Answers
The pH of acid is between 0-7 on pH scale while for base pH range is from 7-14. Therefore, the pH of 1.0 x 10⁻³ M of solution is 7.002. pH is a unitless quantity. Therefore, pH of acid is 1.88 and pOH of base is 12.12.
What is pH?pH is a measurement of amount of hydronium ion H₃O⁺ in a given sample. More the value of hydronium ion concentration, more will be the solution acidic.
On subtracting pH from 14, we get pOH which measures the concentration of hydroxide ion in a given solution. pH depend on the temperature. At room temperature pH scale is between 0 to 14. pH of neutral solution is 7.
LiOH + HCl \(\rightarrow\) LiCl + H\(_2\)O
moles of Lithium =7 grams÷6.941 g/mol
=1.00mole
total volume of solution = 7 liters + 0.5L
= 7.5l
concentration of HCl =1.00/7.5l=0.13mol/l
pH=-log [OH⁻ ]
substituting the values
pH=-log 0.13
pH=1.88
pOH= 14-pH
= 14-1.88
=12.12
Therefore, pH of acid is 1.88 and pOH of base is 12.12.
To learn more about pH, here:
https://brainly.com/question/27945512
#SPJ1
Related Questions
How many atoms are in 80.7 g of pure aluminum?
Answers
Answer:
Like Roman said Avogadro's number is the key to your problem. The value that you'll use on paper is 6.022x10^23. Luckily in our case a mol specifically refers to the amount of atoms. Its a weird concept but 1.97 mol of Aluminum is the exact same amount of atoms as say helium. The only differences lay in the total mass of the sample.
Drag each positive ion to bond it with a negative ion to form the neutral ionic compound indicated.

Answers
Answer:
1. NaCl
2. NH4F
3. MgO
4.LiCl
5. KI
6. CaO
Explanation:
In that order
A rectangular cube has a length of 1.2 cm, a width of 2.4
cm, and a height of 3.0 cm. Its mass is 21.6 g. What is its
density
Answers
Density = mass ÷ volume
Density = 21.6g ÷ (1.2 x 2.4 x 3.0)
Density = 21.6g ÷ 8.64cm3
Density = 2.5g/cm3 -> 2500kg/m3
Hope this makes sense! :)
A rectangular cube has a length of 1.2 cm, a width of 2.4 cm, and a height of 3.0 cm. Its mass is 21.6 g. The density of the cube is 2500kg/m3.
What is a cube?A cube is a three-dimensional shape that has six faces. It contains six faces of squares. The cube has 6 faces, 12 edges, and 8 vertices.
Density is the mass per unit volume. It is a scalar quantity, and it is calculated by dividing mass by volume.
Given the length is 1.2 and width is 2.4 and the height is 3.0 cm. So the volume of the cube will be multiplying these values.
The mass is given 21.6 g.
Density = mass / volume
Putting the values in the formula
Density = 21.6g / (1.2 x 2.4 x 3.0)
Density = 21.6g / 8.64cm3
Density = 2.5g/cm3 = 2500 kg/m3
Thus, the density of the cube is 2500 kg/m3.
To learn more about density of cube, refer to the link:
https://brainly.com/question/14585774
#SPJ6
Oxygen is represented by a red sphere. Nitrogen is represented by a blue sphere. Hydrogen is represented by a white sphere. In the reactants there are five molecules that contain two white spheres and four molecules that contains one red and one blue sphere. In the products there are two molecules that contain two blue spheres, four molecules that contain one red and two white spheres, and one molecule that contains two white spheres.
What is the chemical formula for the limiting reactant in the reaction shown?
Answers
Answer:
5H2 + 4NO = 2N2 +4H2O + H2
Explanation:
Reactants:
Five molecules that contains two hydrogen = 5H2
Four molecules that contains one nitrogen and one oxygen = 4NO
Products:
Two molecules that contains two nitrogen = 2N2
Four molecules that contains one oxygen and two hydrogen = H2O
One molecules that contains two hydrogen = 1H2 or H2
The balanced chemical equation for the reaction will be: 5 H₂ + 4 NO → 2 N₂ + 4 H₂O + H₂, and there is no limiting reactant in this reaction.
To write the balanced chemical equation for the reaction, let's first identify the reactants and products based on the information provided:
Reactants:
5 molecules contain 2 white spheres (Hydrogen): 5 H₂
4 molecules contain 1 red (Oxygen) and 1 blue (Nitrogen) sphere: 4 NO
Products:
2 molecules contain 2 blue spheres (Nitrogen): 2 N₂
4 molecules contain 1 red (Oxygen) and 2 white (Hydrogen) spheres: 4 H₂O
1 molecule contains 2 white spheres (Hydrogen): 1 H₂
Now, let's write the balanced chemical equation by ensuring that the number of atoms of each element is the same on both sides of the equation:
Reactants: 5 H₂ + 4 NO
Products: 2 N₂ + 4 H₂O + 1 H₂
The balanced chemical equation for the reaction is:
5 H₂ + 4 NO → 2 N₂ + 4 H₂O + H₂
Now, let's determine the limiting reactant:
From the balanced equation, we can see that the stoichiometric ratio between H₂ and NO is 5:4. This means that for every 5 molecules of H₂, we need 4 molecules of NO for the reaction to proceed completely.
Since we have 5 molecules of H₂ and 4 molecules of NO, both reactants are present in the exact stoichiometric ratio required for the reaction. Therefore, neither H₂ nor NO is in excess, and both will be fully consumed during the reaction. As a result, there is no limiting reactant in this reaction.
To know more about limiting reactant here
https://brainly.com/question/33417913
#SPJ2
--The given question is incomplete, the complete question is
"Oxygen is represented by a red sphere. Nitrogen is represented by a blue sphere. Hydrogen is represented by a white sphere. In the reactants there are five molecules that contain two white spheres and four molecules that contains one red and one blue sphere. In the products there are two molecules that contain two blue spheres, four molecules that contain one red and two white spheres, and one molecule that contains two white spheres. What is the chemical formula for the limiting reactant in the reaction shown? chemical formula: Write the balanced chemical equation for the reaction, using lowest whole-number coefficients."--
"Calculate the pH of a 0.15 M HBr solution AND classify the solution of acidic, neutral, and basic"
a. 0.82 basic
b. 0.82 acidic
c. 13.9 basic
d.13.9 acidic
Answers
Answer:
la respuesta es la letra: b
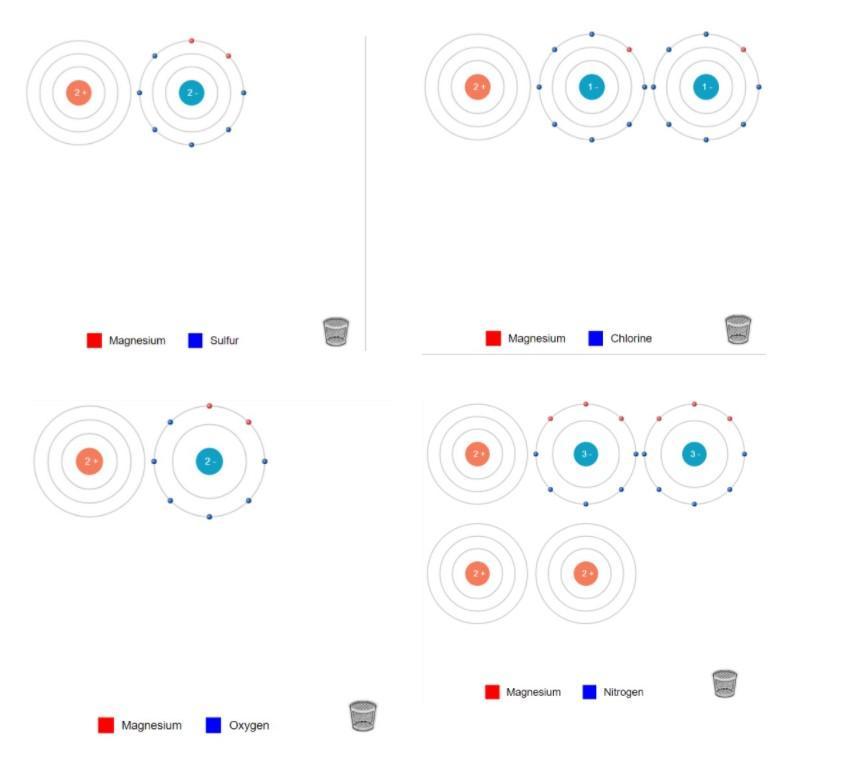
can someone help me with my chemistry homework please???

Answers
1.) Lithium and Sulfide:
Formula: \(\bold{Li_{2}S}\)Ion Charges: \(\bold{Li~1+,~Li~1+,~S~2-}\)2.) Lithium and Chlorine:
Formula: \(\bold{2LiCl}\)Ion Charges: \(\bold{Li~1+, Li~1+,Cl~1-,Cl~1-}\)3.) Lithium and Oxygen:
Formula: \(\bold{Li_{2}O}\)Ion Charges: \(\bold{Li~1+,Li~1+,O~2-}\)4.) Lithium and Nitrogen:
Formula: \(\bold{Li_{3}N}\)Ion Charges: \(\bold{Li~1+,Li~1+,Li~1+,N~3-}\)5.) Magnesium and Sulfur:
Formula: \(\bold{MgS}\)Ion Charges: \(\bold{Mg~2+,S~2-}\)6.) Magnesium and Chlorine:
Formula: \(\bold{MgCl_2}\)Ion Charges: \(\bold{Mg~2+,Cl~1-,Cl~1-}\)7.) Magnesium and Oxygen:
Formula: \(\bold{MgO}\)Ion Charges: \(\bold{Mg~2+,O~2-}\)8.) Magnesium and Nitrogen:
Formula: \(\bold{Mg_3N_2}\)Ion Charges: \(\bold{Mg~2+,Mg~2+,Mg~2+,N~3-,N~3-}\)Explanation:______________________________
Lithium and Sulfur: In order to make Lithium Sulfide, There must be 2 Lithium and 1 Sulfur. You transfer the electrons from both Lithium's to the Sulfur.Lithium and Chlorine:In order to make Lithium Chloride, There must be 2 Lithium and 2 Chlorine. You transfer the electrons from both Lithium's to the Chlorines, (One electron for each chlorine.)Lithium and Oxygen:In order to make Lithium Oxide, There must be 2 Lithium and 1 Oxygen. You transfer the electrons from both Lithium to Oxygen. Lithium and Nitrogen:In order to make Lithium Nitride, There must be 3 Lithium and 1 Nitrogen. You transfer the electrons from all 3 Lithium to Nitrogen. Magnesium and Sulfur:In order to make Magnesium Sulfide, There must be 1 Magnesium and 1 Sulfur. You transfer the both electrons from Magnesium to Sulfur. Magnesium and Chlorine:In order to make Magnesium Chloride, There must be 1 Magnesium and 2 Chlorine. You transfer on electron to each Chlorine. Magnesium and Oxygen:In order to make Magnesium Oxide, There must be 1 Magnesium and 1 Oxygen. You transfer both electrons from Magnesium to Oxygen. Magnesium and Nitrogen:In order to make Magnesium Nitride, There must be 3 Magnesium and 2 Nitrogen. You transfer 3 electrons from Magnesium to Nitrogen.______________________________

A 1.50 gram sample of contain 3.32g of CO2 1.58g of N2O5 and 1.865g of H2O .Its molar mass is 102.2g/mol. Determine the emperical and molecular formulas.
Answers
The correct answer for emperical and molecular formula = C5H14N2 .
3.23 g x (12.011 / 44.0098) = 0.8815 g carbon
1.865 g x (2.016 / 18.0152) = 0.2087 g Hydrogen
1.58 g x (28.014 / 108.009) = 0.4098 g Nitrogen
1.50 g minus (0.8815 + 0.2087 + 0.4098) = 0.
Convert each element's mass to moles.
0.8815 g/12.011 g/mol = 0.0734 mol carbon
0.2087 g 1.008 g/mol = 0.207 mol Hydrogen
0.4098 g 14.007 g/mol = 0.02926 mol Nitrogen
Step three is to calculate the ratio of molar amounts expressed in the smallest, whole numbers.
0.0734 mol 0.02926 mol = 2.51 carbon
7.07 mol hydrogen = 0.207 mol 0.02926 mol
Nitrogen: 0.02926 mol = 1 Nitrogen: 0.02926 mol = 1
Doubling each value yields C = 5, H = 14.14, and N = 2, resulting in the empirical formula C5H14N2.
Learn more about emperical formula here :-
https://brainly.com/question/22890345
#SPJ9
What is the molarity of a solution containing 2.50 moles in 35 mL of solution?
Answers
Answer: 71.43 M.
Explanation: To calculate the molarity of a solution, we divide the number of moles of solute by the volume of the solution in liters. The volume of the solution in this case is given in milliliters, so we need to convert it to liters before we can calculate the molarity.
35 mL = 0.035 L (since 1 mL = 0.001 L)
So, the molarity of the solution can be calculated as follows:
Molarity = moles of solute / volume of solution in liters
Molarity = 2.50 moles / 0.035 L
Molarity = 71.43 M
Therefore, the molarity of the solution is 71.43 M.
Given Kc = 2367 at 999 K, calculate Kp for the reaction at equilibrium: CS₂(g) + 3Cl₂(g) → S₂Cl3(g) + CCl4(8) R = 0.08206 L atm K-¹ mol-¹
Answers
The value of Kp for the given reaction at equilibrium is approximately 192,986.689 L atm mol⁻¹.
To calculate the equilibrium constant Kp for the given reaction, we can use the relationship between Kc and Kp, which is expressed as:
Kp = Kc * (RT)^Δn
Where:
- Kp is the equilibrium constant in terms of partial pressures.
- Kc is the equilibrium constant in terms of concentrations.
- R is the ideal gas constant (0.08206 L atm K⁻¹ mol⁻¹).
- T is the temperature in Kelvin.
- Δn is the change in the number of moles of gas (sum of products - sum of reactants).
In this case, the reaction involves four moles of gas on the left-hand side (reactants) and five moles of gas on the right-hand side (products). Therefore, Δn = 5 - 4 = 1.
Given that Kc = 2367 at 999 K, we can substitute these values into the equation:
Kp = 2367 * (0.08206 L atm K⁻¹ mol⁻¹ * 999 K)^1
Simplifying the expression:
Kp = 2367 * (81.367 L atm mol⁻¹)
Calculating the product:
Kp ≈ 192,986.689 L atm mol⁻¹
Therefore, the value of Kp for the given reaction at equilibrium is approximately 192,986.689 L atm mol⁻¹.
For more questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
Heredity Lab Report Instructions:
In the Heredity lab, you investigated how hamsters inherit traits from their parents. Record your observations in the lab report below. You will submit your completed report.
Name and Title: Include your name, instructor's name, date, and name of lab.
Objective(s): In your own words, what was the purpose of this lab?
Hypothesis: In this section, please include the if/then statements you developed during your lab activity.
These statements reflect your predicted outcomes for the experiment.
Test One: If I breed a short fur, FF female with a short fur, Ff male, then I will expect to see (all short fur; some short and some long fur; all long fur) offspring.
Test Two: If I breed a short fur, Ff female with a short fur, Ff male, then I will expect to see (all short fur; some short and some long fur; all long fur) offspring.
Test Three: If I breed a long fur, ff female with a long fur, ff male, then I will expect to see (all short fur; some short and some long fur; all long fur) offspring.
Procedure: The procedures are listed in your virtual lab. You do not need to repeat them here.
Please be sure to identify the test variable (independent variable) and the outcome variable (dependent variable) for this investigation. Remember, the test variable is what is changing in this investigation.
The outcome variable is what you are measuring in this investigation.
Test variable (independent variable): Outcome variable (dependent variable): Data: Record the data from each trial in the data chart below. Be sure to fill in the chart completely. Test One Parent 1: FF Parent 2: Ff Phenotype ratio: ________ : ________ short fur : long fur Test Two Parent 1: Ff Parent 2: Ff Phenotype ratio: ________ : ________ short fur : long fur Test Three Parent 1: ff Parent 2: ff Phenotype ratio: ________ : ________ short fur : long fur Conclusion: Your conclusion will include a summary of the lab results and an interpretation of
Answers
For Test One, phenotype ratio is Short fur : Long fur = 2 : 0; For Test Two, the phenotype ratio is Short fur : Long fur = 3 : 1; For Test Three, the phenotype ratios will be Short fur : Long fur = 0 : 2
What are the phenotype ratios from the test crosses?For Test One:
Parent 1: FF (homozygous dominant for short fur)
Parent 2: Ff (heterozygous for short fur)
The Punnett square for this cross will give the following genotype ratios:
FF : Ff = 1 : 1
And the corresponding phenotype ratios will be:
Short fur : Long fur = 2 : 0 or 100% short fur
For Test Two:
Parent 1: Ff (heterozygous for short fur)
Parent 2: Ff (heterozygous for short fur)
The Punnett square for this cross will give the following genotype ratios:
FF : Ff : ff = 1 : 2 : 1
And the corresponding phenotype ratios will be:
Short fur : Long fur = 3 : 1 or 75% short fur and 25% long fur
For Test Three:
Parent 1: ff (homozygous recessive for long fur)
Parent 2: ff (homozygous recessive for long fur)
The Punnett square for this cross will give the following genotype ratios:
ff : ff = 1 : 0
And the corresponding phenotype ratios will be:
Short fur : Long fur = 0 : 2 or 100% long fur
For this investigation, the test variable is the breed of hamster and the outcome variable is the phenotype of the hamster.
Learn more about heredity at: https://brainly.com/question/930755
#SPJ1
Calculate the number of molecules of Freon-12 in 4.75 mg of Freon-12. What is the mass of chlorine in 4.75 mg of Freon-12?
Answers
The mass of chlorine in 4.75 mg of Freon-12 is approximately 1.39 x \(10^{-3}\)g.
What is Mass?
Mass is a fundamental property of matter that measures the amount of substance or material present in an object. It is a scalar quantity and is typically measured in units such as grams (g) or kilograms (kg) in the metric system, or pounds (lb) or ounces (oz) in the imperial system.
Freon-12, also known as dichlorodifluoromethane, is a chlorofluorocarbon (CFC) compound that was used as a refrigerant and propellant in aerosol products. Its chemical formula is CCl2F2.
The atomic masses of carbon (C), chlorine (Cl), and fluorine (F) are approximately 12.01 g/mol, 35.45 g/mol, and 18.998 g/mol, respectively.
The molar mass of Freon-12 (CCl2F2) is:
Molar mass of Freon-12 = 12.01 g/mol (C) + 2 x 35.45 g/mol (Cl) + 2 x 18.998 g/mol (F)
= 120.91 g/mol
Next, we use the formula:
moles = mass / molar mass
to calculate the number of moles of Freon-12 in 4.75 mg:
moles of Freon-12 = 4.75 mg / 120.91 g/mol
= 3.92 x 10^-5 mol
Finally, we use Avogadro's number to calculate the number of molecules of Freon-12:
Number of molecules = moles of Freon-12 x Avogadro's number
= 3.92 x\(10^{-5}\) mol x 6.022 x\(10^{23\) molecules/mol
= 2.35 x \(10^{19}\) molecules
To calculate the mass of chlorine in 4.75 mg of Freon-12, we can use the molar mass of chlorine (Cl) and the moles of Freon-12 calculated above.
The molar mass of chlorine (Cl) is 35.45 g/mol.
Mass of chlorine in Freon-12 = moles of Freon-12 x molar mass of chlorine
= 3.92 x \(10^{-5}\) mol x 35.45 g/mol
= 1.39 x \(10^{-3}\) g
Learn more about Mass from the given link
https://brainly.com/question/86444
#SPJ1
Engineers find a new metal that is stronger than steel.. (so on)
Answer is : D "Collaborate with other engineers in the design of a plane made from this metal".
Answers
Answer:
Explanation:
same thing as normal plane. just replace normal metal with this new sick metal :cool:
also make sure it weighs a good amount and won't fall from weight... im confused was this already answered???
Answer:
Collaborate with other engineers in the design of a plane made from this metal
Explanation:
<3
Calculate the number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water.
Answers
The number of moles of gas produced from the reaction of 2.00g of potassium with an excess amount of water is 0.025 moles.
The reaction of potassium with an excess amount of water is:
2K + 2H\(_2\)O \(\rightarrow\) 2KOH + H\(_2\)
To calculate the moles of hydrogen gas first we need to calculate moles of potassium in 2.00g
No. of moles = (mass) / (molecular mass)
The mass given is 2.00 g and the Molecular mass is 39.09 units
∴ No. of moles = (2) / (39.09) = 0.05
From the above reaction, we get that 2 moles of potassium give 1 mole of hydrogen gas. Thus, 0.05 moles of potassium gives 0.025 moles of hydrogen gas.
Therefore, the no. of moles of hydrogen gas produced is 0.025 moles.
To learn more about potassium,
brainly.com/question/13321031
A box of pills claims that they will make your cold go away sooner. Who
would be the most impartial source for information on this claim?
A. The company that makes the medicine
B. The store that sells the medicine
C. A company that makes a competing medicine
.
D. A university study that tests medicine
Answers
Answer:D
Explanation:
Which of the following is not a base :CaSO4, Al(OH)3, Ca(OH)2, KOH
Answers
Answer:
among these which is not a base is CaSO4
Brainly!!!
which element has three occupied quantum shells with 4 electrons in the outermost shell
Answers
Answer:
Argon. If you look at the periodic table, the noble gases (column on the furthest right of the table - labeled column 18) have fully filled electron shells. This is why noble gases (helium, neon, argon, krypton, xenon, and radon) are not very reactive with other elements - they are happy with their fully filled electron shells. So, the third element down on the noble gas column (Argon) has 3 fully filled electron shells. Periodic table courtesy of Wikipedia
(taken from Quora)
Write a balanced nuclear equation for the beta decay of 234/90Th

Answers
The balanced nuclear equation for the beta decay of 234/90Th can be represented as follows: ^234/90Th --> ^234/91Pa + e^0/-1β
In this equation, the nucleus of thorium-234 (234/90Th) undergoes beta decay. During beta decay, a neutron within the nucleus is converted into a proton, resulting in the emission of an electron (beta particle). As a result, the thorium-234 nucleus is transformed into protactinium-234 (234/91Pa) by gaining one proton.
The beta particle emitted during the decay process is represented as e^0/-1β, where the superscript 0 denotes that the electron has no charge (neutral), and the subscript -1 indicates that the electron carries a negative charge of -1.
It is important to note that in a nuclear equation, the total atomic mass and atomic number on both sides of the equation must be equal to maintain a balanced equation and conserve mass and charge.
For more such questions on balanced nuclear equation visit:
https://brainly.com/question/31505524
#SPJ8
Which of the following gives the correct numbers of protons, neutrons, and
electrons, respectively, in a neutral atom of Potassium?
A) 19 protons, 20 neutrons, 19 electrons
B) 39 protons, 20 neutrons, 39 electrons
C) 20 protons, 19 neutrons, 19 electrons
D) 39 protons, 20 neutrons, 19 electrons
Answers
Answer:
A
Explanation:
because in neutral proton equals to electron
The correct option is A. Potassium has 19 protons, 20 neutrons, and 19 electrons.
What is potassium?
Potassium is an element. It has protons and electrons. potassium has a nucleus. The nucleus contains protons and neutrons. Electrons revolve around the nucleus.
Proton is a positively charged atom and an electron is negatively charged and revolves around the atom. Neutron is neutral in charge.
The atomic number of potassium is 19. So it has 19 protons and 20 neutrons and 19 electrons.
Therefore, The correct option is A. Potassium has 19 protons, 20 neutrons, and 19 electrons.
To learn more about potassium refer to the link:
https://brainly.com/question/13321031
#SPJ2
I would really appreciate someone could answer this question for mee! I will mark brainliest aswell. :)
Which statement is true about the total mass of the reactants during a chemical change?
A)It is destroyed during chemical reaction
B)It is less than the total mass of the products.
C)It is equal to the total mass of the products.
D)It is greater than the total mass of the products.
Answers
Answer:
c
Explanation:
mass is like matter it cant be created or destroyed so mass stays the same.
Answer:
C) It is equal to the total mass of the products.
Explanation:
The law of conservation of mass states that in a chemical reaction, the total mass of reactants is equal to the total mass of products.
- profparis
A teaspoon of dry coffee crystals dissolves when mixed in a cup of hot water. This process produces a coffee solution. Which term best identifies how the original crystals are classified?
-Reactant
-product
-solvent
-a solute
Answers
Answer:
The correct answer is - solute.
Explanation:
A solute is one half of a solution that is dissolved in the other half of the solution, known as the solvent. Solute particles are always less or lower in quantity or concentration than the solvent in a solution.
In this case, coffee crystals are dissolved in hot water so the solute will be coffee crystals and the amount of coffee crystals are also less than the amount of solvent.
Answer:
solute
Explanation:
You make Kool- Aid. Unfortunately, you misread the directions and made 0.512 L at a concentration of 13.2 M. In order for it to taste good it needs to be only 2.00 M. How much water do you need to add to make taste good? Show your work.
Answers
To dilute the Kool-Aid solution to a concentration of 2.00 M, we need to calculate the volume of water (Vw) that needs to be added.
The initial concentration of Kool-Aid solution is 13.2 M, and the initial volume is 0.512 L. Let's assume that Vw is the volume of water we need to add to dilute the solution to a concentration of 2.00 M.
Since the total volume after dilution will be the sum of the initial volume of Kool-Aid solution and the volume of water added, we can set up the following equation based on the dilution formula:
C1V1 = C2V2
where
C1 = initial concentration of Kool-Aid solution = 13.2 M
V1 = initial volume of Kool-Aid solution = 0.512 L
C2 = final concentration of Kool-Aid solution = 2.00 M
V2 = final volume of Kool-Aid solution after adding water = 0.512 L + Vw
Substituting the given values into the equation, we get:
13.2 M x 0.512 L = 2.00 M x (0.512 L + Vw)
Simplifying the equation, we get:
6.7584 L·M = 1.024 L·M + 2.00 M·Vw
Subtracting 1.024 L·M from both sides, we get:
5.7344 L·M = 2.00 M·Vw
Dividing both sides by 2.00 M, we get:
Vw = 2.8672 L
Therefore, we need to add 2.8672 L of water to 0.512 L of 13.2 M Kool-Aid solution to dilute it to a concentration of 2.00 M.
Know more about Kool-Aid solution here:
https://brainly.com/question/27297342
#SPJ11
Write the name of the alkane with six carbon atoms
Answers
Hexane is an alkane with six carbon atoms.
Hexane is an organic substance having the chemical formula C6H14. It is a straight-chain alkane containing six carbon atoms. When pure, it is an odorless, colourless liquid with a boiling point of roughly 69 °C (156 °F). It is a broadly applicable non-polar solvent that is quite safe, mainly unreactive, affordable, and easily evaporable.
Learn more about Hexane here:
https://brainly.com/question/12368227
#SPJ9
if a solution has a pOH of 2.85 what is the hidrogen ion?a. 1.38x10^-3mb. 7.24x10^-12mc. 724md. 3.50x10^-15m
Answers
To find the concentration of hydrogen ion from the pOH we need two important informations, first is that pOH + pH = 14, this is a constant number, therefore, the pH in our question needs to be pH = 14 - 2.85
pH = 11.15, now we have the pH for the question, now we can use to find the concentration of H+, which represents the acidity of a solution.
In order to find which was the correct option I have used the "pH = -log [H+]" formula, which tells us that the pH is equal to the negative log of the concentration of hydrogen ions.
a. pH = -log [1.38*10^-3] =
pH = 2.86
b. pH = -log [7.24*10^-12]
pH = 11.14
c. pH = -log [7.24*10^2]
pH = -2.86
d. pH = -log [3.50*10^-15]
pH = 14.4
From this data, we can see that letter B is the correct one
Another trick that helped me know the answer before the calculations, was to have in mind that the pOH was low, therefore the pH must be a high value, and therefore the concentration of H+ has to be a really small concentration, the one that fitted best was letter B
A student is asked to calculate the density of a small rock. An electronic scale
measures the mass of the rock as 12.1 grams. The student puts the rock in a large
graduated cylinder that is partially filled with water. The water level in the
graduated cylinder before the rock is added is 24.2 ml, and after the rock is added,
the level rises to 28.8 mL. What is the density of the rock, stated in grams per cubic
centimeter?
Answers
Answer:
\(\boxed {\boxed {\sf \rho \approx 2.63 \ g/cm^3}}\)
Explanation:
The density of an object is found by dividing the mass by the volume.
\(\rho= \frac {m}{v}\)
The mass is 12.1 grams, but we are not given the volume. Instead, we are told that the water before is 24.2 millilitres and the water after is 28.8 millilitres.
So, the volume was taken using water displacement. We can find the volume by subtracting the initial volume from the volume after the object was added.
v= with object - initial v= 28.8 mL-24.2 mL=4.6 mLNow we know the mass and volume:
\(m= 12.1 \ g \\v= 4.6 \ mL\)
Substitute the values into the formula.
\(\rho= \frac {12.1 \ g }{ 4.6 \ mL}\)
Divide.
\(\rho= 2.63043478261 \ g/mL\)
Let's round to the nearest hundredth. The 0 in the thousandth place tells us to leave the 3.
\(\rho \approx 2.63 \ g/mL\)
1 milliliter is equal to 1 cubic centimer, so the density can also be written as:
\(\rho \approx 2.63 \ g/cm^3\)
The density of the rock is approximately 2.63 grams per cubic centimeter.
a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
Convert 2.40 x 10 23 molecules of an anonymous substance with a molar mass of 18.02 g/mol to its mass in grams.
Answers
The mass of 2.40 × 10²³ molecules of anonymous substance with molar mass of 18.02g/mol is 7.18grams.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles in the substance by its molar mass as follows:
mass = no of moles × molar mass
First, we convert the number of molecules in the anonymous substance to moles as follows:
2.40 × 10²³ ÷ 6.02 × 10²³ = 0.39moles
mass of anonymous substance = 0.39 moles × 18.02g/mol = 7.18grams
Learn more about mass at: https://brainly.com/question/13320535
#SPJ1
Which characteristic is used to classify metamorphic rocks as foliated or non-foliated?
Answers
Answer: d. Arrangement of grains
Explanation:
Edge:)))
According to the law of conservation of matter, what cannot change during a chemical reaction?.
Answers
Answer:The law of conservation of mass states that in a chemical reaction mass is neither created nor destroyed. ... The carbon atom changes from a solid structure to a gas but its mass does not change. Similarly, the law of conservation of energy states that the amount of energy is neither created nor destroyed.
Explanation:
Determine the pH of each of the following solutions.
a. a solution that is 5.2×10−2 M in HClO4 and 5.2×10−2 M in HCl
b. a solution with a density of 1.01 g/mL that is 1.04% HCl by mass
Answers
a. To find the pH of a solution that is 5.2x10^-2 M in HClO4 and 5.2x10^-2 M in HCl, we need to first determine the concentration of H+ ions in the solution. Both HClO4 and HCl are strong acids, which means they completely dissociate in water to form H+ and Cl- ions. Therefore, the concentration of H+ ions in the solution will be equal to the concentration of the acids.
[H+] = [HClO4] + [HCl]
[H+] = 5.2x10^-2 M + 5.2x10^-2 M
[H+] = 1.04x10^-1 M
Now we can use the definition of pH to find the pH of the solution:
pH = -log[H+]
pH = -log(1.04x10^-1)
pH ≈ 0.983
Therefore, the pH of the solution is approximately 0.983.
b. To find the pH of a solution with a density of 1.01 g/mL that is 1.04% HCl by mass, we need to first convert the mass percent to molarity. We can assume that the density of the solution is equal to the density of water (1.00 g/mL) for the purpose of this calculation.
The mass of HCl in 100 g of the solution is:
100 g x 1.04/100 = 1.04 g
The molar mass of HCl is 36.5 g/mol, so the number of moles of HCl is:
1.04 g / 36.5 g/mol = 0.0285 mol
The volume of the solution is:
100 g / 1.01 g/mL = 99.01 mL = 0.09901 L
Therefore, the concentration of HCl in the solution is:
[HCl] = 0.0285 mol / 0.09901 L
[HCl] ≈ 0.288 M
Now we can use the definition of pH to find the pH of the solution:
pH = -log[H+]
pH = -log[HCl]
pH = -log(0.288)
pH ≈ 0.540
Therefore, the pH of the solution is approximately 0.540.
Write a structural formula for the principal organic product formed in the reaction of methyl bromide with each of the following compounds:
1. Sodium hydroxide
2. Potassium ethoxide
3. Sodium benzoate
4. Lithium azide
Answers
Answer:
1. CH3Br + NaOH --------> H3C---OH + Na---Br
Explanation: