if 16 g of a radioactive substance are present, what mass (in grams) will be present after two half-lives?your answer should have two significant figures.
Answers
After one half-life, half of the original radioactive substance will remain, which is 8 grams. After another half-life (totaling two half-lives), half of the remaining 8 grams will remain, which is 4 grams.
Therefore, after two half-lives, 4 grams of the radioactive substance will be present.
It is important to note that the concept of half-life is used to describe the rate at which a radioactive substance decays. It is the amount of time it takes for half of the original sample to decay. The remaining amount will continue to decay at the same rate until there is none left. The half-life of a substance can vary greatly, ranging from fractions of a second to billions of years.
In conclusion, if 16 g of a radioactive substance are present and two half-lives have occurred, the mass of the substance remaining would be 4 grams with two significant figures.
To learn more about half-life, refer:-
https://brainly.com/question/24710827
#SPJ11
Related Questions
Determine the expected mass of the copper after 24 hours

Answers
The expected mass of copper element after 24 hours as per graph is 288 grams.
What is an element?It is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
The number of protons in the nucleus is the defining property of an element and is related to the atomic number.All atoms with same atomic number are atoms of same element.In every 10 minutes 2 mg of copper is produced thus after 24 hours that is 1440 minutes copper amount will be 1440×2/10=288 mg.
Thus, the expected mass of copper element after 24 hours as per graph is 288 grams.
Learn more about element,here:
https://brainly.com/question/14347616
#SPJ5
what mass (in g) of potassium chlorate is required to supply the proper amount of oxygen needed to burn 131.8 g of methane? assume 100% yield for both reactions. enter to 0 decimal places. are the equations balanced?
Answers
The balanced chemical equation for the complete combustion of methane is: CH4 + 2O2 -> CO2 + 2H2O. the mass of potassium chlorate needed to burn 131.8 g of methane is 999.47 g.
The number of moles of methane can be calculated as follows:
131.8 g methane / 16.04 g/mol = 8.22 moles
From the balanced chemical equation, we can see that 2 moles of oxygen are required to burn 1 mole of methane, so the number of moles of oxygen needed to burn 8.22 moles of methane is 2 * 8.22 moles = 16.44 moles.
The balanced chemical equation for the decomposition of potassium chlorate to produce oxygen is: 2KClO3 -> 2KCl + 3O2
So, for every 2 moles of potassium chlorate decomposed, 3 moles of oxygen are produced. Hence, to produce 16.44 moles of oxygen, we need 16.44 moles / 3 moles/2 moles of KClO3 = 8.22 moles of KClO3.
The mass of potassium chlorate required can be calculated as follows:
8.22 moles * 122.55 g/mol = 999.47 g
So, the mass of potassium chlorate required to supply the proper amount of oxygen needed to burn 131.8 g of methane is 999.47 g.
Learn more about methane here:
https://brainly.com/question/2127750
#SPJ4
Ba(CN)2
What is the name of the compound?
Answers
Answer:
Barium cyanide
Explanation:
I don't no
The name of the compound Ba(CN)₂ is called barium cyanide.
The compound Ba(CN)₂ is called barium cyanide. It is a white crystalline solid that is soluble in water. Barium cyanide is a toxic compound and can be fatal if ingested. It is used in electroplating and other metallurgical processes.
The name of the compound is derived from the names of the elements that make up the compound. Barium is named after the Latin word for "heavy", and cyanide is named after the Greek word for "blue". The -ide suffix indicates that the compound is an ionic compound, with barium as the cation and cyanide as the anion.
The chemical formula for barium cyanide can be written in a few different ways. The most common way is to write it as Ba(CN)₂. This formula shows that there is one barium atom for every two cyanide atoms. The formula can also be written as BaCN₂, but this is less common.
To know more about compound here
https://brainly.com/question/34151797
#SPJ6
true or false, Characteristic line spectra only appear in the visible region of light.
Answers
Characteristic line spectra only appear in the visible region of light.
This is true because this is the only region we can see the line spectra.
Visible light or white light comprises of seven colours which can produce wavelengths and frequencies of Spectra by either emission or absorption of light/ radiation on matter.
To identify elements,we use the visible lines emitted from the excited atoms since they all have their own emission spectrum unique to each of them.
See related answer here:https://brainly.com/question/18498298
The video uses visual representations to show the generation and travel of radio waves. What did you learn about radio waves from these representations?
Answers
Answer: I learned that almost every technology has radio waves that spread out from their sources.
Explanation:
Methyl orange might be selected as an indicator for the titration of a weak base with a strong acid. Can you estimate the ph at the equivalence point?.
Answers
Yes. The pH of 3.7 should be the equivalency point.
Methyl orange is employed as a strong acid and weak base indication for what reason?Methyl orange transforms from a basic to an acidic color during a strong acid weak base titration, making it a useful chemical indicator. In acidic and basic media, respectively, methyl orange exhibits red and yellow colors. In titration, the solution appears yellow at first because the pH is high.
Methyl orange is a frequently used pH indicator in titrations because of its easy-to-see color shift. Because it changes color at a weak acid's pH, it is frequently employed in acid titrations. Methyl orange turns red in an acidic medium while turning yellow in a basic one.
To know more about indicator visit:
https://brainly.com/question/29359841
#SPJ4
1. On a train ride cross-country, the trees, buildings, and people appear to be speeding by the window. This is because the train is our _____
2. Scientists use the ______
system for making measurements.
3. Physical science studies the relationships between ____,____,
and ______.
4. If 6.6 tyrannosaurs weigh 30,000 kg, then what does one tyrannosaur weigh?
5. For most activities, the ___
makes a handy frame of reference.
Answers
On a train ride cross-country, the trees, buildings, and people appear to be speeding by the window. This is because the train is moving in the forward direction.
Scientists use the shared system for making measurements. This system is also called system of international.
Physical science studies the relationships between natural but non-living objects. Physical science is ordinarily thought of as consisting of four broad areas: astronomy, physics, chemistry, and the Earth sciences.
If 6.6 tyrannosaurs weigh 30,000 kg, then one tyrannosaur weigh about 4545 kg. For most activities, the earth makes a handy frame of reference.
Learn more about science here: https://brainly.com/question/17216882
#SPJ1
Which method of finding slope do you prefer? Why?
(slope formula, table, other)
Answers
At 25 oC the solubility of magnesium carbonate is 3.16 x 10-3 mol/L. Calculate the value of Ksp at this temperature. Give your answer in scientific notation to 2 SIGNIFICANT FIGURES (even though this is strictly incorrect)
Answers
The value of Ksp at 25°C for magnesium carbonate is 9.98 x \(10^{(-6)\)\(mol^2/L^2\), rounded to 2 significant figures.
The solubility product constant (Ksp) represents the equilibrium constant for the dissolution of a sparingly soluble compound in water. For magnesium carbonate, the balanced equation for its dissolution can be written as:
\(MgCO_3\)(s) ⇌ \(Mg^{(2^+) }(aq) + CO3^{(2-) }(aq)\)
The solubility of magnesium carbonate is given as 3.16 x \(10^{(-3)\) mol/L. In this case, the concentrations of \(Mg^{(2^+)\) and \(CO_3^{(2^-)\)ions in the equilibrium expression are both equal to the solubility value, which can be represented as \([Mg^{(2^+)}] = [CO_3^{(2^-)}] = 3.16 * 10^{(-3)\) mol/L.
The equilibrium expression for the dissolution reaction can be written as:
Ksp =\([Mg^{(2^+)}] *[CO_3^{(2^-)}]\)
Substituting the solubility value into the equation, we have:
Ksp = (3.16 x \(10^{(-3)\) mol/L) * (3.16 x \(10^{(-3)\)mol/L) = 9.98 x \(10^{(-6)\)\(mol^2/L^2\)
Thus, the value of Ksp at 25°C for magnesium carbonate is 9.98 x\(10^{(-6)} mol^2/L^2\), rounded to 2 significant figures.
Learn more about solubility product constant here:
https://brainly.com/question/1419865
#SPJ11
How many pKa values are there for a monoprotic acid ?
a. 4
b. 2
c. 1
d. 3
Answers
Answer:
Only one, so option c
Explanation:
Because monoprotic as it says, had only one proton to lose giving it only one pka for example Hydrochloric Acid (H-Cl). unlike a diprotic acid which would have two pkas like carbonic acid for example (H2CO3).
Drag each tile to the correct image. Match each hydrocarbon class to its structure. carboxylic acid amine halocarbon alcohol

Answers
Answer:
1. Amine.
2. Alcohol.
3. Carboxylic Acid.
4. Halocarbon.
Explanation:

The correct answer according to the tile are Amine, Alcohol, Carboxylic acid, Halocarbon.
How can hydrocarbons be classified based on their structure?
Hydrocarbons can be classified as either aromatic or aliphatic compounds, depending on the presence of a benzene ring.
What is the most common classification of hydrocarbons?
Alkanes are hydrocarbons in which all of the bonds are single bonds. Alkenes are hydrocarbons that contain a carbon-carbon double bond.
Learn more about hydrocarbon here : brainly.com/question/490217
#SPJ2
Suppose that the bond market and the money market both start out in equilibrium, then the Federal Reserve decreases the money supply. The result will be a ______________ in the money market and a _________________ in the bond market, which will push bond prices _________________ and interest rates will ___________________ until a new equilibrium is reached.
Answers
The result will be a decrease in the money market and an increase in the bond market, which will push bond prices down and interest rates will rise until a new equilibrium is reached.
Suppose the Federal Reserve decreases the money supply, this action will lead to a decrease in the money market equilibrium and an increase in the bond market equilibrium. As a result, bond prices will drop, and interest rates will rise until a new equilibrium is established. This shift will occur because as the money supply decreases, the demand for money will increase, leading to an increase in interest rates.
This increase in interest rates will then cause bond prices to drop as the cost of borrowing rises, and investors will demand a higher yield. Ultimately, a new equilibrium will be established where the demand for money equals the supply, and the bond market is in equilibrium with the new interest rate.
More on markets: https://brainly.com/question/32114875
#SPJ11
any single orbital can hold a maximum of electrons when full
Answers
Answer:
2 electrons
Explanation:
I literally learned this in class just now.
Answer:
There can be two electrons in one orbital maximum. The s sublevel has just one orbital, so can contain 2 electrons max. The p sublevel has 3 orbitals, so can contain 6 electrons max.
UwU I might be wrong
Which of the following is the strongest intermolecular interaction? Group of answer choices A, dipole-dipole interactions B, London dispersion force C, covalent bonding D, ionic bonding
Answers
Covalent bonding is the strongest intermolecular interaction. However, it is important to note that covalent bonding is not an intermolecular force, but rather an intramolecular force.
Intermolecular forces are the interactions between molecules, while intramolecular forces are the interactions within a molecule.
Of the options given, ionic bonding is the strongest intermolecular force. It involves the complete transfer of electrons from one atom to another, resulting in the formation of ions that are held together by electrostatic attraction. Dipole-dipole interactions and London dispersion forces are weaker than ionic bonding, but they are still important intermolecular forces. Dipole-dipole interactions involve the attraction between the positive end of one polar molecule and the negative end of another polar molecule.
London dispersion forces are the weakest intermolecular force and arise from the temporary dipoles that form due to the movement of electrons within a molecule.
Learn more about intermolecular interaction here:
https://brainly.com/question/29690903
#SPJ11
Why is it not possible to use solid sodium hydroxide directly to make a standard NaOH
solution?
Answers
Answer:
Sodium hydroxide is deliquescent (absorbs moisture from the atmosphere) solid. It cannot be weighed accurately. Therefore, it is not possible to prepare a standard solution of sodium hydroxide of accurately known concentration by weighing NaOH.
In pole beans, green pods (g) are a dominant trait, while yellow pods (g) are a recessive trait. a bean plant with a gg genotype is crossed with a second plant that has the gg genotype. if this cross produces 500 offspring, approximately how many of the offspring will have green pods? responses a 00 b 125125
c 250250 d 500
Answers
The GG genotype of one bean plant is crossed with the gg genotype of another plant. 500 offspring this hybrid will generate will also have green pods on them The dominant gene will be present in all 500 of offspring, making them all green.
What exactly are dominant and recessive traits?Even though the dominant trait only exists in one copy, it is always manifested when the linked allele is dominant. Only when both of the related alleles are recessive do recessive features manifest themselves. The related trait is much less likely to develop when one of the genes is dominant.
An easy dominant attribute is what?An inheritance pattern with simple dominance is defined as a trait with two separate qualities, where one allele dominates the other and the trait is governed by a single gene. Humans receive 2 pairs of 23 pairs of chromosomes, each from each parent, one from each parent's side of the family.
To know more about dominant visit:
https://brainly.com/question/2808776
#SPJ4
2. If a student drops a 2.3 g piece of magnesium into a flask of hydrochloric acid, this reaction occurs: Mg + 2HCl MgCl2 + H2
How many liters of hydrogen can be produced at a pressure of 2 atm and a temperature of 298 K?
Answers
1.2 L of hydrogen can be produced at a pressure of 2 atm and a temperature of 298 K.
What is an ideal gas equation?The ideal gas law (PV = nRT) relates the macroscopic properties of ideal gases. An ideal gas is a gas in which the particles (a) do not attract or repel one another and (b) take up no space (have no volume).
Step 1: Write the balanced equation
Mg + 2 HCl ⇒ MgCl₂ + H₂
Step 2: Calculate the moles corresponding to 2.3 g of Mg
The molar mass of Mg is 24.31 g/mol.
2.3 g × 1 mol ÷24.31 g = 0.095 mol
Step 3: Calculate the moles of H₂ produced
0.095 mol Mg × 1 mol H₂ ÷ 1 mol Mg = 0.095 mol H₂
Step 4: Calculate the volume occupied by the hydrogen
We will use the ideal gas equation.
P × V = n × R × T
V = n × R × T÷P
V = 0.095 mol × (0.0821 atm.L/mol.K) × 298 K÷2 atm
V = 1.2 L
Learn more about the ideal gas here:
https://brainly.com/question/27691721
#SPJ1
A student is driving her car when an insect strikes her windshield. Which statement correctly describes the forces in this situation?
Answers
Answer:
The insect exerts no force on the windshield, and the windshield strikes the insect with a large force.
Explanation:
:#D
the 5p orbitals fill immediately after the 4d orbitals and immediately before the 6s based on: (select all that apply)
Answers
The 5p orbital fills immediately after the 4d and immediately before the 6s orbital. True.
Orbital electron confirgurationThe orbital electron configuration is the arrangement of electrons via the available orbitals in the atoms of elements. This arrangement follows certain rules. These include:
Aufbau principle: this states that low energy level orbitals are first filled before higher energy level orbitals.Hund's rule: states that electrons are distributed singly in orbitals with more than one degenerate before pairing starts.Following these rules, the order of filling the different orbitals that exist, including the degenerates is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p. 6s, etc.
In other words, the 5p orbital comes after the 4d orbital but before the 6s orbital. This means that the 5p orbital will be filled with electrons immediately after the 4d orbital and before the 6s orbital.
More on orbital electron configurations can be found here: https://brainly.com/question/29215691
#SPJ1
A friend suggests the following question for a science experiment. Discuss ways that the question could be reworded to make it a more appropriate scientific question. How much pollution is in urban streams?
Answers
Answer:
I don't think so
Explanation:
many pollution
solve for x 5 to the power 2 x + 4 - 25 to the power x - 1 is equals to
Answers
Answer:
[][][][][][][][][][][][][][]
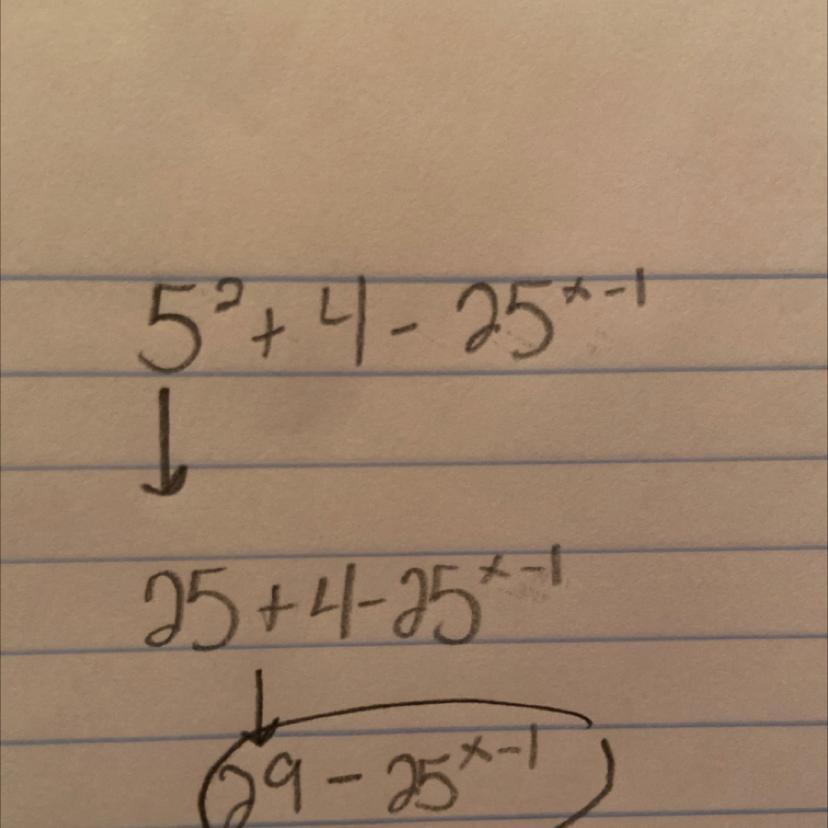
Which of the following is true about distillation boiling point determination? More than one answer may be correct.
O Keep boiling the sample until there is nothing left in the flask.
O The reduced pressure needs to be recorded if performing the distillation under vacuum.
O Impurities in the sample will not affect the boiling point.
O The sample should be as pure as possible before boiling point determination.
O The thermometer should be positioned with the tip immersed in the liquid.
Answers
The statements which true about distillation boiling point determination are:
The reduced pressure has to be recorded if performing the distillation under vacuum.The sample must be as pure as possible before boiling point determination.The thermometer must be positioned with the tip immersed in the liquid.What is distillation?Distillation is the process which happens when a liquid sample is volatilized to produce a vapor which is subsequently condensed to a liquid richer in the more volatile components of the initial sample. It is symbolized at its simplest when steam from a kettle turns deposited as drops of distilled water on a cold surface.
Regarding the distillation boiling point determination, the temperature at where a liquid's vapor pressure reaches equilibrium with atmospheric pressure is called the liquid's boiling point. To accomplish distillation at decreased pressure, it is needed to record the external pressure. The sample have to be as pure as is practical. When the contaminants are dissolved, the liquid's boiling point increases. Thus, the recorded boiling point will not be exact. And to measure temperature changes properly, the thermometer's tip has to be placed in the liquid.
Learn more about distillation at: https://brainly.com/question/29037176
#SPJ4
What is force? Why?
Answers
which pictogram is not mandatory for chemical labels and sds?
Answers
OSHA would only require the use of eight of the nine pictograms that the GHS employs. Although not required, the environmental pictogram could be employed to provide supplementary information.
Anything without risks to one's physical safety and health is not dangerous and does not need a safety data sheet (SDS). Be aware that many dusts, including flour as well as hardwood dusts, contain potential health risks and demand an SDS.
Wood or wood-based items. the definition of explosives in the Explosives Act. radioactive nuclear substances as defined by the Nuclear Safety as well as Control Act. hazardous waste which is being bought and sold either through recovery, recycling, and disposal.
To know more about pictograms
https://brainly.com/question/29898314
#SPJ4
An ion has 28 protons, 24 electrons, and 30 neutrons. What is its ionic
charge?
Answers
To answer this we look only at the protons and electrons. Because there are 4 more protons than electrons we know that this ion has 4 less electrons than it would need to have to have a neutral charge. Therefore, the ionic charge is +4
the air within a piston equipped with a cylinder absorbs 565 j of heat and expands from an initial volume of 0.10 l to a final volume of 0.85 l against an external pressure of 1.5 atm. what is the change in internal energy of the air within the piston? (
Answers
Air within the piston experiences a 490 J shift in internal energy.
The internal energy (U) of a system in thermodynamics is the total energy contained in the system and may be thought of as the sum of the energy in the form of work (w, supplied to or released by the system) and energy in the form of heat (q, given to or released by the system) . This value is positive when the system takes heat from its surroundings, whereas it is negative when it releases heat into its surroundings. When the environment works with the system, there is a positive value; but, when the system works against the environment, there is a negative value.
ΔU = q + w
The negative product of the change in volume (V) and the external pressure (P) is known as w. [w = - P·ΔV]
⇒ ΔU = q - P·ΔV
The cylinder, the piston, and the trapped air make up the system in this puzzle (Attached)
1) q= 565 J (a positive value because the surroundings transferred heat to the cylinder) (a positive value because the environment delivered heat to the cylinder)
2) P = 1 atm.
3) V = 0.86 atm.L - 0.12 atm.L = 0.74 atm.L - q + w - U = 565 J - 1 atm - 0.74 atm.L - U = 565 J - 0.74 atm.L
We must use the same heat unit—the joule—to indicate the work value. In light of the fact that one joule is = 0.00987 atm.L, 0.74 atm.L x (1 J/0.00987 atm.L) = 74.97 J. U = q + w U = 565 J - 74.97 J U = 490 J.
Learn more about Internal energy here:
https://brainly.com/question/11742607
#SPJ4
Question 14 of 34
for a reaction, ah = 2 kj. for which value of tas is the reaction
spontaneous?
oa. -2 kj
b. 3 kj
oc. 2 kj
od. -3 kj
Answers
Option B is correct answer, when TΔS is 3 KJ the reaction is spontaneous reaction.
What is spontaneous reaction?
A spontaneous process in thermodynamics is one that happens without the system receiving any outside input. The time-evolution of a system in which it releases free energy and transitions to a lower, more thermodynamically stable energy state is a more technical definition (closer to thermodynamic equilibrium). Following the general convention for thermodynamic measurements, the sign convention for changes in free energy is as follows: a release of free energy from the system corresponds to a negative change in the system's free energy and a positive change in the surrounding free energy.
The reaction's spontaneity is determined by the Gibbs Free Energy (ΔG) value.
This value is calculated using the following equation, which depicts the link between temperature, entropy (ΔS), enthalpy (ΔH), and the Gibbs free energy (ΔG):
ΔG = ΔH - T(ΔS)
A positive ΔG value represents a non-spontaneous reaction,
A negative ΔG value represents a spontaneous reaction.
Given ΔH = 2 KJ,
We get ΔG negative only when TΔS is 3 KJ.
To know more about spontaneous reaction, go to link
https://brainly.com/question/24376583
#SPJ4
copper
tin (IV) sulfite
copper(1) sulfite
+ tin
Answers
Experiments conducted flow of liquid through eylinder Shows following correlation for a dissolving solute ( mass tramien Sh=0.023(Re)0.83(3e)1/3;Sh=5herwood number, 3e = benimidt number colburn Asbuming chilton-analogy, write the corresponding heat transfer correlation.
Answers
The Chilton-Colburn analogy, also known as Reynolds Analogy, provides a connection between heat transfer and mass transfer of a fluid.
Hence, the corresponding heat transfer correlation, assuming the Chilton-Colburn analogy for mass transfer is:
Sh = 0.023(Re)0.83(3e)1/3
The corresponding heat transfer correlation by Chilton-Colburn analogy is:
Nu = 0.023 (Re)0.83 (Pr)n, where n = 0.4 to 0.6.
Assuming that the Chilton-Colburn analogy holds good for the present situation, the above correlation can be used as a heat transfer correlation.
Learn more about Reynolds Analogy
brainly.com/question/31298157
#SPJ11
What is the answer
A 2
B 2.5
C 3
