identify the initial and final states if an electron in hydrogen emits a photon with a wavelength of 656 nm .
Answers
The initial and final states if an electron in hydrogen emits a photon with a wavelength of 656 nm is n = 3 to n = 2.
given that :
wavelength of the photon emitted λ = 656 nm = 656 × 10⁻⁹ m
the energy of the photon is given as :
E = hc / λ
= (6.626 × 10⁻³⁴ × 3 ×10⁸ ) / 656 × 10⁻⁹
= 3 × 10⁻¹⁹ J = 1.9 eV
En = - 13.6 / n² eV
where n = 1,2,3 ....
1) n = 2 to n = 1
E2 - E1 = - 13.6 eV ( 1 / 4 - 1/ 1)
= 10.2 eV
2) n = 3 to n = 2
E3 - E2 = -13.6 eV ( 1/3² - 1/ 2² )
= 1.9 eV
Thus the initial and the final states are n = 3 to n = 2.
To learn more about wavelength here
https://brainly.com/question/29892125
#SPJ4
Related Questions
I NEED HELP! The quarter is ending for me tomorrow and I have to have this in, please help if you can.
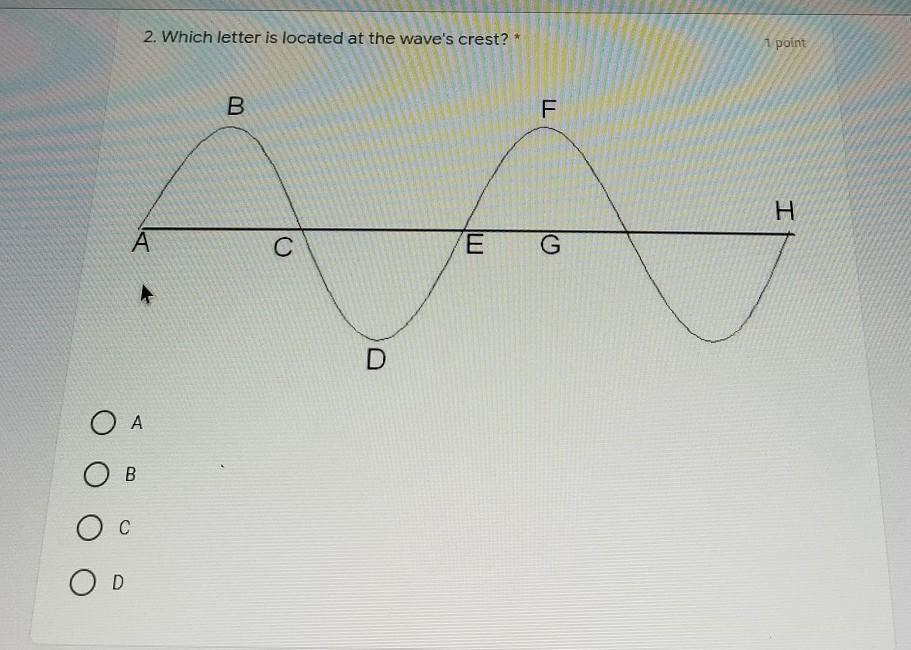
Answers
Answer:
The answer is B
someone please help, here is the question.
calculate the grans of Mg needed, when reacted with excess HCI, to produce 40.0 ml of hydrogen gas at 760.mm Hg 23°c?
Answers
0.040 grams of Mg are needed when reacted with excess HCl to produce 40.0 mL of hydrogen gas at 760 mm Hg and 23°C
To calculate the grams of Mg needed, we will use the ideal gas law (PV=nRT) and stoichiometry.
Given: volume of H₂ = 40.0 mL, pressure = 760 mm Hg, temperature = 23°C.
1. Convert volume to L: 40.0 mL × (1 L/1000 mL) = 0.040 L
2. Convert temperature to K: 23°C + 273.15 = 296.15 K
3. Convert pressure to atm: 760 mm Hg × (1 atm/760 mm Hg) = 1 atm
Using the ideal gas law, PV=nRT:
n = PV/RT = (1 atm × 0.040 L) / (0.0821 L atm/mol K × 296.15 K) = 0.00165 mol H₂
The balanced chemical equation is:
Mg + 2HCl → MgCl₂ + H₂.
From the stoichiometry, 1 mol of Mg produces 1 mol of H₂.
Therefore, moles of Mg needed = 0.00165 mol.
Finally, to find the grams of Mg needed, multiply by the molar mass of Mg (24.31 g/mol):
0.00165 mol × 24.31 g/mol = 0.040 g
Learn more about ideal gas law at
https://brainly.com/question/29883434
#SPJ11
What volume of a 2.0M NaOH(aq) is needed to completely neutralize 24 milliliters of 0.5M HCl(aq)?
Show numerical setup and answer.
Answers
Answer:
\(V_{base}=6.0mL\)
Explanation:
Hello there!
In this case, by considering that the reaction between sodium hydroxide and hydrochloric acid is in a 1:1 mole ratio of these two reactants, we are able to use the following equation relating the concentration and volume of each one:
\(M_{acid}V_{acid}=M_{base}V_{base}\)
In such a way, by solving for the volume of the base, we will obtain:
\(V_{base}=\frac{M_{acid}V_{acid}}{M_{base}} \\\\V_{base}=\frac{0.5M*24mL}{2.0M}\\\\V_{base}=6.0mL\)
Regards!
Tornadoes can negatively affect an ecosystem when
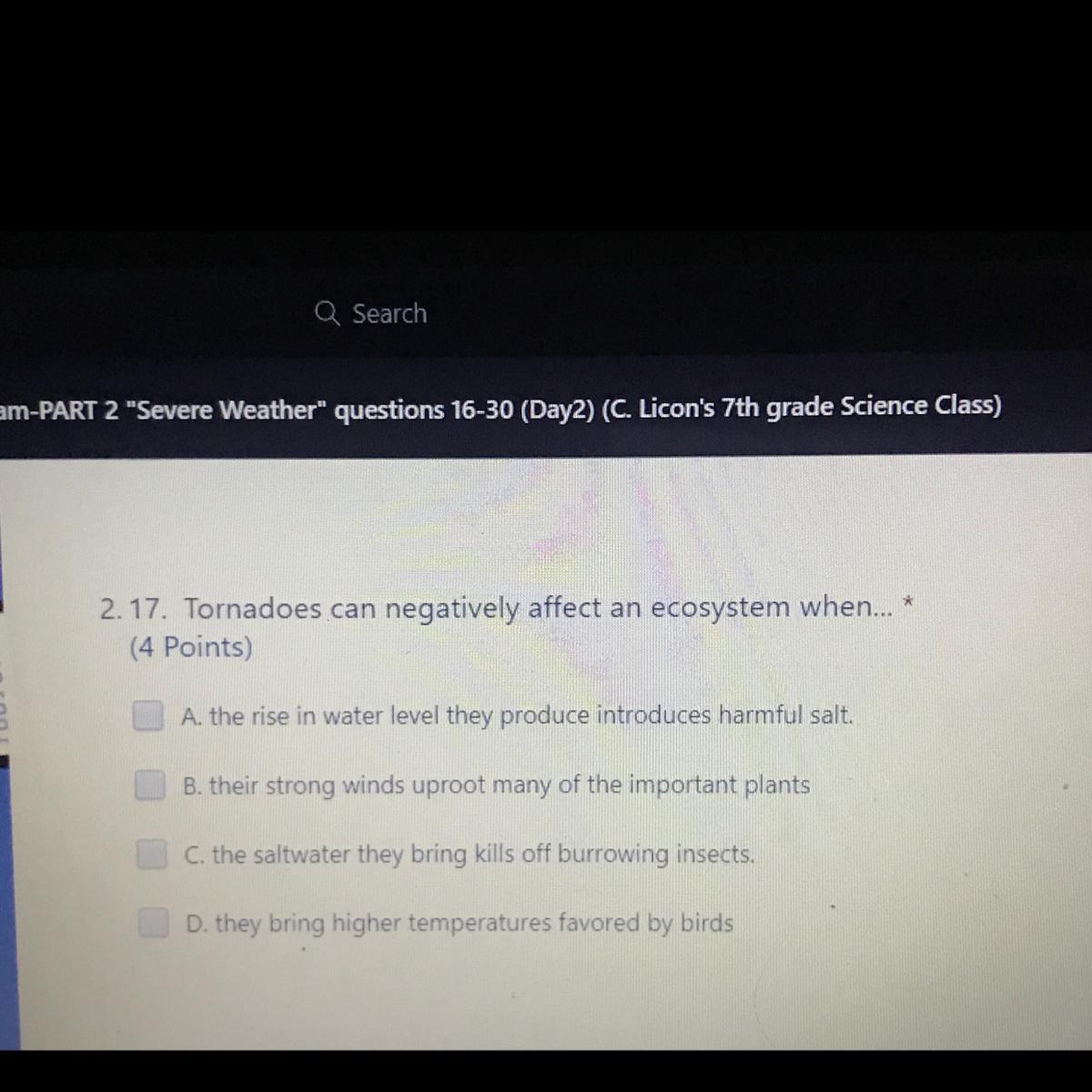
Answers
Answer:
b, their strong winds uproot many of the important plants
Explanation:
Which formula can be used to find velocity if kinetic energy and mass are known?
V=
-(KE)(m)
V=
2 m
KE
V=
KE(m)
2 KE
V=
m

Answers
Answer:
\(v = \sqrt{ \frac{2ke}{m} } \)
option D is the correct option.
Here,
If an object of mass 'm' moving with a velocity'v' then,
\(kinetic \: energy = \frac{1}{2} m {v}^{2} \\ \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: {v}^{2} = \frac{2ke}{m} \\ \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: \: {v}^{2} = \sqrt{ \frac{2ke}{m} } \)
hope this helps...
Good luck on your assignment..
Answer:
it's D on edge2021
Explanation:
Balance the following reaction. Choose "blank" if no coefficient is needed.
C 2 H 5 + O 2 → CO 2 + H 2 O
Answers
Answer:
4 C2H5 + 13 O2 -> 8 CO2 + 10 H2O
Explanation:
What I do is I make a chart of how many elements the product side and reactant sides have:
Reactants-
2 C
5 H
2 O
Products-
1 C
3 O
2 H
It's easier to see how many coefficients are needed for the reaction to be balanced then. Hope this helped.
How do these momenta compare the ballon rocket?
Answers
Hypothesis: If you can measure the pH of a range of acids and bases using a universal pH indicator, then you can use those values to calibrate a cabbage pH indicator. To determine the pH of a solution using a pH indicator paper, you need a .
Answers
To determine the pH of a solution using a pH indicator paper, you need a color chart or a color scale that corresponds to different pH values.
This color chart or scale is used to compare the color of the pH indicator paper after it has been immersed in the solution. The pH indicator paper is impregnated with a universal pH indicator, which is a chemical compound that changes color depending on the acidity or alkalinity of the solution.
The indicator undergoes a chemical reaction with the hydrogen ions (H+) or hydroxide ions (OH-) present in the solution, resulting in a color change.
By comparing the color of the pH indicator paper with the color chart or scale, you can determine the approximate pH of the solution. The color chart usually provides a range of colors corresponding to different pH values, allowing you to match the observed color to the nearest pH value.
In the hypothesis mentioned, the aim is to calibrate a cabbage pH indicator using the pH values obtained from a universal pH indicator. Therefore, in addition to the pH indicator paper and color chart, you would also need a range of solutions with known pH values to establish a calibration curve specific to the cabbage pH indicator.
In summary, to determine the pH of a solution using a pH indicator paper, you need a color chart or scale that correlates the observed color of the pH indicator paper with different pH values. This chart or scale serves as a reference for interpreting the color change and determining the pH of the solution.
Know more about hypothesis here:
https://brainly.com/question/31293943
#SPJ8
Answer: COLOR KEY
Explanation: CS
PLEASE HELP ME ASAP!!
In a chemical change, _________ materials are formed that are different from the starting materials.
Answers
To solve such this we must know the concept of chemical reaction. Therefore, in a chemical change, different materials are formed that are different from the starting materials.
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved.
There are so many types of chemical reaction reaction like combination reaction, double displacement reaction. Chemical reaction is a chemical process in which different materials are formed that are different from the starting materials.
Therefore, in a chemical change, different materials are formed that are different from the starting materials.
Learn more about the chemical reactions, here:
brainly.com/question/3461108
#SPJ1
what is usually needed for a decomposition reaction to take place?
Answers
Energy input in the form of heat, light, or electricity is necessary for the majority of decomposition reactions.
Do elements always result from a reaction of decomposition?
Decomposition reactions usually produce discrete elements as their byproducts. An addition reaction occurs when a reactant splits into two or more products. This breakdown reaction is 2AI2O3 4AI + 3O2.
What is frequently required for a decomposition process to take place?
Energy input in the form of heat, light, or electricity is necessary for the majority of decomposition reactions. Only two elements can be found in binary compounds. When a binary chemical breaks down into its constituent parts, it undergoes the simplest type of breakdown reaction.
Learn more about binary compounds:
brainly.com/question/2951729
#SPJ4
which of the compounds should test positive for the 2,4-dnp test? which of the compounds should test positive for the 2,4-dnp test? p-anisaldehyde menthol benzaldehyde menthone
Answers
p-anisaldehyde and benzaldehyde are the compounds should test positive for the 2,4-dnp test.
For the qualitative determination of ketone or aldehyde functional group carbonyl functionality, 2,4-Dnp or Dinitrophenylhydrazine can be utilised. Dinitrophenylhydrazone, a precipitate that might be yellow, orange, or red, forms when the test is successful. positive A negative test indicates that your unknown does not contain an aldehyde or ketone, whereas a positive test indicates that it does. A positive test is actually the result of the hydrazine group's reaction with the aldehyde or ketone.
Learn more about 2,4-dnp here:
https://brainly.com/question/16899050
#SPJ4
write out the structure of the cofactor required for each of the following reactions
Answers
Enzymes are biological catalysts that facilitate chemical reactions in living organisms. Many enzymes require the assistance of cofactors, which are non-protein molecules that aid in the enzyme's function. There are two types of cofactors: inorganic cofactors and organic cofactors, also known as coenzymes.
Now, for each of the following reactions, I will provide the structure of the cofactor required:
1. Alcohol dehydrogenase: This enzyme facilitates the conversion of alcohol to aldehyde. The cofactor required for this reaction is NAD+ (nicotinamide adenine dinucleotide), which is an organic cofactor. Its structure consists of two nucleotides joined by a phosphate group, with a nicotinamide group attached to one of the nucleotides.
2. Carbonic anhydrase: This enzyme facilitates the conversion of carbon dioxide and water into bicarbonate ions. The cofactor required for this reaction is a zinc ion, which is an inorganic cofactor. Its structure consists of a single zinc atom coordinated by four nitrogen atoms in a tetrahedral arrangement.
3. Cytochrome P450: This enzyme facilitates the oxidation of various organic compounds, including drugs, toxins, and steroids. The cofactor required for this reaction is heme, which is an organic cofactor. Its structure consists of an iron ion coordinated by a porphyrin ring.
4. DNA polymerase: This enzyme facilitates the synthesis of new DNA strands. The cofactor required for this reaction is magnesium ion, which is an inorganic cofactor. Its structure consists of a single magnesium atom coordinated by six water molecule.
To know more about Biological Catalysts visit:
https://brainly.com/question/13493167
#SPJ11
The rate constant for a first-order reaction is 0.54 s-1. What is the half-life of this reaction if the initial concentration is 0.39 M
Answers
The half life time of first order reaction is 1.3 sec
Given:
First order rate constant, K = 0.54 M⁻¹s⁻¹
Initial concentration= 0.39 M
From the formula of first order half life time,
K = \(0.693/t_{1/2}\)
So half life time is given by, sec
t = 0.693/0.54 = 1.3 sec
What is half life ?Half-life, in radioactivity, is the amount of time needed for half of a radioactive sample's atomic nuclei to decay (change spontaneously into other nuclear species by emitting particles and energy), or, alternatively, the amount of time needed for a radioactive material's rate of disintegrations per second to decrease by half.
Cobalt-60, a radioactive isotope used in radiotherapy, has a half-life of 5.26 years, for instance. As a result, after that time, a sample that had 8 g of cobalt-60 at first would only have 4 g of cobalt-60 and would produce half as much radiation.
To view more about half life, refer to:
https://brainly.com/question/15418153
#SPJ4
period of repolarization of the neuron during which it cannot respond to a second stimulus
Answers
The period of repolarization of a neuron during which it cannot respond to a second stimulus is known as the refractory period. It typically lasts for about 1-2 milliseconds.
The Refractory Period: How Neurons Reset and Respond to StimuliThe refractory period is a crucial part of the neuron's normal functioning. This period of time allows the neuron to recover from the initial stimulus and reset itself before it can respond to a second stimulus. During the refractory period, the neuron's voltage and other properties return to their resting state, allowing it to be responsive to a second stimulus. This short period of time typically lasts for 1-2 milliseconds, after which the neuron can respond to a second stimulus.
Learn more about Neurons: https://brainly.com/question/26387085
#SPJ4
When heated, calcium carbonate decomposes according to the equation given below:
CaCO3 --> CaO + CO2
If 50.0 g of CaCO3 react but only 20.0 g of CO2 are recovered, what is the percent yield of this reaction?
90.9%
40.0%
66.7%
250%
Answers
Answer:
Percent yield = 90.9%
Explanation:
Given data:
Mass of CaCO₃ = 50.0 g
Mass of CO₂ produced = 20.0 g
Percent yield = ?
Solution:
Chemical equation:
CaCO₃ → CaO + CO₂
Number of moles of CaCO₃:
Number of moles = mass/molar mass
Number of moles = 50.0 g/ 100.1 g/mol
Number of moles = 0.5 mol
Now we will compare the moles of CO₂ with CaCO₃.
CaCO₃ : CO₂
1 : 1
0.5 : 0.5
Mass of CO₂: Theoretical yield
Mass = number of moles × molar mass
Mass = 0.5 mol × 44 g/mol
Mass = 22 g
Percent yield:
Percent yield = ( actual yield / theoretical yield ) × 100
Percent yield = (20.0 g/ 22.0 g) × 100
Percent yield = 0.909 × 100
Percent yield = 90.9%
Is this correct?????? Pls helpppp :)

Answers
Answer:
i think all is correct but maybe switch 3 and 11? to me it makes way more sense.
Explanation:
What are some possible factors that must remain constant during the testing
Answers
Answer:
Four basic components that affect the validity of an experiment are the control, independent and dependent variables, and constants. These basic requirements need to be present and identified to consider an experiment valid.
Is the pressure of an ideal gas directly proportional to its absolute temperature?
Answers
The pressure of an ideal gas is directly proportional to its absolute temperature.
How are pressure of an ideal gas and temperature related?
The pressure of an ideal gas is directly proportional to absolute temperature at constant volume.The volume of a gas is also directly proportional to it's absolute temperature at constant pressure.
When a constant volume of gas is held, the pressure of gas increases as the temperature increases. Mathematically it can be expressed as:
P∞T
In order to remove proportionality sign an equal sign is introduced with a proportionality constant 'K',thus the equation becomes
P=KT
On dividing both sides of above equation by T , equation becomes
P/T=K
When a plot of pressure versus temperature is plotted a straight line graph is obtained passing through the origin.
To know more about pressure-temperature relation of ideal gas click here:
https://brainly.com/question/1190311
#SPJ4
what is 10 times 10
Answers
Answer:
\(\huge{\textbf{\textsf{{\color{skyblue}{An}}{\blue{sw}}{\red{er}}{\color{red}{:}}}}}\)
10 times 10
\(10 \times 10 \\ = 100\)
100 is the correct answer.
Answer:
\({\boxed{\boxed{\tt { ⎆ Answer :- }}}} \ \)
⏩ \(10 \: \: times \: \: 10 \\ = 10 \times 10 \\ = 100\)
ʰᵒᵖᵉ ⁱᵗ ʰᵉˡᵖˢ
\( GuMiHo \) ❦
Why does Oxygen (O - 8) have a higher ionization energy than Tellurium (Te - 52)?
Answers
compare and contrast the four types of crystals
Answers
The four types of crystals are ionic crystals, metallic crystals, covalent crystals, and molecular crystals.
What are crystals?Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry.
An ionic crystal is a crystalline ionic compound. They are solids consisting of ions bound together by their electrostatic attraction into a regular lattice.
A crystalline solid in which the atoms are held together by metallic bonds. Metallic crystals are found in some interstitial compounds as well as in metals and alloys.
Covalent Crystals also called atomic crystals are the molecular solids in which the same or different atoms are joined together by covalent bonding.
Molecular crystals are substances that have relatively weak intermolecular binding, such as dry ice.
Thus, there are four types of crystals such as ionic crystals, metallic crystals, covalent crystals, and molecular crystals.
Learn more about crystals here:
https://brainly.com/question/1951877
#SPJ2
Animal cells do NOT
have cell walls. Why do
you think this is an
advantage?
Answers
Answer:
Because they don't need them , cell wall which are found in cell, maintain cell shape almost as if each cell has it's own exoskeleton ame this them to stand upright without the need for bones .
Explanation:
Organize the reaction steps in the best order for the synthesis of the compound shown from benzene. 1. bromination 2. nitration 3. reduction.
Answers
The best order for the synthesis of the compound shown from benzene would be 1. nitration, 2. bromination, and 3. reduction.
Firstly, nitration involves adding a nitro group (-NO₂) to the benzene ring, which is achieved by reacting benzene with a mixture of concentrated nitric and sulfuric acid. This reaction is highly exothermic and can result in the formation of undesirable by-products if not carefully controlled. However, once the nitro group is added, it provides a suitable handle for subsequent functionalization reactions.
Next, bromination involves adding a bromine atom (-Br) to the benzene ring, which is achieved by reacting the nitrobenzene with a bromine source such as iron(III) bromide. This reaction replaces the nitro group with a bromine atom, which is more reactive and can undergo further reactions.
Finally, reduction involves converting the bromonitrobenzene to the desired compound by reducing the nitro and bromine groups to amine and alkyl groups, respectively. This can be achieved using a reducing agent such as hydrogen gas and a metal catalyst such as palladium on carbon. The reduction reaction must be carefully controlled to avoid over-reduction or under-reduction of the functional groups.
In summary, the best order for the synthesis of the compound shown from benzene is nitration, bromination, and reduction. This order ensures that the functional groups are added and modified in a controlled manner to achieve the desired product.
To know more about synthesis, refer to the link below:
https://brainly.com/question/3471911#
#SPJ11
1. For the reaction below, which change would cause the equilibrium
to shift to the right?
CH,(g) + 2H,S(g) + heat --
CS,(g) + 4H,(g)
(a) Decrease the concentration of H,S.
(b) Increase the pressure on the system.
(c) Increase the temperature of the system.
(d) Increase the concentration of CS,
(e) Decrease the concentration of CH.
Answers
option c is the correct answer
because the gas there will be evolved and caused the equilibrium to shift to right side
ionisation, the first important work for percussion ensemble, was composed by:____
Answers
The composition "Ionisation" was composed by Edgard Varèse. It is considered a groundbreaking and influential piece in the realm of percussion ensemble music.
Completed in 1931, "Ionisation" is significant because it was one of the first compositions to exclusively focus on percussion instruments, without the inclusion of traditional melodic or harmonic elements.
Varèse explored the vast potential of percussion sounds, utilizing various instruments including drums, cymbals, sirens, and even unconventional objects like anvils and whistles.
The composition's rhythmic complexity, use of polyrhythms, and exploration of timbre pushed the boundaries of traditional musical conventions and expanded the possibilities for percussion ensemble compositions in the future.
To know more about Ionisation, refer here:
https://brainly.com/question/20658080#
#SPJ11
How many milliliters of a 0.900% (m/v) normal saline solution can be prepared from 3.00 g of sodium chloride, NaCl
Answers
We can prepare approximately 333.33 milliliters of a 0.900% (m/v) normal saline solution from 3.00 g of sodium chloride.
To determine the volume of a 0.900% (m/v) normal saline solution that can be prepared from 3.00 g of sodium chloride (NaCl), we need to calculate the amount of NaCl required to make this solution.
The term "0.900% (m/v)" means that there are 0.900 g of NaCl dissolved in 100 mL of solution. So, if we have 3.00 g of NaCl, we can use a proportion to find the volume of solution:
(3.00 g NaCl) / (0.900 g NaCl/100 mL solution) = (3.00 x 100) / 0.900 mL solution
= 333.33 mL solution
Therefore, we can prepare approximately 333.33 milliliters of a 0.900% (m/v) normal saline solution from 3.00 g of sodium chloride.
To know more about normal saline solution visit:
https://brainly.com/question/29120657
#SPJ11
How do the ramp heights of the different objects compare? How does the ramp height relate to the strength of the frictional force between the book and the object?
Answers
The height of a ramp does not directly determine the strength of the frictional force between a book and an object.
How do they compare?The strength of the frictional force between a book and an object is not directly influenced by the height of a ramp. The nature of the surfaces in contact, the force forcing the surfaces together (normal force), and the coefficient of friction are some of the variables that affect the frictional force between two surfaces.
The coefficient of friction between the book and the object plays a major role in determining the strength of the frictional force.
Learn more about frictional force:https://brainly.com/question/30280206
#SPJ1
A gas occupies 37. 5 mL at 102. 3 kPa. At 27. 5 mL, what will the pressure be?
Answers
The pressure will be 139.92 kPa at a volume of 27.5 mL.
To answer this question, we will use Boyle's Law formula, which states that the product of the initial pressure (P1) and volume (V1) of a gas is equal to the product of the final pressure (P2) and volume (V2) when the temperature remains constant.
Step 1: Identify the initial pressure (P1), initial volume (V1), and final volume (V2).
P1 = 102.3 kPa
V1 = 37.5 mL
V2 = 27.5 mL
Step 2: Apply Boyle's Law formula, which is P1 * V1 = P2 * V2. We need to find the final pressure (P2).
102.3 kPa * 37.5 mL = P2 * 27.5 mL
Step 3: Solve for P2.
P2 = (102.3 kPa * 37.5 mL) / 27.5 mL
Step 4: Calculate the value of P2.
P2 ≈ 139.64 kPa
At 27.5 mL, the pressure of the gas will be approximately 139.64 kPa.
Learn more about pressure at https://brainly.com/question/28012687
#SPJ11
What is the equation for lactic fermentation after glycolysis?.
Answers
The equation for lactic fermentation after glycolysis is:
pyruvic acid + NADH → lactic acid + NAD⁺
Skeletal muscles are where lactic acid fermentation takes place. When there is not enough oxygen, lactate dehydrogenase converts pyruvate to lactic acid. Lactic acid accumulation in the muscles causes fatigue. Muscle cells and other bacterial and animal cells engage in a type of anaerobic fermentation.
The metabolic process known as lactic acid fermentation turns glucose or other six-carbon sugars into the metabolite lactate, which is the lactic acid in solution, and cellular energy.
The creation of many food products involves the bacterial process known as lactic fermentation. It serves a critical function in food safety by giving the finished products distinctive scents and textures.
To know more about lactic fermentation visit the link:
https://brainly.com/question/2095032?referrer=searchResults
#SPJ4
How much more average Kinetic Energy do molecules have at 50°C compared to 25°C?
Answers
The kinetic energy is 0.5177 × 10⁻²¹ J more at 50°C compared to 25°C.
The average kinetic energy of a molecule is directly proportional to the absolute temperature of a gas.
KE = ( 3/2 ) ( R / Nₐ ) T
Where T is the temperature of the molecule, R is the gas constant, and Nₐ is Avogadro's number.
Now, R = 8.314 J/mol.K
Avogadro's number, Nₐ = 6.022 × 10²³ atoms/ mol
The average kinetic energy at 50° C is:
T = 50° C = 323 K
KE₁ = ( 3/2 ) × ( R / Nₐ ) × T₁
KE₁ = ( 3 × 8.314 × 323 ) / ( 2 × 6.022 × 10²³ )
KE₁ = 668.90 × 10⁻²³ J
KE₁ = 6.6890 × 10⁻²¹ J
The average kinetic energy at 25°C is:
KE₂ = ( 3/2 ) × ( R / Nₐ ) × T₂
KE₂ = ( 3 × 8.314 × 298 ) / ( 2 × 6.022 × 10²³ )
KE₂ = 617.13 × 10⁻²³ J
KE₂ = 6.1713 × 10⁻²¹ J
Now,
The average kinetic energy of the molecules at 50° C compared to 25° C is:
KE = KE₁ - KE₂
KE = 6.6890 × 10⁻²¹ - 6.1713 × 10⁻²¹
KE = 0.5177 × 10⁻²¹ J
Hence, the average kinetic energy is 0.5177 × 10⁻²¹ J more at 50° C compared to 25° C.
Learn more about kinetic energy here:
https://brainly.com/question/8101588
#SPJ9