if 200 g of carbon reacts with excess sulfur dioxide to produce 225 g of carbon disulfide (cs2 ; molar mass 76.139 g/mol), what is the percent yield for the reaction?
Answers
The percent yield for the reaction is approximately 17.74%.
To calculate the percent yield for the reaction, we need to first determine the theoretical yield and then compare it with the actual yield.
1. Balanced equation for the reaction:
C + 2SO2 → CS2 + 2SO
2. Calculate moles of carbon:
200 g C × (1 mol C / 12.01 g C) = 16.66 mol C
3. Calculate theoretical yield:
16.66 mol C × (1 mol CS2 / 1 mol C) × (76.139 g CS2 / 1 mol CS2) = 1268.5 g CS2
4. Calculate percent yield:
Percent yield = (actual yield / theoretical yield) × 100
Percent yield = (225 g CS2 / 1268.5 g CS2) × 100
≈ 17.74%
To know something about the percent yield, click below.
https://brainly.com/question/17042787
#SPJ11
Related Questions
1. What is Science?
A. The goal of science is to investigate and understand the natural world, to explain events in the natural
world, and to use those explanations to make useful predictions.
Answers
If I have 6.02 x 1023 particles of O2, how many
moles do I have?
Answers
Answer:
You have about 6158 Molecules of 02.
Explanation:
write a balanced chemical equation showing the products of the dissolution of cr(clo3)3. (include states-of-matter under the given conditions in your answer. use the lowest possible whole number coefficients.)
Answers
Chromium(III) chlorate (Cr(ClO3)3) is a solid (s) that dissolves in water to form aqueous chromium(III) ions (Cr^3+, aq) and 3 aqueous chlorate ions (ClO3^-, aq). The coefficients represent the lowest possible whole numbers to balance the equation.
When Cr(ClO3)3 is dissolved in water, it dissociates into its respective ions. The balanced chemical equation for the dissolution of Cr(ClO3)3 can be written as:
Cr(ClO3)3(s) → Cr3+(aq) + 3ClO3-(aq)
This equation shows that one molecule of solid Cr(ClO3)3 dissociates into one Cr3+ ion and three ClO3- ions in aqueous solution. The state-of-matter for Cr(ClO3)3 is solid (s), while the state-of-matter for Cr3+ ion and ClO3- ions is aqueous (aq). The coefficients in the equation are already in their lowest possible whole number form.
To know about Dissolution visit:
https://brainly.com/question/29882264
#SPJ11
Select the correct answer. which substance in this redox reaction is the oxidizing agent? cu 2agno3 → 2ag cu(no3)2 a. n b. agno3 c. cu d. no3− e. cu(no3)2
Answers
AgNO₃ will act as the oxidising agent.
For the given chemical equation:Cu + 2AgNO₃ → 2Ag + Cu(NO₃)₂
Half reactions for the given chemical reaction:
Reducing agent:
Cu → Cu²⁺ + 2e⁻
Copper is a reducing agent because it is losing 2 electrons, which causes an oxidation process.
Oxidising Agent:
Ag⁺ + e⁻ → Ag
The silver ion undergoes a reduction process and is regarded as an oxidizing agent since it is acquiring one electron per atom.
Hence, AgNO₃ is considered as an oxidizing agent and therefore the correct answer is Option B.
Oxidising and Reducing agentsAn oxidizing agent is a substance that reduces itself after oxidizing another material. It passes through a reduction process in which it obtains electrons and the substance's oxidation state is decreased.A reducing agent is a chemical that oxidizes after reducing another material. It passes through an oxidation process in which it loses electrons and the substance's oxidation state increases.To know more about the process of Oxidation and Reduction, refer to:
https://brainly.com/question/4222605
#SPJ4
a) Draw the Jablonski diagram and explain the fates of molecules in excited states b) Explain why fluorescence spectroscopy is more informative compared to absorption spectroscopy
Answers
Fluorescence spectroscopy provides more detailed information compared to absorption spectroscopy due to its ability to detect both the excitation and emission wavelengths of a sample.
In absorption spectroscopy, only the absorption of light by the sample is measured, while in fluorescence spectroscopy, the absorbed light is re-emitted at a longer wavelength, providing additional data. This emission spectrum can reveal structural and environmental information about the molecules in the sample, such as their chemical composition, electronic states, and interactions.
Thus, fluorescence spectroscopy offers a more comprehensive understanding of molecular properties, making it a powerful tool in various scientific fields, including biochemistry, materials science, and environmental analysis.
For more information on spectroscopy visit: brainly.com/question/31594990
#SPJ11
the vapor pressure of liquid carbon disulfide, , is 100. mm hg at 268 k. a 0.0979 g sample of liquid is placed in a closed, evacuated 370. ml container at a temperature of 268 k. assuming that the temperature remains constant, will all of the liquid evaporate?
Answers
Yes, all the liquid will evaporates,
given that :
vapor pressure of liquid carbon disulfide = 100mmHg = 0.1315 kpa
temperature = 268 K
volume of the container = 370 mL = 0.370 L
using ideal gas equation we get :
PV = n R T
n = P V / R T
n = ( 0.315 × 0.370 ) / ( 0.0823 × 278)
n = 0.0050 mol = 5.0 × 10⁻³ mol
number of moles of CS₂ = mass / molar mass
= 0.0979 / 76.139
= 0.00128 mol
= 1.2 × 10⁻³ mol
it is clear that , the vapor pressure of liquid carbon disulfide, , is 100. mm hg at 268 k. a 0.0979 g sample of liquid is placed in a closed, evacuated 370. ml container at a temperature of 268 k. yes the temperature remains constant, all of the liquid evaporate.
To learn more about vapor pressure here
https://brainly.com/question/14664529
#SPJ4
What is the oxidation number of manganese (Mn) in potassium permanganate (KMnO4)?
a. +5
b. +7
c. +8
d. +9
Answers
The oxidation number of Mn in potassium permanganate is +7.
The oxidation number, also known as the oxidation state, is a measure of the degree of oxidation of an atom in a chemical compound. In potassium permanganate, the potassium ion (K+) has an oxidation state of +1, and each oxygen atom (O) has an oxidation state of -2. To determine the oxidation number of Mn, we can use the fact that the sum of the oxidation states in a neutral molecule must be zero. Therefore, we have:
(+1) + x + 4(-2) = 0
Simplifying this equation, we get:
x - 7 = 0
Solving for x, we get:
x = +7
Therefore, the oxidation number of Mn in KMnO₄ is +7. Answer: b. +7.
To learn more about potassium permanganate refer to:
brainly.com/question/29526566
#SPJ4
Can a stable compound be made from lithium and oxygen
Answers
Answer:
No, because oxygen wants two electrons to become stable and lithium will only give up 1 electron.
What is the pressure in atm of 0.47mol of a gas in a 1.7L container at 276K?
Answers
2. A sample of gas is placed in a container at 25oC and 2 atm of pressure. If the temperature is raised to 50oC, what is the new pressure? P = 2.17 atm
3. At 1 atm of pressure water boils at 100oC, if the sample was placed under 2 atm of pressure, what would be the temperature? (This would be like a pressure cooker).
T = 746 K = 473oC = 883oF
4. At what temperature would water boil if the pressure is 600 torr? (Use information from problem 3: this shows why food doesn't cook well at higher elevations)
T = 294 K = 21.5oC = 70.7oF
5. Calculate the volume of 40.6 g of F2 at STP. V = 23.9 L
6. A sample of 2.0 moles of hydrogen gas is placed in a container with a volume of 10.4 L. What is the pressure of the gas in torr if the gas is at 25oC? P = 4.70 atm = 3576
7. The tire pressure is 32 psi. What is the pressure in torr if 1 atm = 14.7 psi?
P = 1654 torr
8. A gas is placed in a balloon with a volume of 3.0 L at 28oC and 900 torr. What would be the new volume for the gas if placed under STP? V = 3.2 L
9. How many moles of gas would occupy a volume of 14 L at a pressure of 700 torr and a temperature of 30oC? n = 0.52 mol
10. Calculate the volume of 24.0 g of HCl at STP. V = 14.8 L
11. What is the volume of one mole of acetylene gas at STP? V =22.414 L
12. What is the volume of 0.75 mol of gas at 72oC and 2 atm? V = 10.6 L
13. After eating beans, a student collects a sample of gas at 0.97 atm and 26oC which occupies a volume of 3.5 L, calculate its volume at STP. V = 3.1 L
14. Ammonia (NH3) is placed in 1.5 L flask at 25oC. If the pressure of the gas is 0.899 atm, what is the density? d = 0.626 g/L
15. A mixture of Ar and CO gases is collected over water at 28oC and an atmospheric pressure of 1.05 atm. If the partial pressure of Ar is 600 torr, what is the partial pressure of CO? (vapor pressure of water at 28oC is 28.3 mmHg) PCO = 0.223 atm
16. Determine the partial pressures of each of the gases in the following mixture: 17.04 g NH3, 40.36 g Ne and 19.00 g F2. The gases are at 1.5 atm of pressure.
PNH3 = 0.428 atm; PNe = 0.857 atm; PF2 = 0.2124 atm
17. Potassium chlorate decomposes under heat as follows:
2 KClO3 (s) -------> 2 KCl (s) + 3 O2 (g)
The oxygen gas is collected over water at 25oC. The volume of gas is 560 mL measured at 1 atm. Calculate the number of grams of KClO3 used in the reaction. (vapor pressure of water = 0.0313 atm) nO2 = 0.022 mol; 1.81 g KClO3
Can someone help me answer these chemistry problems?
1. How many moles of helium are required to fill a 5.10 L balloon to a pressure of 1.1 atm at 22 degrees C? Show your work to receive full credit.
2. If there are 4.00 moles of a gas at a pressure of 5.60 atm and a volume of 12.0 liters, what is the temperature? Show your work to receive full credit.
3. If I contain 3 moles of gas in a container with a volume of 60 liters and at a temperature of 400 K, what is the pressure inside the container? Show your work to receive full credit.
4. You are given a 3.00 mol sample of krypton with a volume of 7.00 L. The temperature is held at 300. K. What is the pressure of the krypton sample? Show your work to receive full credit.
5. The air at the top of Mount Everest has pressures of 201.0 torr N2, 50.0 torr O2, 2.0 torr Ar, and 0.5 torr CO2. What is the total air pressure at the top of the highest peak in the world? Show all work to receive full credit.
6. A mixture of 1.20 mols He, 2.40 mols Ne, 4.80 mols Kr, and 0.60 mols Ar has a total pressure of 600.0 mm Hg. What is the partial pressure of the Kr? Show all work to receive full credit.
Answers
Answer:
Q.1
To calculate moles of helium (n)=?
given
p=1.1 Atm
V=5.10 L
T =22°C
R =8.314 (universal gas constant)
Now use,
PV = nRT
1.1×5.10 =n×8.314×22
n= 5.61÷182.9
n= 0.030 moles
Use the same method to solve further questions!
The chemical equation below represents an unbalanced chemical reaction:
Fe + 0, → Fe,o,
When the equation is balanced, what coefficient is needed for Fe2O3?
A
1
B
2
С
3
D
4
Answers
Answer:
2
Explanation:
The phenomenon in which electrons that are closer to the nucleus slightly repel those that are farther out, is known as: select the correct answer below: - shielding - deflecting - building up - converging
Answers
The phenomenon in which electrons that are closer to the nucleus slightly repel those that are farther out is known as Shielding.
Electrons in an atom are negatively charged particles, and they are attracted to the positively charged nucleus. However, the outer electrons of an atom are also repelled by the inner electrons that are closer to the nucleus. This repulsion is due to the negative charges of the electrons, and it partially cancels out the attraction of the nucleus for the outer electrons.
Shielding is the phenomenon in which electrons that are closer to the nucleus slightly repel those that are farther out. This makes it possible for electrons in higher energy levels to be farther from the nucleus, so they are less strongly attracted and easier to remove.
Learn more about Shielding here: https://brainly.com/question/27985711
#SPJ11
which of the following statements are correct? Rewrite each false statement to make it true. (a) Elements listed in rows on the periodic table are in the same family. (b) Elements in the same column of the periodic table exhibit the same physical properties. (c) Elements in the same group are in the same family. (d) Elements that are side by side on the periodic table belong to the same period.
Answers
Answer:
c)elements in the same group are in the same family
The elements in the same column are in the same family.
The periodic table is arranged in rows and the periods. The elements in the same row has the same number of shells. The elements in the same column are in the same family.
The correct statements are;
Elements in the same column of the periodic table exhibit the same physical properties. Elements in the same group are in the same family. Elements that are side by side on the periodic table belong to the same period.Learn more: https://brainly.com/question/20906233
Structures and Forces - What is force and external forces.
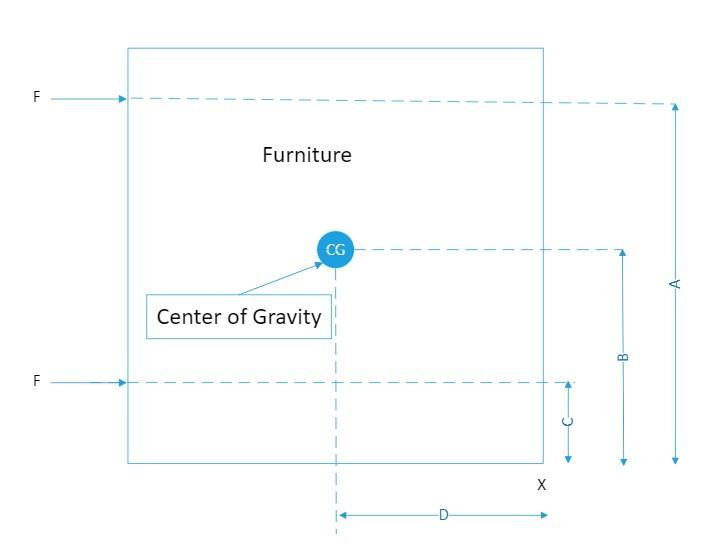
Question 1) Jacob and Ravleen are moving furniture. They need to move the filing cabinet over 6 feet and it is very heavy. Jacob wants to apply force and push it by putting his hands on point A because he thinks it would be easier to move it from the top as there is less weight on the top. Ravleen says it's better to push at point B because the center of gravity is lower. Which person is correct and EXPLAIN why and what may happen if it is pushed in the wrong spot.
Answers
Answer:
The restoring force that prevents the furniture from tipping over when pushing the furniture towards the right, F= W × D
Where;
W = The weight of the furniture
D = The horizontal distance from the center of gravity of the furniture, CG and the to the lower right corner of the furniture
Given that A > D, for the furniture to tip over when pushing above the CG, we have;
F1 × A ≤ W × D
∴ F1 << W or for a force much smaller than W, the weight of the furniture applied above the center of gravity, the furniture will tip over
However when the force F2 is applied, where C < D, we have for no tipping over;
F2 × C ≤ W × D, therefore, given that C < D, when a force much higher than the weight, W, is applied at F2, the furniture will remain upright
Therefore, it is better push at a point lower than or equal to the center of gravity, whereby the point B is lower than the center of gravity, it is better to push at point B, Ravleen is correct and it is better to push at point B because the center of gravity is lower
If the furniture is pushed at the wrong spot it may tip over
Please see attached drawing
Explanation:
Rank the following solutions from lowest to highest vapor pressure. Rank from lowest to highest. To rank items as equivalent, overlap them
1. 20 g. of glucose C6H12O6 in 100ml h2o
2. 10 g. of protassium acetate KC2H3O2 in 100ml h2o
3. 20 g. of sucrose C12H22O11 in 100ml h2o
Answers
The solutions will be ranked as follows from lowest to highest vapor pressure:
20 g. of glucose C6H12O6 in 100ml h2o
20 g. of sucrose C12H22O11 in 100ml h2o
10 g. of potassium acetate KC2H3O2 in 100ml h2o
What factors affect the vapor pressure?The vapor pressure depends on the concentration and nature of the solute. A nonvolatile solute like sugar will decrease the vapor pressure of the solvent, while a volatile solute such as acetate salt , will increase it
Both glucose and sucrose are non-volatile solutes, so they will decrease the vapor pressure of the solvent and thus will have lower vapor pressure than the pure solvent. However, sucrose has a lower molecular weight than glucose which means that it will have a greater effect on the vapor pressure of the solution and thus have a slightly lower vapor pressure.
To know more about sucrose, visit:
brainly.com/question/29186350
#SPJ1
Is oxygen and potassium an ionic bond?
Answers
Ionic bond: Metal & Nonmetal
Brainliest is appreciated
What is the total number of neutrons in the nucleus of a neutral atom that has 19 electrons and a mass number of 39
Answers
Answer:
NO OF NEUTRON =MASS NO -ATOMIC NO
=39-19
=20
bcz atomic no is equal to no of electrons
mark as brainliest
During radioactive decay, atomic nuclei of unstable isotopes
a.
give off nuclear radiation.
b.
are broken down by radioactive bacteria.
c.
form chemical bonds.
d.
are unchanged.
Answers
Which elements are
considered "Noble Metals"?
Answers
Answer:
ruthenium (Ru), rhodium (Rh), palladium (Pd), osmium (Os), iridium (Ir), platinum (Pt), gold (Au), silver (Ag).
Explanation:
The term "Noble Metals" traditionally refers to a group of metals that are resistant to corrosion and oxidation in moist or chemically aggressive environments. The elements commonly considered noble metals are Gold, Platinum, Palladium, Palladium, etc.
Gold is perhaps the most well-known noble metal. It is highly resistant to corrosion and oxidation. Platinum is another widely recognized noble metal. It is extremely resistant to corrosion and has a high melting point.
Palladium is a noble metal that exhibits excellent chemical stability and resistance to corrosion.
To learn more about Noble Metals, follow the link:
https://brainly.com/question/15775417
#SPJ6
What properties do compounds with covalent bonds have?
High melting point
Solid only at room temperature
Solid, liquid, or gas at room temperature
Low electrical conductivity
High electrical conductivity
Low melting point
Answers
Answer:
properties of compounds with covalent bonds include:
They are powerful chemical bonds that exist between atoms.
Covalent bonds rarely break on their own after they are formed.
A covalent bond forms when two non-metal atoms share a pair of electrons.
Covalent bonds are strong – much energy is needed to break them.
Compounds with giant covalent structures have high melting and boiling points. The large number of strong covalent bonds involved means that a large amount of energy is required to break them apart.
Compounds with covalent bonds may be solid, liquid or gas at room temperature depending on the number of atoms in the compound. Since most covalent compounds contain only a few atoms and the forces between molecules are weak, most covalent compounds have low melting and boiling points.
Covalent compounds do not conduct electrical currents. This is because they lack free ions. The movement of charge carriers is the reason why water is conductive. In contrast, covalent compounds do not contain ions and are not soluble in water. However, there are several examples of covalent compounds that do conduct electricity. These include graphite, a metal with a single free electron.
hope that was helpful! :D
Which statement about exothermic reactions is TRUE?
A.In an exothermic reaction, energy is absorbed, bonds are broken, and the temperature decreases.
B. In an exothermic reaction, energy is released, bonds are formed, and the temperature increases.
Answers
Which statement about exothermic reactions is TRUE?
A.In an exothermic reaction, energy is absorbed, bonds are broken, and the temperature decreases.
B. In an exothermic reaction, energy is released, bonds are formed, and the temperature increases.3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many moles of water can be made when 170.2 grams of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Nitrogen
14
Copper
63.5
Oxygen
16
Answers
When 170.2 grams of HNO3 are consumed, 1.35 moles of water can be created. The answer, rounded to the closest tenth, is 1.4 moles of water.
Molar mass: What Is It?The mass in grams of one mole of a chemical is its molar mass. The quantity of atoms, molecules, and ions contained in a substance is measured in terms of moles. A mole of any substance contains 6.022 x 1023 molecules.
We must first calculate how many moles of HNO3 are present in 170.2 grams.
We may convert grams to moles using the molar mass of HNO3:
molar mass of HNO3 = (1 x 1) + (14 x 1) + (3 x 16) = 63 g/mol
moles of HNO3 = 170.2 g / 63 g/mol = 2.70 mol
To calculate the quantity of water produced, we can use the following ratio:
4 moles H2O / 8 moles HNO3 = x moles H2O / 2.70 moles HNO3
After finding x, we obtain:
x = (4 moles H2O / 8 moles HNO3) * 2.70 moles HNO3 = 1.35 moles H2O.
To know more about molar mass visit:-
https://brainly.com/question/22997914
#SPJ1
what type of mixtures can be seperated by using fractional distillation? Give any two examples
Answers
Answer:
liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation and separation of various components of crude oil. This method works because the liquids in the mixture have different boiling points.
Explanation:
please give 5 star
Arrange the gases in order of decreasing density when they are all under STP conditions.
Neon , Helium, Florine, Oxygen
Answers
The correct order of decreasing density of the gases under STP conditions is Fluorine, Oxygen, Neon, and Helium.
Under STP conditions, the gases can be arranged in order of decreasing density as follows:
Fluorine > Oxygen > Neon > Helium
Fluorine has the highest density due to its relatively heavy atomic mass compared to the other gases. Oxygen follows next with a density higher than Neon and Helium. Neon has a density higher than Helium as it has a larger atomic size and atomic mass than Helium. Helium, being the lightest gas, has the lowest density among the given gases under STP conditions.
The higher the molar mass, the greater the density of the gas. Here are the molar masses of the given gases:
1. Helium (He) - 4 g/mol
2. Neon (Ne) - 20 g/mol
3. Fluorine (F2) - 38 g/mol
4. Oxygen (O2) - 32 g/mol
Using the molar masses, we can arrange the gases in decreasing order of density:
1. Fluorine (F2)
2. Oxygen (O2)
3. Neon (Ne)
4. Helium (He)
Under STP conditions, the gases in decreasing order of density are: Fluorine, Oxygen, Neon, and Helium.
Learn more about STP conditions here:-
https://brainly.com/question/31609879
#SPJ11
explain why a water or carbon dioxide fire extingisher might not be effective in putting out a sodium fire
Answers
Water and carbon dioxide are ineffective extinguishing agents for a sodium fire because they react with sodium to produce hydrogen gas and carbon monoxide, which can ignite and worsen the fire.
When sodium metal is exposed to air or water, it reacts vigorously and can ignite, producing a bright yellow flame. Sodium is highly reactive and has a low ignition temperature, so even a small amount of heat or moisture can cause it to ignite.
Water is not appropriate because it reacts with sodium to produce hydrogen gas, which can ignite and make the fire worse. This reaction also releases a large amount of heat, which can further fuel the fire.
Carbon dioxide can also be ineffective for extinguishing a sodium fire. While carbon dioxide does displace oxygen, it can also react with the sodium metal to produce carbon monoxide and sodium oxide. These products can also ignite and potentially increase the intensity of the fire.
Therefore, in the case of a sodium fire, specialized extinguishing agents such as dry powder, sand or graphite are recommended. These agents can help to smother the fire and prevent the sodium from coming into contact with air or moisture, which can further fuel the fire. It is important to note that sodium fires should be handled with extreme caution and should only be extinguished by trained professionals using the appropriate equipment and techniques
For more such questions on Sodium
https://brainly.com/question/20482524
#SPJ4
Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
Acid rain may have 355 ppm of dissolved carbon dioxide, which contributes to its acidity. Calculate the molar concentration of carbon dioxide in a 4.26L sample of acid rain
Answers
The molar concentration of CO2 in a 4.26 L sample of acid rain is 0.0081 mol/L.Acid rain is caused by air pollution.
The molar concentration of carbon dioxide in a 4.26L sample of acid rain is calculated as follows:First, we will calculate the number of moles of carbon dioxide in the sample. The formula to calculate the number of moles is:Number of moles = mass of the substance/molar mass of the substanceThe molar mass of CO2 is 44.01 g/mol. 355 ppm means 355 parts per million, which is equivalent to 355 mg/L.To calculate the mass of carbon dioxide in 4.26 L of acid rain:Mass of CO2 = Volume × ConcentrationMass of CO2 = 4.26 L × 355 mg/L = 1510.3 mg = 1.51 gTherefore, the number of moles of CO2 in the sample is:Number of moles of CO2 = Mass of CO2/Molar mass of CO2 = 1.51 g/44.01 g/mol = 0.0344 molTo calculate the molar concentration of CO2:Molar concentration of CO2 = Number of moles of CO2/Volume of solution in litersMolar concentration of CO2 = 0.0344 mol/4.26 L = 0.0081 mol/L, When sulfur dioxide and nitrogen oxide pollutants combine with atmospheric moisture, they form acidic compounds that can travel long distances and fall as acid rain. Acid rain can damage forests, kill fish in streams, and corrode buildings and monuments, among other things.
for more such questions on concentration
https://brainly.com/question/28564792
#SPJ11
The decomposition of N2O5 dissolved in carbon tetra chloride occurs followingly at constant temperature. N2O5(solution)⇌2NO2(solution)+1/2 O2(g)
This reaction is of first order and its rate constant is 5×10^−4 sec^−1? If initial concentration of N2O5 is 0.4 mol litre^−1 then
(i) What will be the initial reaction rate?
(ii) What will be the half-life period of this reaction?
(iii) What time will be taken to complete 75% reaction?
Answers
(i) The initial reaction rate is \(2*10^{-4} mol litre^{-1} sec^{-1.\)
(ii) The half-life period of the reaction is 1386 seconds.
(iii) The time taken to complete 75% of the reaction is approximately 2772 seconds.
We can use the first-order rate equation:
Rate = k[N2O5]
Where:
Rate is the reaction rate,
k is the rate constant,
[N2O5] is the concentration of N2O5.
Given:
Rate constant (k) = \(5*10^{-4} sec^{-1}\)
Initial concentration of N2O5 =\(0.4 mol litre^{-1}\)
(i) To find the initial reaction rate:
Substitute the given values into the rate equation:
Rate = k[N2O5]
Rate = \((5*10^{-4} sec^{-1})(0.4 mol litre^{-1})\)
Rate = \(2*10^{-4} mol litre^{-1} sec^{-1}\)
The initial reaction rate is \(2*10^{-4} mol litre^{-1} sec^{-1}\).
(ii) To find the half-life period:
The half-life of a first-order reaction is given by the equation:
t(1/2) = (0.693 / k)
Substitute the given value of k into the equation:
t(1/2) = \((0.693 / 5*10^{-4} sec^{-1})\)
t(1/2) = 1386 sec
The half-life period of this reaction is 1386 seconds.
(iii) To find the time taken to complete 75% of the reaction:
The time required to complete a certain percentage of a reaction can be found using the equation:
t = (ln(1 / (1 - x)) / k)
Where x is the fraction of the reaction completed (in this case, 75%).
Substitute the given values into the equation:
t =\((ln(1 / (1 - 0.75)) / 5*10^{-4} sec^{-1})\)
t = 2772 sec
The time taken to complete 75% of the reaction is approximately 2772 seconds.
To know more about reaction rate refer here
https://brainly.com/question/13693578#
#SPJ11
Can you create or destroy energy? If yes why, if no why?
Answers
It can only be converted from one form of energy to another.
I hope this helps! ❤️
Answer:
Energy can be changed from one form to another, but it cannot be created or destroyed. The total amount of energy and matter in the Universe remains constant, merely changing from one form to another
Explanation:
energy cannot be created Or destroyed.
I have little or no rainfall for long periods of time. I cause death to all living things because of lack of water. What am I?
Answers
Answer:
a drought !
- little to no water causes it
- it causes for organisms to die and organisms need water !