Answers
Answer:
The suitable equation for this reaction is
2CO + O₂ -----> 2CO₂
Here, we are given that we have 2 grams of O₂
From the equation, we can see that 2 * Moles of O₂ = Moles of CO₂
Moles of O₂:
2/32 = 1/16 moles
Therefore, the number of moles of CO₂ is twice the moles of O₂
Moles of CO₂ = 2 * 1/16
Moles of CO₂ formed = 1/8 moles
Mass of CO₂ formed = Molar mass of CO₂ * Moles of CO₂
Mass of CO₂ formed = 44 * 1/8
Mass of CO₂ formed = 5.5 grams
Kindly Mark Brainliest, Thanks!!!
Related Questions
PLEASE HELP ASAP!
5 + 6 HNO3 -> H2504 + 6 NO2 + 2H20
In the above equation how many moles of water can be made when 112.6 grams of HNO3 are consumed?
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Hydrogen 1
Nitrogen 14
Sulfur 32
Oxygen 16
Answers
0.595 moles of water can be made at 112.6 grams of \(HNO_{3}\) are consumed
To determine the number of moles of water produced when 112.6 grams of \(HNO_{3}\) are consumed, we use the equation's stoichiometry and molar masses.
To determine the number of moles of water produced when 112.6 grams of \(HNO_{3}\) are consumed, we need to use the molar mass of \(HNO_{3}\) and the stoichiometric coefficients of the balanced chemical equation.
The molar mass of \(HNO_{3}\) is calculated as follows:
1 mole of hydrogen (H) = 1 g/mol
1 mole of nitrogen (N) = 14 g/mol
3 moles of oxygen (O) = 3 × 16 g/mol = 48 g/mol
Adding these together, the molar mass of \(HNO_{3}\) is 1 + 14 + 48 = 63 g/mol.
Now, we can set up a conversion factor using the stoichiometry of the balanced equation:
From the equation: 5 + 6 \(HNO_{3}\) -> \(H_{2}SO_{4}\) + 6 \(NO_{2}\) + 2 \(H_{2}O\)
From the coefficients: 6 moles of \(HNO_{3}\) produce 2 moles of \(H_{2}O\)
To find the moles of water produced, we use the following calculation:
112.6 g \(HNO_{3}\) × (1 mol \(H_{2}O\) / 63 g \(HNO_{3}\)) × (2 mol \(H_{2}O\) / 6 mol \(HNO_{3}\)) = 0.595 mol \(H_{2}O\)
Therefore, when 112.6 grams of \(HNO_{3}\) are consumed, approximately 0.595 moles of water can be produced according to the given balanced equation and molar masses.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ8
A student placed a straw into the water and blew bubbles into the water for 30 seconds. The pH of the glass of tested again.
Use the pH scale below to determine the pH value of the water in this test. Record the value. Also, determine whether the
pH stayed the same, became more acidic, or became more alkaline compared to the first test.
A student placed a straw into the water and blew - 1
Answers
Record the value. Also, determine whether the pH stayed the same, became more acidic, or became more alkaline compared to the first test.
What is alkaline and is it good for you?Alkaline water has a higher pH level than that of plain tap water. So proponents say that it can neutralize acid in your bloodstream. Some say that alkaline water can help prevent disease, such as cancer and heart disease.
Which foods are alkaline?Most fruits and vegetables, soybeans and tofu, and some nuts, seeds, and legumes are alkaline-promoting foods, so they're fair game. Dairy, eggs, meat, most grains, and processed foods, like canned and packaged snacks and convenience foods, fall on the acid side and are not allowed.
To know more about alkaline visit
https://brainly.com/question/11584594
#SPJ1
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
Example of preparation of soap?
Answers
Answer:
Soap is prepared by hydrolyzing a fat under alkaline (basic) conditions. The reaction is called saponification, and produces one molecule of glycerin and three molecules of soap, for each molecule of fat taken. ... For example, a salt of a saturated, long-chain acid makes a harder, more insoluble soap.
Explanation:
A dam constructed to produce tidal power does so by _____. reducing the range between high tide and low tide harnessing water flow to drive turbines and electric generators protecting a coastal area from large ocean waves preventing saltwater from moving from the ocean into a bay
Answers
A dam constructed to produce tidal power does so by harnessing water flow to drive turbines and electric generators .
What purposes does tidal energy serve?Tidal energy was employed in grain mills to crush grains mechanically, just like wind energy was. grain crushing Here, the tidal energy generated by the turbines was used. Hydroelectric dams, which serve as significant energy storage, also employ tidal energy to store energy.
Tidal power can harm marine life because tidal turbines' whirling blades can cause marine organisms to perish. Fish habitations in tidal power settings may be impacted by noise from the turbines' rotation. Tidal energy can also affect how sediment and water are processed.
Therefore, option B is correct.
Learn more about tidal power at:
https://brainly.com/question/10578402
#SPJ1
The graph below shows how the temperature and volume of a gas vary when
the number of moles and the pressure of the gas are held constant. How can
the volume of the gas be increased if the pressure is constant?
v
т
A. By increasing the temperature
B. By letting the gas expand over time
C. By letting the gas contract over time
D. By decreasing the temperature
Answers
Answer:
D
Explanation:
PV =nRT
So V and T are inversely proportional
how many degrees of unsaturation are present in a molecule with the molecular formula c5h8brno? (1 pts)
Answers
The degree of unsaturation of the compound is 3.
What is the degree of unsaturation?When we talk about the degree of unsaturation, we are referring to the number of the hydrogen atoms that could not be found in the system. We know that when a saturated compound is formed, there is the loss of some of the hydrogen atoms from the molecular formula.
The term unsaturated in itself means that the compound do not have the exact number of the hydrogen atoms or the hydrogen atom compliment that it should normally have.
This would happen as the multiple bonds are formed then a given number of the hydrogen atoms would be lost from the system so that the multiple bond can now be formed in the compound that we are looking at. The more the number of the multiple bonds, the more the degree of unsaturation of the molecule.
Learn more about unsaturation:https://brainly.com/question/12433190
#SPJ1

Which of the following sets of quantum numbers contains an error?Group of answer choices:n = 4, l = 3, ml = -2, ms = -1/2n = 2, l = 0, ml = +2, ms = -1/2n = 3, l = 2, ml = 0, ms = -1/2n = 3, l = 1, ml = +1, ms = +1/2n = 2, l = 1, ml = 0, ms = -1/2

Answers
Answer
n = 2, l = 0, ml = +2, ms = -1/2
n = 3, l = 1, ml = +1, ms = +1/2
Explanation
In atoms, there are a total of four quantum numbers namely;
The principal quantum number (n), n = 1, 2, 3, ....
The orbital angular momentum quantum number (l), for n = 3, l = (n - 1) = 0, 1, 2, The magnetic quantum number (ml), for l = 2, ml = (-l, 0, +l) = -2, -1, 0, +1, +2, and The electron spin quantum number (ms), ms = -1/2, +1/2
Therefore, the following sets of quantum numbers contains an error among the given sets:
n = 2, l = 0, ml = +2, ms = -1/2
n = 3, l = 1, ml = +1, ms = +1/2
When 36.5g Of NaCL(s) is added to enough water to make 550.0 ml of solution, what is the molarity of the solution?
Answers
Answer:
1.1442 moles| litre,NaCl
Explanation:
RAM:56g|mole
number of moles=36.5g÷58g|mole =0.6293moles
0.6293 is contained in 550ml
=1000ml
[1000×0.6293]÷550 =1.1442moles| litre,NaCl
Chemistry..... Reaction Rate
W → U + S Chemistry Reaction Rate use the table to find reaction rate
See reaction Rate Table Picture

Answers
The reaction rates for trial 1 is 8.22 x 10⁻² M⁻² s⁻¹ and 1.10 M⁻² s⁻¹ for trail 2 and 3
How to find reaction rate?Keep the concentration of W constant while varying the concentrations of U and S while measuring the reaction rate in order to determine the reaction rate with regard to U and S.
Select trial 1 as the reference trial and calculate the reaction's rate constant (k) with respect to U and S, assuming that the concentration of W is constant throughout all three trials.
For trial 1:
[W] = 0.13 M
Rate = 4.72 x 10⁻⁴ M/s
For trial 2:
[W] = 0.13 M
Rate = 1.18 x 10⁻² M/s
From the equation rate = k[U][S], set up the following ratio of rates:
Rate2/Rate1 = (k[U]2[S]2)/(k[U]1[S]1)
Simplifying:
k = (Rate2/Rate1) x (1/[U]2) x (1/[S]2) x ([U]1) x ([S]1)
Substituting the values from trials 1 and 2:
k = (1.18 x 10⁻² M/s) / (4.72 x 10⁻⁴ M/s) x (1/0.65 M) x (1/1 M) x (0.13 M) x (1 M)
k = 8.22 x 10⁻²M⁻² s⁻¹
Similarly, for trial 3:
[W] = 0.13 M
Rate = 2.95 x 10⁻¹ M/s
Again, using trial 1 as the reference trial, figure out the reaction's rate constant (k) in relation to U and S:
k = (Rate3/Rate1) x (1/[U]3) x (1/[S]3) x ([U]1) x ([S]1)
k = (2.95 x 10⁻¹ M/s) / (4.72 x 10⁻⁴ M/s) x (1/3.25 M) x (1/1 M) x (0.13 M) x (1 M)
k = 1.10 M⁻² s⁻¹
Therefore, the equation states the reaction rate in relation to U and S is k = 8.22 x 10⁻² M⁻² s⁻¹ and 1.10 M⁻² s⁻¹ for trials 2 and 3, respectively.
Find out more on Reaction Rate here: https://brainly.com/question/24795637
#SPJ1
To _____ means to draw a conclusion based on something you observe
A. Guess
B. Control
C. Model
D. Infer
Answers
Answer: D
Explanation: Infer
Identify reactions types and balancing equations

Answers
Balance the following chemical equations:
1. N2 + 3 H2 → 2 NH3
Ex: Synthesis reaction
2. 2 KClO3 → 2 KCl + 3 O2
Single Replacement reaction
3. 2 NaF + ZnCl2 → ZnF2 + 2 NaCl
Decomposition reaction
4. 2 AlBr3 + 3 Ca(OH)2 → Al2(OH)6 + 6 CaBr2
Double Replacement reaction
5. 2 H2 + O2 → 2 H2O
Combustion reaction
6. 2 AgNO3 + MgCl2 → 2 AgCl + Mg(NO3)2
Synthesis reaction
7. 2 Al + 6 HCl → 2 AlCl3 + 3 H2
Decomposition reaction
8. C3H8 + 5 O2 → 3 CO2 + 4 H2O
Combustion reaction
9. 2 FeCl3 + 6 NaOH → Fe2O3 + 6 NaCl + 3 H2O
Double Replacement reaction
10. 4 P + 5 O2 → 2 P2O5
Synthesis reaction
11. 2 Na + 2 H2O → 2 NaOH + H2
Single Replacement reaction
12. 2 Ag2O → 4 Ag + O2
Decomposition reaction
13. C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Combustion reaction
14. 2 KBr + MgCl2 → 2 KCl + MgBr2
Double Replacement reaction
15. 2 HNO3 + Ba(OH)2 → Ba(NO3)2 + 2 H2O
Double Replacement reaction
16. C5H12 + 8 O2 → 5 CO2 + 6 H2O
Combustion reaction
17. 4 Al + 3 O2 → 2 Al2O3
Synthesis reaction
18. Fe2O3 + 2 Al → 2 Fe + Al2O3
Single Replacement reaction
Learn more about Chemical reactions, here:
https://brainly.com/question/29762834
#SPJ1
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 2.9 g of butane is mixed with 18.5 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.
Answers
Answer:
8.8g of CO₂ could be produced
Explanation:
The reaction of combustion of butane, C₄H₁₀, is:
C₄H₁₀ + 13/2O₂ → 4CO₂ + 5H₂O
We need to find moles of butane and oxygen to obtain the limiting reactant. With moles of limiting reactant and the chemical equation we can determine moles of CO₂ and maximum mass that could be produced:
Moles butane -Molar mass: 58.12g/mol-
2.9g * (1mol / 58.12g) = 0.050 moles butane
Moles Oxygen -Molar mass: 32g/mol-
18.5g * (1mol / 32g) = 0.578 moles oxygen
For a complete reaction of 0.050moles of butane are needed:
0.050moles of butane * (13/2mol O₂ / 1mol butane) = 0.325 moles of oxygen
As there are 0.578 moles, the limiting reactant is butane and moles of carbon dioxide produced are:
0.050moles of butane * (4mol CO₂ / 1mol butane) = 0.20 moles of CO₂
The maximum mass that could be produced is - Molar mass: 44g/mol-:
0.20 moles of CO₂ * (44g/mol) =
8.8g of CO₂ could be producedCalculate the amount of copper in moles in a 27.5g pure copper sheet
Answers
The amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To calculate the amount of copper in moles in a pure copper sheet, we need to use the molar mass of copper and the given mass of the sheet.
The molar mass of copper (Cu) is approximately 63.55 g/mol. This value represents the mass of one mole of copper atoms.
Given that the mass of the pure copper sheet is 27.5 g, we can calculate the number of moles using the following formula:
moles = mass / molar mass
Substituting the values:
moles = 27.5 g / 63.55 g/mol
moles ≈ 0.433 mol
Therefore, the amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To arrive at this result, we divided the given mass of the sheet (27.5 g) by the molar mass of copper (63.55 g/mol). This calculation allows us to convert the mass of the sheet into the corresponding number of moles of copper.
The result tells us that the 27.5 g pure copper sheet contains approximately 0.433 moles of copper atoms. This conversion to moles is useful in various chemical calculations and allows for easier comparison and analysis of quantities on a molecular scale.
for more such question on copper visit
https://brainly.com/question/29176517
#SPJ8
Which of the following is the most plausible explanation for the fact that the saponification of the triacylglycerol in the passage resulted in four different fatty acid salts?
a. The triacylglycerol molecule consisted of four different fatty acid units.
b. Glycerol was transformed into a fatty acid salt under the reaction conditions.
c. One of the fatty acid salts was unsaturated, and it completely isomerized under the reaction conditions.
d. One of the fatty acid salts was unsaturated, and a small percentage isomerized under the reaction conditions.
Answers
Answer: The correct option is C (One of the fatty acid salts was unsaturated, and it completely isomerized under the reaction conditions).
Explanation:
Fats and oils belongs to a general group of compounds known as lipids. Fatty acids are weak acid and are divided into two:
--> Saturated fatty acids: These have NO double bonds in their hydrocarbon chain, and
--> Unsaturated fatty acids: These have one or more double bonds in their hydrocarbon chain.
SAPONIFICATION is defined as the process by which fats and oil is hydrolyzed with caustic alkali to yield propane-1,2,3-triol and the corresponding sodium salt of the component fatty acids. During this process, One hydroxide ion is required to hydrolyze one ester linkage of a triacylglycerol molecule. Because there are three ester linkages in a triacylglycerol, three equivalents of sodium hydroxide will be needed to completely saponify the triacylglycerol. This explains the reason why saponification of the triacylglycerol iresulted in four different fatty acid salts.
The formula for water is H₂O meaning there are 2 Hydrogen atoms and 1 oxygen. What is the atomic mass of one molecule to the nearest hundredth?
A) 17.99
B) 16.99
C) 15.99
D) 18.99
Answers
\( { \bf\implies 17.99u}\)
Step-by-step explanation:We know that water is the combination of two hydrogen atoms and one oxygen atoms.To find atomic mass of water (\(\bf{H_2O}\))
Atomic mass of Hydrogen × 2 + Atomic mass of oxygen × 1We know that,
Atomic mass of Hydrogen = 1Atomic mass of Oxygen = 15.99\(\mapsto\)1 × 2 + 15.99 × 1
\(\mapsto\)2 + 15.99
\(\mapsto\) 17.99u Ans.
Additional information :Learn the Period table of Elements to solve this type of questions. It is very important. I attached the period table of elements. Learn it and you are able to solve it.
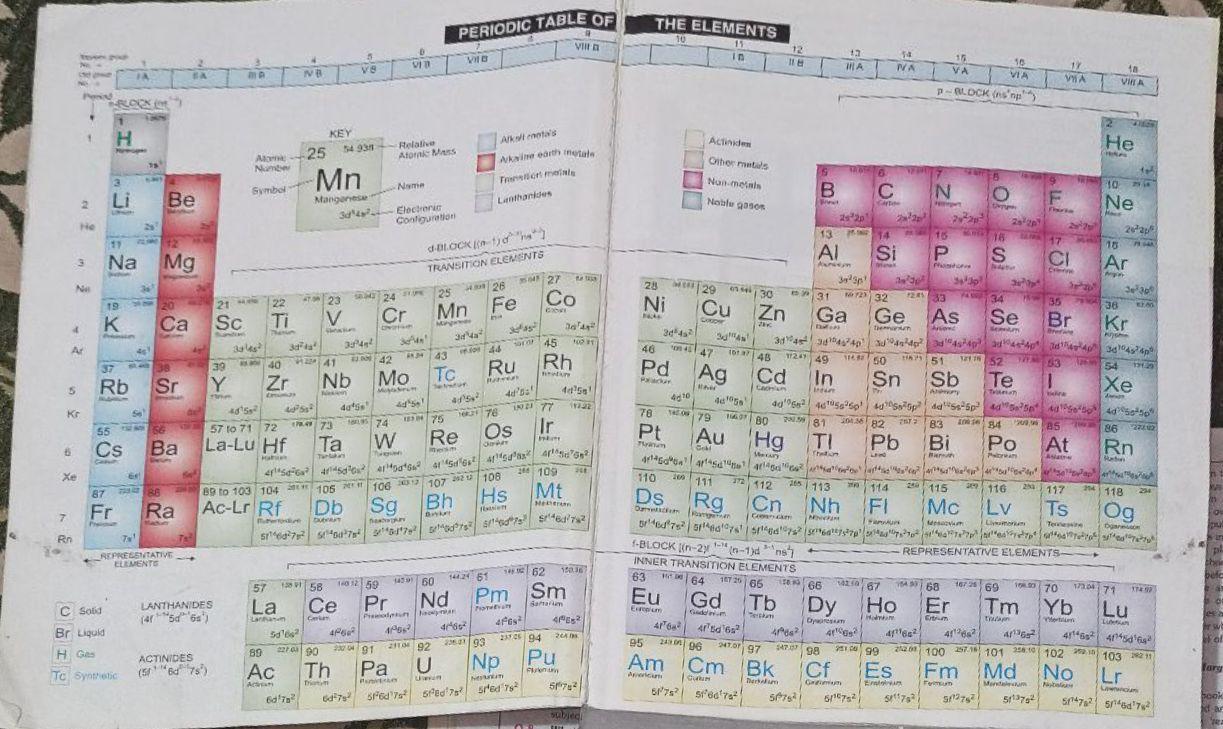
Show your work. 37.2 moles CO2 to oxygen atoms.
Answers

I’m confused on this

Answers
Answer:
B
Explanation:
The scientist had a wrong hypothesis?
Answer:
it would be C.
Explanation:
becuase the scientist would know what to to next time for his next experiment. even if it went wrong, he can try to get it right again.
Ben observes how quickly some dry wood is burning in a campfire.which term best relates to bens observation
Answers
The term that best relates to Ben's observation is the rate of reaction.
What is the rate of reaction?The rate of reaction is a term used to describe how quickly a reaction occurs. In reactions, reactants react to form products. Thus, the rate of a reaction refers to the rate of disappearance of reactants or the rate of appearance of products.
The burning of wood is a form of reaction because the wood burns to produce products such as ashes, heat, smoke, and gases. Thus, the rate of disappearance of the wood would be the rate of the reaction.
Therefore, the term that best relates to Ben's observation is rate of reaction.
More on rate of reactions can be found here: https://brainly.com/question/8592296
#SPJ1
2. You dissolve 0.395g of KMnO, in enough water to give 250 mL of solution. What is the molar concentration of KMnO₂?
Answers
Hope it helps you ... ...


For the reaction 4 FeCl2(aq) + 302(g) → 2Fe2O3(s) + 4Cl2(g), what volume of a
0.945 M solution of FeCl2 is required to react completely with 4.32 x1021 molecules
of O2?
4.23 x 103 mL
09.04 mL
O 5.69 mL
O 10.1 mL
O 5.08 mL
Answers
Answer:
\(V=10.12mL\)
Explanation:
Hello there!
In this case, according to the given chemical reaction, it is possible to evidence the 4:3 mole ratio between oxygen and iron (II) chloride; thus, we can compute the moles of the latter that are consumed by the given molecules of the former:
\(n_{FeCl_2}=4.32x10^{21}molec O_2*\frac{1molO_2}{6.022x10^{23}molec O_2} *\frac{4molFeCl_2}{3molO_2} \\\\n_{FeCl_2}=0.0095molFeCl_2\)
Now, since we have a 0.945-M solution of this iron (II) chloride, the corresponding volume turns out to be:
\(V=\frac{n_{FeCl_2}}{M}\\\\V=\frac{0.00956mol}{0.945mol/L}\\\\V=0.01012L\\\\V=10.12mL\)
Best regards!
Estimate how much heat in joules is released when 25.0 g of water (C = 4.184 J/g°C) is cooled from 80.0°C to 30.0°C?
Answers
Answer:
5230 J
Explanation:
m = 25 g = 0,025 kg
c = 4,184 J /(g * °C) = 4184 J /(kg * °C)
\(t_{1}\) = 80 °C
\(t_{2}\) = 30 °C
The formula is Q = c *m * (\(t_{2} - t_{1}\))
Calculating:
Q = 4184 * 0,025 * (30 - 80) = 5230 J
Note that we get a negative heat (-5230 J). It just means that it is released.
The amount of heat will be 5230 j.
What is heat?
Heat is a type of energy that is transferred between both the system and its surroundings as a result of temperature variations.
Calculation of heat.
Given data:
Mass = 25.0 g = 0.025 kg
C = 4.184 J/g°C
\(T_{1}\) = 80.0°C
\(T_{2}\) = 30.0°C
Q= ?
By using the formula of heat.
Q = MC (\(T_{2} - T_{1}\))
Put the value of given data in heat equation.
Q(heat) = 0.025 × 4.184 ( 30 - 80)
Q(heat) = 5230 J.
Therefore, the amount of heat will be 5230 J.
To know more about heat.
https://brainly.com/question/13860901.
#SPJ2
Cl2 and N2 react according to the following equation 3Cl2(g) + N2(g) rightarrow 2NCl3(g) If 4 L of a stoichiometric mixture of chlorine and nitrogen are converted to nitrogen trichloride under conditions of constant temperature and pressure what is the volume of NCl3(g) produced?
Answers
Answer:
Volume of NCl3 is 3L
Explanation:
Avogadro states: All gases at the same volume under temperature and pressure constant have the same number of moles.
The chemical equation is:
3Cl2(g) + N2(g) → 2NCl3(g)
Where 3 moles of chlorine reacts with 1 mole of nitrogen to produce 2 moles of NCl3.
But using Avogadros law we can say:
3L of chlorine and 1L of nitrogen produce 2L of Nitrogen trichloride.
3L of chlorine and 1L of nitrogen: 4L (The stoichiometric mixture)
That means, volume of NCl3 produced is 3L
Determine the number of neutrons, protons, and electrons for a bromide ion that has a mass number of 77, and a charge of −1.
Answers
Answer: For the bromide ion with a mass number of 79 and a charge of -1, there are 36 electrons, 35 protons, and 44 neutrons present for the element that has a mass
For the element that has a mass number of 56 and 30 neutrons
Explanation:
How many moles of tin, Sn, are in 2500 atoms of tin?
Answers
4.2 × 10⁻²¹ moles Sn
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Using Dimensional AnalysisAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Explanation:Step 1: Define
2500 atoms Sn
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
Set up: \(\displaystyle 2500 \ atoms \ Sn (\frac{1 \ mol \ Sn}{6.022 \cdot 10^{23} \ atoms \ Sn})\)Multiply: \(\displaystyle 4.15144 \cdot 10^{-21} \ moles \ Sn\)Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs.
4.15144 × 10⁻²¹ moles Sn ≈ 4.2 × 10⁻²¹ moles Sn
Which force slows a skateboard when a skateboarder puts a foot down to brake?
O motion
O gravity
O friction
O inertia
Answers
Answer:
It is definitely friction
Aspirin synthesis involves the addition of an acetyl group to salicylic acid in a condensation reaction with an alcohol. The acetyl group could be added with a carboxylic acid but the preferred procedure is to use the acid anhydride. Why is preferable to use an acid anhydride for ester formation with an alcohol rather than a carboxylic acid? Select one: Carboxylic acids react with water in an undesired side reaction. Acid anhydrides are generally easier and safer to handle than carboxylic acids. Carboxylic acids change the pH of the solution too much for the reaction to proceed. Acid anhydrides are less stable than esters so the equilibrium favors the ester product.
Answers
Answer:
The correct appropriate will be Option 1 (Acid anhydrides are less stable than esters so the equilibrium favors the ester product.)
Explanation:
Acid anhydride, instead of just a carboxyl group, is typically favored for esterification. The predominant theory would be that Anhydride acid is somewhat more volatile than acid. This is favored equilibrium changes more toward the right of the whole ester structure. Extremely responsive than carboxylic acid become acid anhydride as well as acyl chloride. Thus, for esterification, individuals were most favored.The other options offered are not relevant to something like the scenario presented. So, the solution here is just the right one.
Using the reactivity series shown in the handbook, tell whether the following reactions
can occur: (YES or NO)
1. Na replaces K?
2. Co replaces Cu?
3. Zn replaces Zn2+?
4. Ni replaces Ba?
Answers
The possibility of metals replacing each other is dependent on the relative positions of the metals in the activity series.
The activity series is an arrangement of elements in order of decreasing reactivity. The elements that are higher in the series can displace the elements that are lower in the series.
Before we make a decision in each case, we must consider what metal is lower or higher in the series;
Between Na and K, Na is lower in the series so the answer is noBetween Co and Cu, Co is higher than Cu in the series so the answer is yesSince Zn and Zn^2+ are just a specie and its ion, the answer is no.Since Ba is higher than Ni, Ni can not replace Ba so the answer is noLearn more: https://brainly.com/question/24058474
Answer:
No
Yes
No
No
Explanation:
All combustion reactions release energy.
-True
-False
Answers
Answer:
true
Explanation:
All combustion reactions are chemical changes which release energy , hence the statement is true.
What are chemical changes?Chemical changes are defined as changes which occur when a substance combines with another substance to form a new substance.Alternatively, when a substance breaks down or decomposes to give new substances it is also considered to be a chemical change.
There are several characteristics of chemical changes like change in color, change in state , change in odor and change in composition . During chemical change there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
There are three types of chemical changes:
1) inorganic changes
2)organic changes
3) biochemical changes
During chemical changes atoms are rearranged and changes are accompanied by an energy change as new substances are formed.
Learn more about chemical changes,here:
https://brainly.com/question/23693316
#SPJ2
Acid catalyzed dehydration -condensation reactions of carboxylic acids and alcohols produce chemicals called esters. Using. Carbon skeletal notation, write the dehydration—condensation reaction that occurs. Between ethaanol and butanoic acid. What is the name of the este
Answers
The acid catalyzed dehydration and condensation reaction of acids and alcohols produce corresponding esters. The reaction of ethanol with butanoic acid produces ethyl butanoate.
What is esterification ?Esterification is the process of formation of esters by the treatment of carboxylic acid with alcohols. The COOH group from the acid and the OH group from the alcohols reacts together to eliminate a water molecule and then condensation occurs to form esters.
Esters are the compounds with the general formula of RCOOR, where the two R can be of same or different alkyl groups.
The butanoic acid and ethanol reacts together forms the ester ethyl butanoate as written below:
\(\rm CH_{3} -CH_{2}-OH + CH_{3} -CH_{2}-CH_{2}-COOH \rightarrow CH_{3} -CH_{2} -COO- CH_{2} -CH_{2}-CH_{3}\)
Find more on esterification :
https://brainly.com/question/30453412
#SPJ1