Answers
If 298.2g of HCL is produced what is the percent yield
To determine the percent yield, we need to know the actual yield and the theoretical yield of the reaction. If this information is not given, we cannot calculate the percent yield.
However, assuming that the question is referring to a specific chemical reaction in which hydrochloric acid (HCl) is produced, we can use the balanced chemical equation for the reaction to calculate the theoretical yield.
For example, if the reaction is:
NaCl + H2SO4 → Na2SO4 + 2HCl
We can see that 1 mole of NaCl reacts with 1 mole of H2SO4 to produce 2 moles of HCl. Therefore, to calculate the theoretical yield of HCl, we need to know the amount of NaCl and H2SO4 used in the reaction.
Once we have the actual yield and theoretical yield of the reaction, we can calculate the percent yield using the formula:
Percent yield = (Actual yield / Theoretical yield) x 100%
Without this additional information, we cannot calculate the percent yield.
Related Questions
The correct chemical equation for the reaction of sodium bromide and lead nitrate is: _________.
a. NaBr(aq) + Pb(NO3)2aq) ---------->PbBr2ts) + NaNO3(aq)
b. NaBr(aq) + Pb(NO3)2----------> PbBr2(aq) + NaNO3(s)
c. 2 NaBr(aq) + Pb(NO3)2(aq)---------> PbBr2(aq) + NaNO3(s)
d. 2 NaBr(aq) + Pb(NO3)2(aq)---------> PbBr2 + NaNO3(aq)
Answers
Answer : The correct chemical equation is:
\(2NaBr(aq)+Pb(NO_3)_2(aq)\rightarrow PbBr_2(s)+2NaNO_3(aq)\)
Explanation :
Balanced chemical equation : It is defined as the equation in which the atoms of individual elements present of reactant side must be equal to the product side.
According to the question, when sodium bromide reacts with lead nitrate then it reacts to give sodium nitrate and lead bromide as a products.
The balanced chemical equation will be:
\(2NaBr(aq)+Pb(NO_3)_2(aq)\rightarrow PbBr_2(s)+2NaNO_3(aq)\)
Table salt contains 39.33 g of sodium per 100 g of salt. The U.S. Food and Drug Administration (FDA) recommends that adults consume less than 2.40 g of sodium per day . A particular snack mix contains 1.30 g of salt per 100 g of the mix .
Answers
471 g, or roughly 16.6 ounces, of the snack mix, can be consumed daily by an adult.
What is sodium?The chemical element sodium has an atomic number of 11 and the symbol Na, which comes from its Latin name, natrium. It is an alkali metal, which is a soft, silvery-white group of elements in the periodic table. Due to its strong reactivity, sodium is not found in nature's elemental form but rather in compounds like sodium hydroxide, sodium bicarbonate, and sodium chloride (table salt) (lye).
How do you determine it?We must first translate the quantity of salt in the snack mix into the quantity of sodium it contains in order to determine how much sodium is in the snack mix:
1.30 g of salt/100 g of mix x 39.33 g of sodium/100 g of salt = 0.51 g of sodium/100 g of mix
As a result, the snack mix has 0.51 g of salt per 100 g.
The following calculation can be used to determine how much of the snack mix an adult can eat without exceeding the FDA's daily salt recommendation:
2.40 g of sodium/day ÷ 0.51 g of sodium/100 g of mix = 4.71 x 100 g of mix.
Therefore, 471 g, or roughly 16.6 ounces, of the snack mix, can be consumed daily by an adult and yet keep under the FDA's daily sodium recommendation.
To know more about sodium, visit:
https://brainly.com/question/30098334
#SPJ1
A gas is at 1.33 atm of pressure and a volume of 682 mL. What will the pressure be if the volume is reduced to 0.419 L?
Answers
At constant temperature, if the volume of the gas decreased to the given value, the pressure increases to 2.16atm.
What is Boyle's law?Boyle's law simply states that "the volume of any given quantity of gas is inversely proportional to its pressure as long as temperature remains constant.
Boyle's law is expressed as;
P₁V₁ = P₂V₂
Where P₁ is Initial Pressure, V₁ is Initial volume, P₂ is Final Pressure and V₂ is Final volume.
Given the data in the question question;
Initial volume of the gas V₁ = 682mL = 0.682LInitial pressure of the gas P₁ = 1.33atmFinal volume of the gas V₂ = 0.419LFinal pressure of the gas P₂ = ?P₁V₁ = P₂V₂
P₂ = P₁V₁ / V₂
P₂ = ( 1.33atm × 0.682L) / 0.419L
P₂ = 0.90706Latm / 0.419L
P₂ = 2.16atm
Therefore, at constant temperature, if the volume of the gas decreased to the given value, the pressure increases to 2.16atm.
Learn more about Boyle's law here: brainly.com/question/1437490
#SPJ1
A 0.750 M Ca(OH)2 solution was prepared by dissolving 48.0 grams of Ca(OH)2 in enough water. What is the total volume of the solution formed?
Answers
The total volume of the solution prepared by dissolving 48.0 grams of Ca(OH)₂ in enough water is 0.865 L
How do i determine the volume of the solution?First, we shall obtain the mole of 48.0 grams of Ca(OH)₂. This is shown below:
Mass of Ca(OH)₂ = 48.0 grams Molar mass of Ca(OH)₂ = 74 g/mol Mole of Ca(OH)₂ =?Mole = mass / molar mass
Mole of Ca(OH)₂ = 48 / 74
Mole of Ca(OH)₂ = 0.649 mole
Finally, we shall obtain the total volume of the Ca(OH)₂ solution. Details below:
Molarity of solution = 0.750 MMole of Ca(OH)₂ = 0.649 moleVolume of solution =?Volume = mole / molarity
Volume of solution = 0.649 / 0.750
Volume of solution = 0.865 L
Thus, we can conclude that the total volume of the solution is 0.865 L
Learn more about volume:
https://brainly.com/question/29144710
#SPJ1
How many moles of NaCl can be produced from 2.5 moles of BaCl_2.
Answers
2.5 moles of BaCl2 can produce 5 moles of NaCl.
What is chemical equation?
Chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas.
The balanced chemical equation for the reaction between barium chloride (BaCl2) and sodium sulfate (Na2SO4) is:
BaCl2 + Na2SO4 → 2 NaCl + BaSO4
From this equation, we can see that for every 1 mole of BaCl2, 2 moles of NaCl are produced.
Therefore, to calculate how many moles of NaCl can be produced from 2.5 moles of BaCl2, we can use the following proportion:
1 mole BaCl2 : 2 moles NaCl = 2.5 moles BaCl2 : x moles NaCl
Where
x is the number of moles of NaCl producedSolving for x, we get:
x = 2.5 moles BaCl2 × (2 moles NaCl / 1 mole BaCl2)
x = 5 moles NaCl
Therefore, 2.5 moles of BaCl2 can produce 5 moles of NaCl.
Learn more about chemical equation here : brainly.com/question/26694427
#SPJ1
which of the following statements about an e1 mechanism is not true? the loss of a proton by the carbocation is a slow step it involves the formation of a carbocation from eliminating a good leaving group it is a two-step process and has the same first step as an sn1 mechanism a common competing reaction is rearrangement of a less stable carbocation to a more stable carbocation
Answers
The statements about an E1 mechanism is not true is that " the loss of a proton by the carbocation is a slow step."
The E1 mechanism is also called as the unimolecular elimination reaction. in the E1 mechanism there are two parts involves that is ionization and deprotonation. In the ionization process the formation of the carbocation takes place as the intermediate. and in the deprotonation steps the protons is lost by the carbocation.
Thus, in the E1 mechanism the loss of the proton by the carbocation is not the slow step. this process happens in the presence of the base.
To learn more about E1 mechanism here
https://brainly.com/question/14787332
#SPJ4
If a gas sample has a pressure of 74 ka at 87 L, what would the new volume be if the pressure changed to 929 kPa?
Answers
Answer:
La ley de los gases ideales relaciona cuatro propiedades macroscópicas de los gases ideales (presión, volumen, número de moles y temperatura). Si conocemos los valores de tres de estas propiedades, podemos utilizar la ley de los gases ideales para conocer la cuarta. En este video, usaremos la ley de los gases ideales para resolver el número de moles (y en última instancia de moléculas) en una muestra de un gas
Explanation:
The "alum" used in cooking is potassium aluminum sulfate hydrate, KAl(SO4)2·x H2O. To find the value of x, you can heat a sample of the compound to drive off all of the water and leave only KAl(SO4)2. Assume you heat 4.74 g of the hydrated compound and that the sample loses 2.16 g of water. What is the value of x?
Answers
The "alum" used in cooking is potassium aluminum sulfate hydrate, KAl(SO4)2·x H2O and the value of x calculated is 12.
What is meant by alum?Alum is a type of chemical compound, usually hydrated double sulfate salt of aluminum having general formula X Al ₂·12 H ₂O, where X is monovalent cation such as potassium or ammonium.
Given mass of KAl(SO₄)₂.XH₂O = 4.74 g
and mass of water = 2.16 g
So, mass of KAl(SO₄)₂ = 4.74 – 2.16 = 2.58 g
Molar mass of KAl(SO₄)₂ ;
= 39 + 27 + 2[32 + (4×26)]
Now, molar mass of KAl(SO₄)₂ = 258 g/mol
Molar mass of H₂O ;
= (2×1) + 16
Now, molar mass of H₂O = 18 g/mol
Hence, KAl(SO₄)₂ = 2.58 / 258 = 0.01
and H₂O = 2.16 / 18 = 0.12
KAl(SO₄)₂ = 0.01 / 0.01 = 1
H₂O = 0.12 / 0.01 = 12
The formula of the compound is obtained as KAl(SO₄)₂.12H₂O
Comparing KAl(SO₄)₂.12H₂O with KAl(SO₄)₂.XH₂O, value of X is 12.
To know more about potassium aluminum sulfate hydrate, refer
https://brainly.com/question/5961763
#SPJ1
When a light bulb is rated at 60 W, it means that
A.each second, the bulb converts 60 J of electrical energy to heat and light.
B.the bulb loses 60 W of potential energy each second.
C.current is traveling at 60 m/s through the filament.
D.the bulb uses 60 J of power when it is lit.
The molecules of a gas are much farther apart than molecules in a solid or liquid.
True
False
Answers
The first question has an answer of A: the bulb turns 60 J of electrical energy into heat and light every second. It is True that a gas's molecules are significantly more spaced away from one another than those of a solid or liquid.
A heated filament or a gas discharge generate light in a light bulb, an electric device. The light of the bulb is produced by a wire filament that is heated by an electric current. Thomas Edison created the first incandescent light bulb that was commercially viable in 1879, and by creating a useful source of artificial light, it completely changed the way we live today. With the introduction of compact fluorescent bulbs, LED lights, and other technologies, light bulbs have developed over time to become more energy-efficient. Light bulbs today are a necessary part of contemporary life, providing illumination for private residences, commercial buildings, and public areas.
Learn more about light bulb here:
https://brainly.com/question/1717583
#SPJ4
on a piece of paper, write balanced molecular, total ionic, and net ionic equations for the reaction between aqueous solutions of sodium hydroxide and nitric acid. select the number that corresponds to the sum of the coefficient for the net ionic equation. [ select ]
Answers
Compose balanced molecule, gross ionic, as well as net ionic formulae for the interaction between sodium hydroxide plus nitric acid in aqueous solutions. The net ionic equation's coefficient sum is three.
What is the purpose of nitric acid?Ammonium nitrate, a crucial component of fertilizers, is created using nitric acid. Additionally, it is used to oxidize materials and to make explosives like trinitrotoluene (TNT) as well as nitroglycerin.
What occurs when nitric acid is touched?The liquid leads to severe burns when it comes in contact with your eyes, which could cause lifelong damage and vision loss. The liquefied or concentrated vapor causes immediate, severe, and profound burns on the skin, while acidic solution result in deep ulcers and leave a brilliant yellow stain.
To know more about nitric acid visit:
https://brainly.com/question/29769012
#SPJ4
The standard emf for the cell using the overall cell reaction below is =2.20 V:
2Al(s) + 3I2(s) ? 2Al3+ (aq) + 6I- (aq)
The emf (voltage) generated by the cell when [Al3+] = 4.0 x 10-3 M and [I-] = 0.015 M
is_______ V
Answers
The cell produces an emf of 2.32 V. For this cell reaction at 25 degrees Celsius, use the Nernst equation. where n represents the number of electrons exchanged during the cellular process.
What is the typical EMF this cell produces?According to measurements, the electromotive force (EMF) is 1.100 V. The standard condition is specified as a concentration of 1 M in an ideal solution, and as a result, 1.100 V is the standard electromotive force, DEo, or standard cell potential for the Zn–Cu galvanic cell.
How is the average Ecell determined?Due to the larger standard reduction potential of the silver half-cell, the reduction will occur. The half-cell of tin will oxidize. The formula E0cell=E0red+E0oxid can be used to get the overall cell potential.
To know more about Electromotive Force (EMF) here:
brainly.com/question/14968481
#SPJ4
Differences between voltage, current and resistance?
Answers
Answer:
Voltage is the measure of electric potential energy per unit charge, current is the flow of electric charge through a circuit, and resistance is the property of a material that opposes the flow of electric current.
Ohm's Law relates these three concepts by stating that current is directly proportional to voltage and inversely proportional to resistance.
Hope this helps!
Which metal will spontaneously react with Zn²⁺(aq), but will not
spontaneously react with Mg²⁺ (aq)?
Answers
Mn(s) metal will spontaneously react with Zn²⁺(aq), but will not
spontaneously react with Mg²⁺ (aq)
The Eo value of an electrochemical cell determines its spontaneity. Positive Eo electrochemical cells are spontaneous, and vice versa.
The relevant Eo of the half-cell in this instance are as follows for Mn(s) metal
Zn2+/Zn = -0.763v for Eo
2.37v for Eo Mg2+/Mg.
Mn2+/Mn = -1.18v for Eo.
Consequently, the equation for an Eo cell (with Zn as one of the half-cells) is: Eo Zn2+/Zn - Eo Mn2+/Mn = -0.763 - (-1.18) = 0.417v.
On the other hand, the equation for an Eo cell (with Mg as one of the half-cells) is: Eo Mg2+/Zn - Eo Mn2+/Mn = -2.37 - (-1.18) = -1.19v.
As a result, Mn(s) metal will spontaneously react with Zn2+(aq), but not with Mg2+ (aq)
To learn more about Mn(s) metal please visit -
https://brainly.com/question/13153267
#SPJ1
Select the correct answer
Which hand is negatively charged?

Answers
Option B is the correct answer .
What is Charge ?
Charge is a basic property of matter that is associated with the presence or absence of electrons. Objects can have positive or negative charge, or they can be neutral, which means they have an equal number of positive and negative charges. In physics, charge is one of the fundamental concepts used to explain the behavior of matter and energy in the universe. The interaction between charges is described by Coulomb's law, which states that the force between two charges is proportional to the product of their charges and inversely proportional to the square of the distance between them.
To learn more about the Charge , click the given link ; https://brainly.com/question/25922783
#SPJ1
A lead ball is added to a graduated cylinder containing 50.6 ml of water, causing the level of the water to increase to 93.0 mL. What is the volume in milliliters of the lead ball, Vieras? Viead =
Answers
42.4 ml is the volume in milliliters of the lead ball if a lead ball is added to a graduated cylinder containing 50.6 ml of water.
What is a graduated cylinder?A tall narrow container with a volume scale is used especially for measuring liquids.
The graduated cylinder contains water
mL is a volume unit.
Water volume = 50.6 ml
The lead ball caused an increase in volume from 50.6 ml to 93.0 mL.
The new volume is the lead ball volume plus the original water volume :
Final volume = Vlead ball+ Water original volume
\(93.0 mL= V_(lead ball) +50.6 ml\)
\(V_(lead ball) = 93.0 mL - 50.6 ml\)
\(V_(lead ball) = 42.4 ml\)
Hence, 42.4 ml is the volume in milliliters of the lead ball.
Learn more about the graduated cylinder here:
https://brainly.com/question/13386106
#SPJ1
1. Does temperature make a difference in how much carbon dioxide gas is needed to
inflate a 60-L air bag using the reaction of sodium bicarbonate and acetic acid? Compare a
winter day at 0°C and standard pressure with a summer day at 35°C and standard pressure.
Calculate the number of moles of carbon dioxide gas required for a 60-L inflation at both
temperatures and then calculate the percent difference in moles.
Answers
Answer:
D
Explanation:
got it right on the test
Help as soon as possible

Answers
The system from the description must be an open system. Option C
What is an open system?
An open system is one that communicates with and is influenced by its surroundings. An open system interacts with its environment by exchanging matter, energy, and information, as opposed to a closed system, which is self-contained and runs autonomously.
The idea of open systems highlights how interconnected and reliant on its environment a system is. For the analysis of system behavior, adaptability, and responses to external forces, understanding these interconnections is essential.
Learn more about open system:https://brainly.com/question/29977128
#SPJ1
A sample of sugar (C12H22O11) contains
1.505 × 1023 molecules of sugar. How many moles of sugar are present in the sample? Answer without doing any calculations.
0.25 mol
0.50 mol
1.00 mol
2.50 mol
Answers
Answer:
0.25 mol
Explanation:
Use the formula n=N/NA
n= number of mols
N = number of particles
Nᵃ = Avogadros constant = 6.02 x \(\\10^{23\)
So, n= \(\frac{1.505 X 10^{23} }{6.02 X 10^{23}}\)
The 10 to the power of 23 cancels out and you are left with 1.505/6.02, which is approximately 1/4. This is the same as 0.25 mol.
Hope this helped :)
How many eight-packs of water would you need if 32 people
are attending your part and each consumed 2 bottles of
water?
Answers
Answer:
You would need 8 eight packs of water
Explanation:
32 x 2 (seeing as this is the amount of water each person consumes)
= 64 divided by 8 (the amount of water in each pack)
= 8 eight packs of water
AlCl₃ has a van't Hoff factor of i = 3.20. What is the concentration of particles in a 0.651 M solution of AlCl₃?
Answers
The concentration of particles in a 0.651 M solution of AlCl₃ is 2.06 M.
This can be calculated by multiplying the molarity of the solution (0.651 M) by the van't Hoff factor (3.20). Therefore, 0.651 x 3.20 = 2.06.
What is molarity?
Molarity is a unit of concentration that is defined as the number of moles of a solute per liter of solution. It is usually represented by the symbol M. The calculation of molarity is done by dividing the number of moles of a solute by the total volume of the solution in liters. It is also often used in dilutions, where the concentration of a solution is reduced by adding more solvent. Molarity is a very important concept in chemistry and is used to predict and measure the amount of substances needed in a reaction.
Therefore, The concentration of particles in a 0.651 M solution of AlCl₃ is 2.06 M.
To learn more about molarity
Here: https://brainly.com/question/17138838
#SPJ1
What is the pH of a 50.0 mL solution of a 0.250 M HC2H3O2 solution after the
addition of 50 mL of 0.250 M NaOH to it? The Ka value for HC2H3O2 is 1.8 x 10-5
.
Answers
A 50.0 mL solution of 0.250 M HC₂H₃O₂ before and after adding 50 mL of 0.250 M NaOH has a pH of 2.87, the pH changes due to the formation of a buffer solution, which can be calculated using the Henderson-Hasselbalch equation.
How to calculate pH of the solution?This problem requires us to calculate the pH of a buffer solution after the addition of a strong base. A buffer solution is one that resists pH changes when modest amounts of acid or base are added. The buffer system in this case is the weak acid, acetic acid (HC₂H₃O₂) and its conjugate base, acetate ion (C₂H₃O₂-).
Before the addition of NaOH, we have a solution of 0.250 M HC₂H₃O₂, which we can assume to be completely dissociated in water:
HC₂H₃O₂ + H₂O ⇌ H₃O+ + C₂H₃O₂-
HC₂H₃O₂ has a Ka value of 1.8 x 10-5. We can use this Ka value to calculate the equilibrium concentration of H3O+ and C₂H₃O₂- in the solution.
First, we need to calculate the initial concentrations of HC₂H₃O₂ and C₂H₃O₂-.
moles of HC₂H₃O₂ = 0.250 M × 0.0500 L = 0.0125 mol
moles of C₂H₃O₂- = 0 mol (since there is no NaOH added yet)
Since HC₂H₃O₂ is a weak acid, it only partially dissociates in water. The equilibrium concentrations of H₃O+ and C₂H₃O₂- can be calculated using the Ka expression:
Ka = [H₃O+][C₂H₃O₂-]/[HC₂H₃O₂]
We can assume that the initial concentration of H3O+ is negligible compared to the amount that will be produced by the dissociation of HC₂H₃O₂, so we can simplify the expression to:
Ka = [H₃O+]²/[HC₂H₃O₂]
[H₃O+]² = Ka × [HC₂H₃O₂]
[H₃O+]² = 1.8 × 10⁻⁵ × 0.0125
[H₃O+] = 1.34 × 10⁻³ M
Now that we know the equilibrium concentration of H₃O+, we can use the pH formula to calculate the pH:
pH = -log[H₃O+]
pH = -log(1.34 × 10⁻³)
pH = 2.87
This is the pH of the buffer solution before the addition of NaOH.
Next, we add 50 mL of 0.250 M NaOH to the solution. NaOH is a strong base, so it completely dissociates in water to produce OH- ions:
NaOH → Na+ + OH-
The OH- ions will react with the acetate ions in the buffer solution to form water and acetate ions:
OH- + C₂H₃O₂- → H₂O + C₂H₃O₂-
This reaction will consume some of the acetate ions in the buffer solution, causing the equilibrium to shift to the left to produce more acetate ions.
To calculate the new concentrations of H₃O+ and C₂H₃O₂-, we need to use the Henderson-Hasselbalch equation:
pH = pKa + log([C₂H₃O₂-]/[HC₂H₃O₂])
where pKa = -log(Ka), [C₂H₃O₂-] is the equilibrium concentration of acetate ions, and [HC₂H₃O₂] is the equilibrium concentration of acetic acid.
To learn more about buffer solution, visit: https://brainly.com/question/8676275
#SPJ1
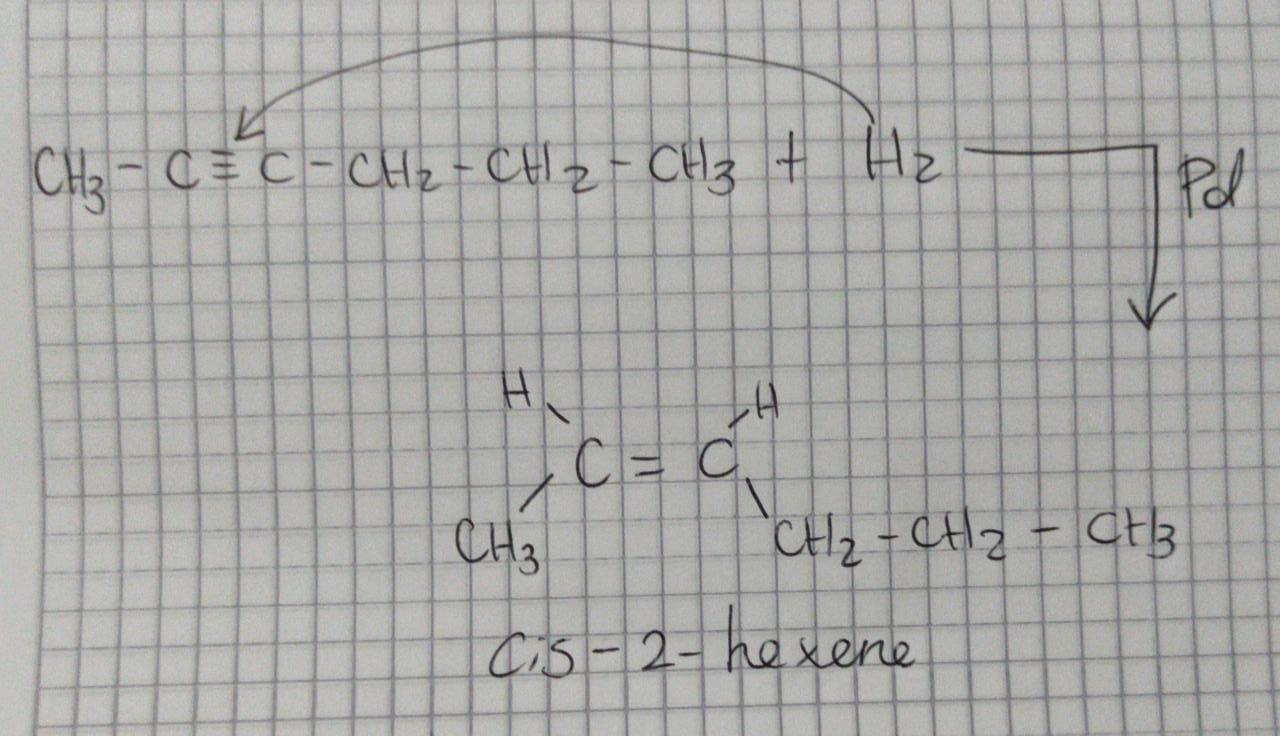
Identify the major product that is obtained when 2-hexyne is treated with H2 and Lindlar's catalyst.
Answers
Answer:
cis-2-hexene
Explanation:
Hello.
In this case, since a Lindlar's catalyst is mainly composed by palladium, which is a transition metal with a very exceptional surface area which makes it a suitable catalyst for hydrogenation reactions by which hydrogen is added to the insaturation in order to make a double bond where the triple bonds was in an alkyne, based on the attached picture we can see that the major product of that reaction is cis-2-hexene.
Best regards!
Look at the diagram of a fuel cell below. A fuel cell with 2 vertical objects labeled A and B connected by an electrical wire through a circle with a M in it. There is an area between the two vertical objects labeled A, and substances flowing to, along, and away from the vertical objects and to the left and right. Which statement describes how electrons move if oxidation occurs on the left side of the cell and reduction occurs on the right side? Electrons move from left to right through Electrons move from right to left through A. Electrons move from left to right through M. Electrons move from right to left through M.
Answers
The electrons move from left to right through the circle labeled "M" to reach the cathode, where reduction takes place.
If oxidation occurs on the left side of the fuel cell and reduction occurs on the right side, the movement of electrons can be described as follows: Electrons move from left to right through the circle labeled "M."
In a fuel cell, the process of oxidation takes place at the anode (labeled A) where the fuel is oxidized, releasing electrons. These electrons then flow through the external electrical circuit, represented by the wire connecting objects A and B. The electrons reach the cathode (also labeled A) on the right side of the cell, where reduction occurs.The circle labeled "M" represents the membrane or electrolyte in the fuel cell. This membrane allows the transport of ions but blocks the movement of electrons. As a result, electrons cannot flow directly through the electrolyte but must travel through the external circuit.
This movement of electrons through the external circuit is what generates an electric current that can be used to power electrical devices or systems.
for such more questions on reduction
https://brainly.com/question/21851295
#SPJ8
Scientific Notation. Change the following number to its scientific notation in standard form (a.bc x 10+y) with THREE significant figures. (4 pts)
0.001512 m
0.01549 L
1.557 g
15 347 mm
Answers
Answer:
1) 1.51 * 10^-3
2) 1.55 * 10^-2
3) cannot be turned into scientific notation
4) If 15,347; 1.53 * 10^4 but If 15.347; 1.53 * 10^1
Explanation:
Scientific notation just means moving the decimal place of a number so that there is only one number in the one's place (the exponent on the ten is the number of times the decimal moved). It is used to write really big or really small numbers without having to write a bunch of zeros.
Balance each of the following equations: _CrF2 + _Al2 (SO4)3 -> _AiF3+ _Cr (SO4)
Answers
Explanation:
lo que tú as puesto es falso
how can two rocks made by the same process be so different in color
Answers
Answer: Although the rocks of the produced by similar processes but their chemical composition may vary dependent upon the type of mineral present in these rocks so the color of the rock also varies depending upon the mineral composition.
Explanation:
The rocks are made by basic steps like disintegration of parent rock material, erosion, crystallization, metamorphism, and sedimentation. These basic steps are common in same kind of rocks but their color may vary because of the mineral composition in them for example, Rhyolite is of light colored because of the presence of quartz mineral present in it.
What is Electron Configuration?
Answers
Electron configuration refers to the distribution of electrons in atoms and molecules.
Electron configuration can be defined as the specific arrangement of negatively charged electrons in different energy levels around atomic nuclei.Electron configuration can also be used to represent the bonds that connect different atoms in a molecule.Moreover, an electron orbital refers to the function that describes the location and behavior of electrons in a given atom.In conclusion, electron configuration refers to the distribution of electrons in atoms and molecules.
Learn more about electron configuration here:
https://brainly.com/question/5524513
Answer:
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
Who developed the equation P1*V1 = P2*V2?
Answers
Answer:
Explanation:
Robert Boyle
differentiate between emulsion,foam and gel
Answers
Answer:
Emulsion:
A colloidal solution in which both the dispersed phase and dispersion medium are liquids is called an Emulsion.
Gel:
Gels is that kind of colloid in which the dispersed phase is a liquid and the dispersion medium is a solid.
Foam:
Foam is a substance in which the particles are gas bubbles and the medium is a liquid.