 = 2.66
The ratio is 1.000 mol of Carbon to 2.66 mol of Hydrogen (\(C_1H_2._6_6\)).
Finally, multiply the ratio by three to get the smallest possible whole number subscripts while still maintaining the correct Carbon-to-Hydrogen ratio :
3 (\(C_1H_2._6_6\)) = (\(C_3H_7._9_8\)) = \(C_3H_8\)
Hence, the Simplest (Empirical) formula for the given alkane is \(C_3H_8\)
Learn more about Empirical formula here ;
https://brainly.com/question/14044066
#SPJ1
First Steps
THE LANDING
As the lander hit, Maria drew a jagged breath, and her chest muscles clenched tight with anxiety. Captain Curran, the group leader, turned around and smiled at Maria and her five friends.
“All right,” he said with forced joviality. “Who wants to be the first of the generations born in space to set foot on a real planet?”
Maria waited for someone else to speak or raise their hand. Next to her, Allen just stared at the floor of the lander muttering, “Not me, not me.”
She glanced at Lily, who Maria had always known to be fearless, but Lily bit her lip and turned away. Javier looked positively gray, and the twins buried their faces in their hands. Someone would have to be first. Maria closed her eyes and took a few meditative breaths, waiting for someone else to volunteer, but the lander was silent.
FINDING COURAGE
The radio crackled to life. “Lander one, this is Mothership, do you copy?”
“Yes,” Captain Curran answered. “We’ve landed safely and are waiting to exit the lander, but there’s just a little…disagreement…over which pioneer wants to be the first to set foot on our new home.”
“Tell them there’s plenty for everyone to see, and they’re going to love it out there,” the voice on the radio responded. “I wish it were me instead of you; I miss the feel of real air on my face.”
Captain Curran flipped off the microphone. “You six have lived your entire lives in space, and it’s a great privilege to be the first of your generation to see a new planet. The others are watching; if you’re afraid, they’ll be afraid. Can’t any of you find the courage to set an example?”
Maria shifted uncomfortably in her seat and thought of her parents; they had traveled across the galaxy to find their family a new home, with clean air and good soil, and she knew this planet was for them. “I’ll do it, Captain,” she mumbled as she slipped out of her harness and rose out of her seat. Maria couldn’t shake the feeling of trepidation as she stood and watched the doors of the lander slide open.
A NEW WORLD
A burst of air hit Maria in the face. She scrunched up her nose as an unfamiliar assortment of odors hit her nostrils. Some smelled sweet, some disgusting, and some were just strange. There were never strange smells on the ship; everything was always the same there. What was it going to be like to live where things changed? The rest of the children gathered around the opening as she climbed down the ladder, and Maria tried to smile as she met their worried eyes. Then, suddenly, something crunched underneath her boots; she was standing on the surface of the planet, and it felt nothing like the smooth metallic halls that she’d known all her life.
Without letting go of the ladder, she turned around to look at the world around the lander. The soil was full of shapes and textures; there were some small, grainy pieces, then larger clumps that she could break with the toe of her boot. One piece was hard and smooth, and she let go of the ladder to pick it up. “Captain,” she called, “I think I’ve found a rock!”
The air was moving, and long, thin, green things bowed and danced. “Grasses,” Maria whispered to herself, remembering the videos in her science lessons. She let go of the ladder and took soft, slow steps and realized her arms and legs felt like they were full of lead. “Natural gravity,” she whispered to herself. She started to walk a little more quickly, getting used to the new sensation. She was doing what others had previously thought impossible—taking steps on a new world.
A creature with gossamer wings landed on her nose, and she crossed her eyes trying to get a good look at it. Something small and furry scurried across her feet as she spun in a slow circle. Inspired, she ran as fast as she could across the foreign soil; she’d never seen somewhere so big, and it was thrilling. Suddenly, a deep, low sound echoed around her.
“That’s the call of a hornbeast,” Captain Curran shouted from the bottom of the ladder. Maria glanced back and saw that he was helping her friends take tentative first steps in the new world. “Walk to your left a little, and there should be a stream—flowing water on the ground; they often go there to drink, the explorers say.”
She started to run in that direction and then paused. “Hurry up!” she yelled, unable to contain her enthusiasm. “Our new home is extraordinary!”
Question
In “First Steps,” which theme is developed by Maria’s volunteering to leave the lander?
Some things cannot be learned in school.
Some things cannot be learned in school.
It takes courage to be the first to try something new.
It takes courage to be the first to try something new.
Taking unnecessary risks is foolish.
Taking unnecessary risks is foolish.
Great tasks can be accomplished by working as a team.
Answers
Answer:
I think it is C. It takes courage to be the first to try something new.
Explanation:
The story showed her courage to the first to try something new.
Hope this helps! :)
what role does the heat capactiy and heat conductivity of the ord play in the accuracy of this measurement? what type of error is this
Answers
According to the temperature difference and mass, a material's thermal conductivity explains its capability to transfer heat, and its specific heat capacity indicates how much heat energy is received or released.
What is measured by thermal conductivity and heat capacity?A material's ability to conduct or transfer thermal energy can be determined by measuring its thermal conductivity, and a material's specific heat capacity specifies the amount of thermal energy needed to raise its temperature.
What are measurements of heat capacity?A physical feature of matter known as heat capacity or thermal capacity is the quantity of heat that must be applied to an object in order to cause a unit change in temperature. Heat capacity is measured in joules per kelvin (J/K), the SI unit.
To learn more about thermal conductivity here:
https://brainly.com/question/7643131
#SPJ4
Question 4 of 10
What effect does adding a solute have on the freezing point of a solution?
A. The solution will not reach a freezing point temperature.
B. The temperature at which the solution freezes is raised.
C. The temperature at which the solution freezes is lowered.
D. The temperature at which the solution freezes is unchanged.
Answers
Answer:
C. The temperature at which the solution freezes is lowered.
A gas sample was produced in the laboratory. The gas was determined to be more dense than air (which is mostly composed of nitrogen). What is the identification of the gas? a)Hydrogen b)Neon c)Methane (CH_4) d)Carbon Dioxide
Answers
The correct option is (d) Carbon Dioxide.
Explanation:
The density of air is around 1.2 g/L, which means that any gas with a density above this value is more dense than air.
Carbon dioxide has a density of approximately 1.98 g/L, which is considerably more dense than air (composed of nitrogen and oxygen).
As a result, if a gas sample is determined to be more dense than air, it is likely to be carbon dioxide (CO2), which has a molecular weight of 44 g/mol.
Carbon dioxide is produced in the laboratory by many chemical reactions and is commonly employed in the food and beverage industries, such as carbonating soda and beer.
To know more about chemical reactions visit;
https://brainly.com/question/29762834
#SPJ11
Does changing the number of neutrons change the identity of the element you have built ?
Answers
Answer:
If you change the number of neutrons somehow, nothing will happen because it carry's no charge at all.
Explanation:
calculate the mass of honh2 required to dissolve in enough water to make 256.9 ml of solution having a ph of 10.02 (kb = 1.1 ✕ 10−8).
Answers
To calculate the mass of HONH2 (hydroxylamine) required to dissolve in enough water to make a 256.9 mL solution with a pH of 10.02, we can use the fact that pH is defined as the negative logarithm of the hydrogen ion concentration ([H+]) in a solution.
We can also use the relationship between pH, pOH, and the ionization constant of water (Kw = 1.0 x 10^-14) to calculate the pOH of the solution.
Given that the pH of the solution is 10.02, the pOH can be calculated as follows:
pH + pOH = 14 (since Kw = 1.0 x 10^-14)
pOH = 14 - pH = 14 - 10.02 = 3.98
Since HONH2 is a weak base, we can use the relationship between pOH, Kb (the base ionization constant), and the concentration of the base to calculate the amount of HONH2 required. The equation for Kb is:
Kb = [OH-]^2 / [HONH2]
Rearranging the equation to solve for [HONH2], we get:
[HONH2] = [OH-]^2 / Kb
Substituting the calculated pOH (3.98) and the given value for Kb (1.1 x 10^-8) into the equation, we can calculate the concentration of HONH2 in the solution.
[OH-] = 10^(-pOH) = 10^(-3.98) = 7.18 x 10^-4
[HONH2] = (7.18 x 10^-4)^2 / (1.1 x 10^-8) = 4.64 mol/L
Finally, we can calculate the mass of HONH2 required to make a 256.9 mL (or 0.2569 L) solution with a concentration of 4.64 mol/L using the formula:
Mass = Concentration x Volume
Mass = 4.64 mol/L x 0.2569 L = 1.19 g
So, the mass of HONH2 required to dissolve in enough water to make a 256.9 mL solution with a pH of 10.02 is 1.19 grams.
To know more about hydrogen ion click here:
brainly.com/question/20309096
#SPJ11
what explains why crystallins in scallops and elephant shrews are both derived from forms of aldehyde dehydrogenase
Answers
Aldehyde dehydrogenase is an enzyme family that is known to play a critical role in many different cellular processes, including detoxification and cellular metabolism. Crystallins are proteins found in the eye lens of various animals, including scallops and elephant shrews.
Aldehyde dehydrogenase is an enzyme family that is known to play a critical role in many different cellular processes, including detoxification and cellular metabolism. Crystallins are proteins found in the eye lens of various animals, including scallops and elephant shrews. These proteins are important for maintaining the transparency and refractive index of the lens.
Crystallins are derived from aldehyde dehydrogenase in scallops and elephant shrews because of convergent evolution. Convergent evolution is a phenomenon where similar traits evolve independently in unrelated species in response to similar environmental pressures. In this case, scallops and elephant shrews both live in environments where high levels of UV light are present. To combat the harmful effects of UV light, these animals have evolved transparent lenses that allow light to pass through and reach the retina without scattering.
To maintain the transparency of the lens, both scallops and elephant shrews have evolved crystallins that are derived from aldehyde dehydrogenase. These proteins have a unique structure that allows them to refract light without scattering it. This is an important adaptation that allows these animals to see clearly in environments where UV light is present.
In conclusion, the reason why crystallins in scallops and elephant shrews are both derived from forms of aldehyde dehydrogenase is due to convergent evolution. These animals have independently evolved similar traits in response to similar environmental pressures, which has led to the development of crystallins that are critical for maintaining the transparency and refractive index of the lens.
To know more about detoxification visit: https://brainly.com/question/30575627
#SPJ11
6. how does a non polar column separate mixture of compounds and how does a polar column separate mixture of compounds
Answers
The capacity of stationary phases used in high performance liquid chromatography (HPLC) to distinguish between polar column and nonpolar chemicals can be used to classify them.
In HPLC, how do you distinguish polar compounds?Extremely polar chemicals, which are difficult to separate under reversed-phase conditions, can be retained and separated successfully using hypercarb columns. This application has shown that: Polar chemical retention is good in hypercarb columns. The techniques created on Hypercarb columns are solid.
How can you distinguish between polar and nonpolar bonds?(We define a bond to be polar if there is a difference between the electronegativity of the atoms in the bond that is more than 0.4. The bond is effectively nonpolar if the difference in electronegativity is smaller than 0.4.) The molecule is nonpolar if there are no polar bonds.
To know more about polar column visit:-
https://brainly.com/question/14221443
#SPJ4
How many molecules of NaOH are in 10.0 g of NaOH? *
Answers
The number of molecules in 10.0 gram of NaOH is 15 * 10²².
To solve this question, we need to understand some terms of mole concept,
Mole - It is the amount of substance containing same number of molecules or atoms as there are atoms in 12 gram of carbon-12 isotope.
Molecules - It is group of atoms bonded together, representing the smallest fundamental unit of a chemical compound taking part in chemical reaction.
Molecular weight - The sum of atomic masses of all atoms in molecules.
Avogadro number - It is the number of atoms, ions, electrons, molecules in one mole of substance. It is represented as NA.
NA = 6.0 * 10²³ (approx)
To calculate the number of molecules, we apply the formulae,
no. of molecules = moles * NA
moles = weight / molecular weight
moles = 10.0 / 40
= 0.25
Substituting this value to calculate number of molecules,
no. of molecules = 0.25 * 6.0 * 10²³
= 15 * 10²²
Therefore the number of molecules of in 10.0 g of NaOH is 15 * 10²².
To know more about moles and molecules,
https://brainly.com/question/29367909
How many atoms of each element are in one molecule of 2-heptanone?
Answers
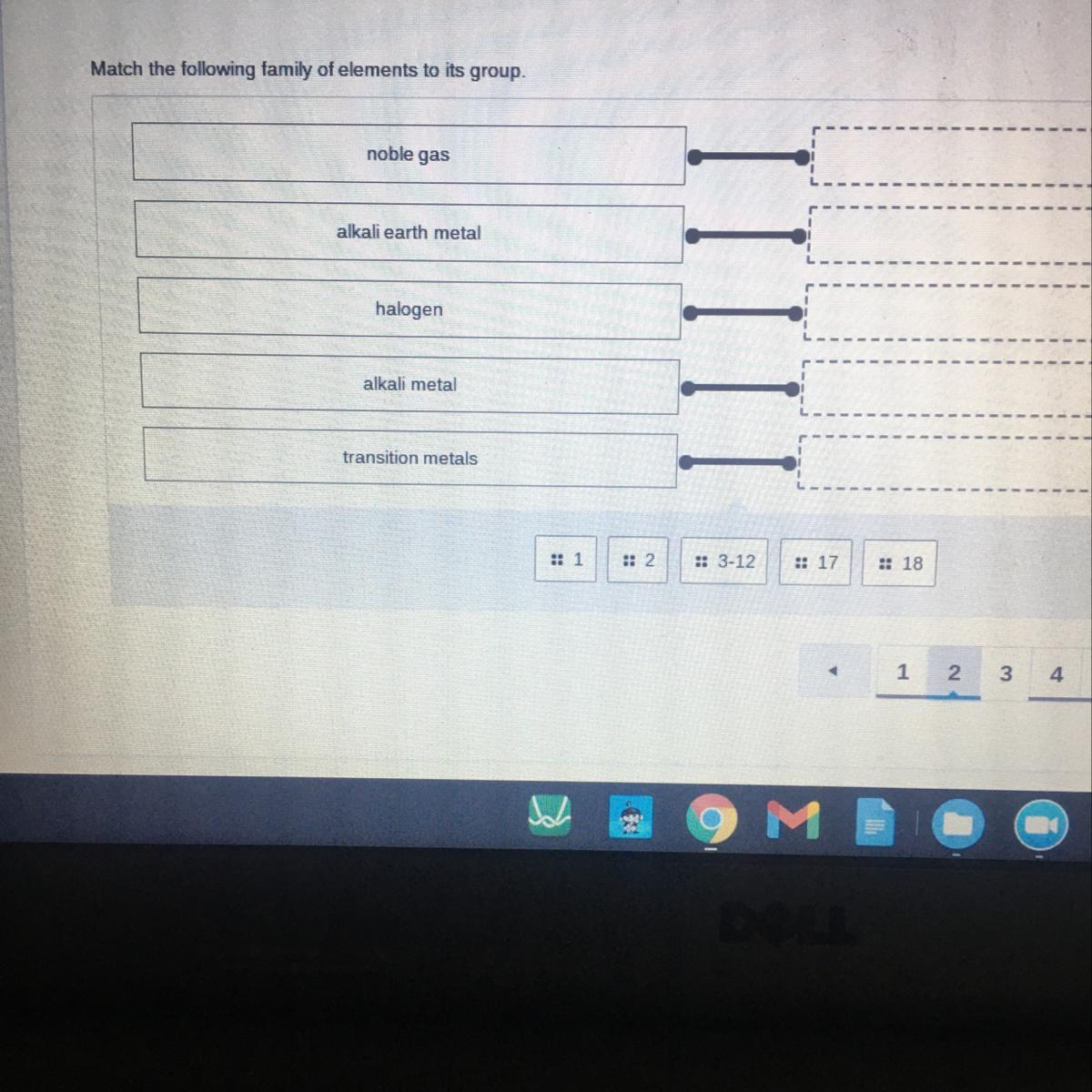
Match the following family of elements to its group.
Answers
Explanation:
To solve this problem, we simply use the periodic table of elements which groups elements based on their atomic numbers.
The atomic number of an element is the number of protons it contains. The protons are the positively charged particles within an atom.
The vertical arrangement of elements on the periodic table is the group. The horizontal arrangement of elements is the period.Now;
Noble gases belongs to group 18
Alkali earth metals belongs to group 2
Halogens belongs to group 17
Alkali metals belongs to group 1
Transition metals belongs to group 3-12
Argon (Ar): (Ne) 3s2 3p6
O longhand notation
O noble-gas notation
Answers
Answer:
It is Noble-gas notation
Explanation:
Just did it on Edgenuity 2020
Two or more than two atoms with different physical or chemical properties can not combine together to form an element. Therefore, the given electronic configuration represents noble-gas notation.
What is element?Element generally consist of atoms or we can atoms combine to form element. Atoms of an element is always same, means all the properties of all atoms of one type of element is same.
The systematic distribution of electrons in the various atomic orbitals is called its electronic configuration. The atomic number of argon is 18. The electronic configuration of argon is (Ne) 3s² 3p⁶. 1,2,3 represents the number of shells and s and represents the orbitals. The superscripts represents the number of electrons in each orbitals. The given electronic configuration represents noble-gas notation.
Therefore, the given electronic configuration represents noble-gas notation.
To know more about element, here:
brainly.com/question/8460633
#SPJ2
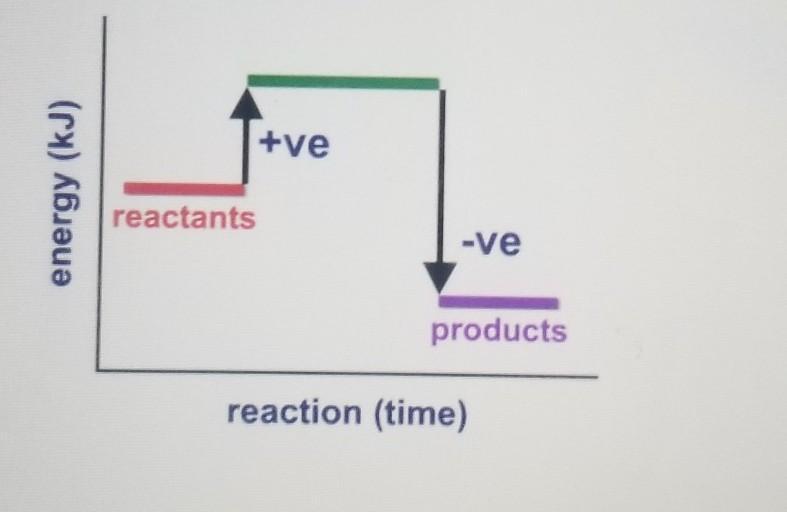
What does the following energy
diagram represent?
A. Exothermic reaction
B. Activation energy
C. Endothermic reaction
D. Specific heat capacity
Answers
Answer:
Option A) Exothermic Reaction
Explanation:
In exothermic reaction, the energy is released. The reactants are at high energy while the products are at low energy as shown in the graph.
Answer:
A.) Exothermic reaction
Explanation:
I got it correct on founders edtell
What effect could the pollution of Groundwater have on a nearby River, Lake or Stream?
Answers
Answer:
please give me brainlist and follow
Explanation:
Contamination of ground water can result in poor drinking water quality, loss of water supply, degraded surface water systems, high cleanup costs, high costs for alternative water supplies, and/or potential health problems. The consequences of contaminated ground water or degraded surface water are often serious.
Which atom (magnesium or chlorine) is larger? _______________________(you should also be prepared to answer the question if asked for the smaller atom)3a. Explain why the atom is larger. Include the following terms in your answer: protons, electrons, shells or layers, columbic attractions
Answers
Answer:
The magnesium atom is larger.
Explanation:
The magnesium atom is larger because it is on the left side of the Periodic Table (period 3 and group 2) where the atomic radius is larger.
Atomic radius is the distance from the center of the nucleus of an atom to the most external electron shell.
The greater the columbic attractions, the closer the protons in the nucleus are to the electrons in the outer layers, making the size of the atom smaller.
if you burn a 1.49g sample of b2h6, and if you collect the water vapor in a 4.25 l flask at 30*c, what will be the pressure of h2o(g) be in the glass?
Answers
Boron hydride (B2H6) reacts with oxygen to produce water and boron trioxide. If a 1.49 g sample of B2H6 is burned and the water vapor is collected in a 4.25 L flask at 30°C, what will the pressure of the H2O(g) be in the flask?Solution: The amount of water formed from B2H6 can be calculated using the balanced chemical equation.
The chemical equation:
2 B2H6(g) + 6 O2(g) → 4 H2O(g) + B2O3(s)
The molar mass of
B2H6 is (2 x 10.81 g/mol) + (6 x 1.01 g/mol) = 27.98 g/mol.
The number of moles of B2H6 in 1.49 g can be calculated by dividing the mass by the molar mass:1.49 g / 27.98 g/mol = 0.0532 mol.Since each mole of B2H6 reacts to form 4 moles of H2O, the number of moles of H2O formed will be:
0.0532 mol B2H6 x (4 mol H2O / 2 mol B2H6) = 0.106 mol H2O
The volume of the flask is 4.25 L and the temperature is 30°C, which is 303 K. The ideal gas law can be used to calculate the pressure of H2O(g) in the flask:P = nRT/V where P is the pressure, n is the number of moles of gas, R is the gas constant (0.08206 L atm/mol K), T is the temperature in Kelvin, and V is the volume of the container. Plugging in the values:
P = (0.106 mol)(0.08206 L atm/mol K)(303 K)/(4.25 L) = 0.639 atm
In this problem, we are asked to determine the pressure of H2O(g) in a flask after a 1.49 g sample of B2H6 is burned and the water vapor is collected. We can use stoichiometry to determine the number of moles of water formed from the combustion of B2H6, and the ideal gas law to determine the pressure of H2O(g) in the flask.Let's start by writing the balanced chemical equation for the combustion of B2H6:
2 B2H6(g) + 6 O2(g) → 4 H2O(g) + B2O3(s)
From this equation, we can see that each mole of B2H6 reacts to form four moles of H2O. The molar mass of B2H6 is: (2 x 10.81 g/mol) + (6 x 1.01 g/mol) = 27.98 g/mol.
The number of moles of B2H6 in the sample is:
1.49 g / 27.98 g/mol = 0.0532 mol
Since each mole of B2H6 produces four moles of H2O, the number of moles of H2O produced is:
0.0532 mol B2H6 x (4 mol H2O / 2 mol B2H6) = 0.106 mol H2O
The volume of the flask is 4.25 L, and the temperature is 30°C, which is 303 K. We can use the ideal gas law to calculate the pressure of H2O(g) in the flask:P = nRT/V where P is the pressure, n is the number of moles of gas, R is the gas constant (0.08206 L atm/mol K), T is the temperature in Kelvin, and V is the volume of the container.Substituting the values we have calculated, we get:
P = (0.106 mol)(0.08206 L atm/mol K)(303 K)/(4.25 L) = 0.639 atm
Therefore, the pressure of H2O(g) in the flask is 0.639 atm.
The pressure of H2O(g) in the flask after a 1.49 g sample of B2H6 is burned and the water vapor is collected in a 4.25 L flask at 30°C is 0.639 atm.
To learn more about stoichiometry visit:
brainly.com/question/28780091
#SPJ11
recommended material for building
Answers
Answer:
Steel is a popular material for building because it is strong without being extremely heavy. This makes it the ideal material for large, multi-story buildings and manufacturing and industrial facilities. Unlike wood, steel can stand up to moisture and is not susceptible to termites and fire
Explanation:
mark me brainliest!!
determine the percent yield of a reaction that produces 22.6 g of fe when 50.00 g of fe2o3 react with excess al according to the following reaction. Molar Mass Fe2O3 = 159.7 g/mol
Answers
The percent yield of the reaction is 82%
First step: make the chemist equation.
2 Al (s) + Fe2O3 (s) → 2 Fe (s) + Al2O3 (s)
As the statement says that aluminium is in excess, the limiting reactant is the Fe₂O₃.
Second step: Find out the moles in the reactant.
Molar weight Fe2O3: 159.7 g/m
Mass / Molar weight = moles
50 g /159.7 g/m = 0.313 moles
Third step: Analyse the reaction. 1 mol of Fe2O3 makes 2 moles of Fe.
1 mol Fe2O3 ____ 2Fe
0.313 mol Fe2O3 ____ 0.626 moles
Molar weight Fe = 55.85 g/m
Moles . molar weight = mass
55.85g/m . 0.626m = 34.9 grams
This will be the 100% yield of the reaction but we only made 22.6 g
34.9 g ____ 100%
22.6 g ____ 64.75 %
Hence, the percent yield is 64.75 %.
Learn more about percent yield from the link given below.
https://brainly.com/question/14903519
#SPJ4
Which of the following is NOT correct?A. Calorimetry involves an insulated container holding a liquid, usually water.B. An object of high temperature is placed into the calorimeter.C. The temperature change of the object and the water will be equal.D. The heat absorbed by the water is equal to the heat lost by the object.E. Calorimetry is a process that measures the heat released or absorbed during a chemical or physical change.
Answers
Among the given options, the statement that is NOT correct is "B. An object of high temperature is placed into the calorimeter."
An object of high temperature is placed into the calorimeter is not correct. Calorimetry is a process that measures the heat released or absorbed during a chemical or physical change. It involves an insulated container holding a liquid, usually water. The temperature change of the object and the water will be equal. The heat absorbed by the water is equal to the heat lost by the object. Calorimetry is an experimental method used to measure the heat energy produced or absorbed during a chemical or physical change. Calorimetry works on the principle of heat transfer between a reaction or physical process and a heat sink or calorimeter. Calorimetry involves an insulated container holding a liquid, usually water, and a thermometer used to measure the change in temperature of the water caused by the addition of heat. When heat is added to or removed from a system, it causes a temperature change in the system. Calorimetry is used in various fields such as food and beverage, medical research, material science, and others.
Learn more about calorimetry on:
https://brainly.com/question/3609481
#SPJ11
process of heat treatment and it's meaning
Answers
Answer:
Heat treatment is the process of heating metal without letting it reach its molten, or melting, stage, and then cooling the metal in a controlled way to select desired mechanical properties. Heat treatment is used to either make metal stronger or more malleable, more resistant to abrasion or more ductile.
Under certain conditions the formation of ammonia from nitrogen and hydrogen has a 7.82% yield. Under these conditions, how many grams of NH3 will be produced from the reaction 25.0 g N2 with 2.00 g H2?
Answers
The grams of NH₃ produced in the reaction is approximately 0.879 g.
To calculate the grams of NH₃ produced, we need to determine the limiting reactant and then calculate the theoretical yield.
1. Calculate the moles of N₂:
Molar mass of N₂ = 28.02 g/mol
moles of N₂ = mass / molar mass = 25.0 g / 28.02 g/mol ≈ 0.892 mol N₂
2. Calculate the moles of H₂:
Molar mass of H₂ = 2.02 g/mol
moles of H₂ = mass / molar mass = 2.00 g / 2.02 g/mol ≈ 0.990 mol H₂
3. Determine the mole ratio between N₂ and NH₂:
From the balanced equation for the formation of ammonia (NH3) from nitrogen (N₂) and hydrogen (H₂):
N₂ + 3H₂ -> 2NH₂
The mole ratio between N₂ and NH₃ is 1:2.
4. Identify the limiting reactant:
Compare the moles of N₂ and H₂ to identify the limiting reactant. The reactant that produces fewer moles of NH₃ will be the limiting reactant.
moles of NH₃ formed from N₂ = moles of N₂ × (2 moles of NH₃ / 1 mole of N₂) ≈ 0.892 mol N₂ × (2 mol NH₃ / 1 mol N₂) ≈ 1.784 mol NH₃
moles of NH₃ formed from H₂ = moles of H₂ × (2 moles of NH₃ / 3 moles of H₂) ≈ 0.990 mol H₂ × (2 mol NH₃ / 3 mol H₂) ≈ 0.660 mol NH₃
Since the moles of NH₃ formed from N₂ (1.784 mol NH₃) are higher than the moles of NH₃ formed from H₂ (0.660 mol NH₃), N₂ is the limiting reactant.
5. Calculate the theoretical yield of NH₃:
Theoretical yield of NH₃ = moles of NH₃ formed (limiting reactant) × molar mass of NH₃
Theoretical yield of NH₃ = 0.660 mol NH₃ × 17.03 g/mol ≈ 11.258 g NH₃
6. Calculate the actual yield:
The actual yield is given as 7.82% of the theoretical yield.
Actual yield of NH₃ = theoretical yield of NH₃ × (7.82 / 100) ≈ 11.258 g NH₃ × (7.82 / 100) ≈ 0.879 g NH₃
Therefore, the grams of NH₃ produced in the reaction is approximately 0.879 g.
Know more about ammonia:
https://brainly.com/question/29519032
#SPJ4
Identify which properties are common to each of the following chemical families
(a) alkali metals
(b) alkaline earth metals
(c) halogens
(d) noble gases
Answers
The noble gases have a full outer shell of valence electrons, making them stable and unreactive. They are colorless, odorless gases at room temperature and have very low boiling points. Their lack of reactivity makes them useful in a variety of applications, including lighting and welding.
The properties that are common to each of the following chemical families include:
(a) Alkali metals The alkali metals have a single valence electron in their outermost shell, which is easily lost to form an ion with a charge of +1. They are the most reactive metals, reacting with water and air to produce hydrogen gas and an oxide layer, respectively. They are silvery-white and have a soft texture.
(b) Alkaline earth metals The alkaline earth metals have two valence electrons in their outermost shell, which they readily lose to form ions with a charge of +2. They are less reactive than the alkali metals, but they still react with oxygen to form an oxide layer on their surface. They are also silvery-white in color and have a harder texture than the alkali metals.
(c) Halogens The halogens have seven valence electrons in their outermost shell, making them highly reactive nonmetals. They readily form ions with a charge of -1 by gaining an electron. They are diatomic molecules at room temperature and can be found in a variety of colors and states of matter.
(d) Noble gases The noble gases have a full outer shell of valence electrons, making them stable and unreactive. They are colorless, odorless gases at room temperature and have very low boiling points. Their lack of reactivity makes them useful in a variety of applications, including lighting and welding. These properties are common to each of the following chemical families.
To know more about noble gases visit:-
https://brainly.com/question/19024000
#SPJ11
write the formulas of alkanes that contain three, five, seven, and nine carbon atoms
Answers
Answer:
Explanation:
Name Number of Carbon Atoms Molecular Formula
methane 1 CH4
ethane 2 C2H6
propane 3 C3H8
butane 4 C4H10
Match each part of the atom with its identity from the list below.

Answers
Answer:
Nucleus: Choice C
Electron: Choice E
Proton: Choice A
Neutron: Choice B
Energy Level: Choice D
Explanation:
1. Nucleus contains the protons and neutrons.
2. Electrons surround the nucleus and have a negative charge.
3. Protons are positively charged and found in the nucleus.
4. Neutrons have a neutral charge and are found in the nucleus.
5. The energy level refers to the electron orbital.
in water, 1 mol of al(clo3)3 (aq) will dissociate into which ions?
Answers
In water, 1 mol of Al(ClO₃)₃ (aq) will dissociate into two moles of Al³⁺ ions and three moles of ClO³⁻ions.
In water, 1 mol of Al(ClO₃)₃ (aq) will dissociate into the following ions: Al³⁺and ClO³⁻.
When Al(ClO₃)₃ is dissolved in water, it will dissociate into its constituent ions. The Al³⁺ ion will separate from the three ClO³⁻ ions. This process is known as dissociation and is a common occurrence when ionic compounds are dissolved in water.
The resulting solution will contain Al³⁺ and ClO³⁻ions that are free to move around and interact with other ions in the solution. In summary, 1 mol of Al(ClO₃)₃ (aq) will dissociate into 1 mol of Al³⁺ ions and 3 mol of ClO³⁻ ions in water.
Learn more about dissociate at https://brainly.com/question/9187417
#SPJ11
What are the properties of covalent bonds and how do they differ from ionic bonds?
Answers
Answer:
Covalent bonds are formed by the sharing of electrons between two non-metallic atoms. These bonds are typically stronger and more stable than ionic bonds and are found in compounds such as water, hydrogen, and carbon dioxide. Covalent bonds differ from ionic bonds in that they do not involve the transfer of electrons from one atom to another.
Explanation:
Write a question you have about Titan or about methane that could help you determine what happened to the lake
Answers
Answer: what would swimming in mathane lake on titan feel like.
Explanation:
Both methane and titan are gases that are combustible in nature.
Those gases which are inflammable are called combustible gases.
For example:-
MethaneEthaneThese gases have various uses in daily life. these uses as follows:-
CookingDecompositionUsed as fuelThe gas will mix in the lake which harms the aquatic animals. The aquatic animals have a hard time to breath.
Hence, the lake will be polluted.
For more information, refer to the link:-
https://brainly.com/question/25068613
Darwin told us that science:
saves lives
Answers
Answer:yup!!
Explanation:
How many moles of silver are present in 30. 5 g of silver? Show your solution:))
Answers
Answer:
0.28 moles
Explanation:
molar mass of silver= 107.86
\(30.5 g Ag*\frac{1 mole Ag}{107.86 g Ag} =0.28 moles\)