Answers
Answer:
P4 + 5O2 → P4O10
Explanation:
White Phosphorus - P4
Dioxygen - O2
Tetraphosphorus Decaoxide - P4O10
Related Questions
The correct number of significant figures for the following set of numbers; 1.200, 0.00340, 55000, 80.080 would be
Responses
2, 3, 5, 5
2, 3, 5, 5
4, 5, 2, 4
4, 5, 2, 4
2, 6, 5, 5
2, 6, 5, 5
4, 3, 2, 5
Answers
4,3,2,5
Explanation:
All zero placed to the right of a decimal point in a number are significant.
1.200 = two significant figures
The zero written to the left of the first non-zero digits in a number are not significant.
0.00340 = three significant figures.
The zero written without digits and after non-zero digits are not significant.
55000 = two significant figures.
The zero between two non-zero digits in a number are significant.
80.080 = five significant figures.
Write the objectives for the topic "Synthetic Fibre & Plastics"
Answers
Answer:
1. To understand the differences between natural and synthetic fibers and their properties.
2. To learn about the manufacturing process of synthetic fibers and plastics and the different types of synthetic fibers and plastics available.
3. To explore the various applications of synthetic fibers and plastics in different industries such as fashion, automotive, construction, and electronics.
4. To investigate the environmental impact of synthetic fibers and plastics and the importance of recycling and proper disposal methods.
5. To evaluate the advantages and disadvantages of synthetic fibers and plastics compared to natural materials and their impact on society and the economy.
6. To analyze the future trends and innovations in the field of synthetic fibers and plastics and their potential impact on different industries and the environment.
if an atom has 22 protons 28 neutrons 20 electrons what would its atomic number be
Answers
Answer:
22
Explanation:
The atomic number indicates the amount of protons, so it would be 22
Enter the nuclear equation for the alpha decay of polonium-216 (21684Po).
Express your answer as a nuclear reaction.
Answers
Through the reaction Po Pb + ta, the Po-216 undergoes alpha decay and transforms into lead (Ph-212).
What's Po's nuclear equation?The Polish scientist Marie Curie and her husband Pierre discovered polonium in 1898, the first naturally occurring unstable element that was isolated. Po21284, Pb20882, and He42 are released when it decomposes. An oxygen isotope called 17O was the first nuclide to be created artificially.
Physical half-life for polonium-210 is 138 days. As lead-206 is produced by radioactive decay, alpha particles are released. Although the alpha particles have a relatively small range, they are quite energetic.
learn more about polonium-210
https://brainly.com/question/16979893
#SPJ4
Can someone please help me with this question. I got half of the question and I am stuck on the rest.

Answers
The mean of the data set is approximately 4.0626, and the 90% confidence interval is [4.060925, 4.064275].
What is the mean and 90% confidence interval of the given data?The sample mean (x) is calculated as follows:
x = (4.0620 + 4.0550 + 4.0650 + 4.0740 + 4.0550 + 4.0660) / 6
x ≈ 4.0626 (rounded to four decimal places)
The 90% confidence interval is calculated as follows;
Standard deviation (s):
(4.0620 - 4.0626)² = 0.00000036
(4.0550 - 4.0626)² = 0.00000576
(4.0650 - 4.0626)² = 0.00000006
(4.0740 - 4.0626)² = 0.00001328
(4.0550 - 4.0626)² = 0.00000576
(4.0660 - 4.0626)² = 0.00000012
average of the squared differences:
(0.00000036 + 0.00000576 + 0.00000006 + 0.00001328 + 0.00000576 + 0.00000012) / 6 ≈ 0.00000624
s = √(0.00000624)
s ≈ 0.002496
the standard error of the mean (SEM):
SEM = 0.002496 / √6
SEM ≈ 0.001018
For a 90% confidence interval, the z value is approximately 1.645.
ME = 1.645 * 0.001018 ≈ 0.001675
CI = x ± ME
CI = 4.0626 ± 0.001675
CI ≈ [4.060925, 4.064275]
Learn more about mean and confidence intervals at: https://brainly.com/question/20309162
#SPJ1
7.0×107 ÷ 2.0×104
turn into a proper scientific notation. PLS HELP
Answers
The expression 7.0x\(10^7\) ÷ 2.0x\(10^4\) can be expressed in proper scientific notation as 3.5x10^3.
To express the division 7.0x\(10^7\) ÷ 2.0x\(10^4\) in proper scientific notation, we need to perform the division and adjust the result to the appropriate format.
Dividing the numbers, we get:
7.0x\(10^7\) ÷ 2.0x\(10^4\)= 3.5x\(10^{(7-4)\)= 3.5x\(10^3\)
The result of the division is 3.5, and we adjust the exponent by subtracting the exponent of the divisor from the exponent of the dividend (7 - 4 = 3).
Therefore, the proper scientific notation representation of the division 7.0x\(10^7\) ÷ 2.0x\(10^4\) is 3.5x\(10^3\).
Scientific notation is a way to express numbers using a coefficient (in this case, 3.5) multiplied by a power of 10 (in this case, 10^3). It allows for more concise representation of very large or very small numbers.
In this case, the division resulted in a number that is smaller than the dividend and has a positive exponent, indicating a smaller magnitude compared to the original numbers. The coefficient represents the significant digits of the result, while the power of 10 represents the scale or magnitude of the number.
For more such questions on scientific notation visit:
https://brainly.com/question/28468914
#SPJ8
The electrolysis of molten AlCl3 for 2.50 hr with an electrical current of 15.0 A produces ________ g of aluminum metal. Group of answer choices
Answers
Answer:
The correct answer is 12.58 grams.
Explanation:
Based on the given information, the electrolysis equation will be,
Al³⁺ + 3e⁻ ⇔ Al
1 mol of Al needs 3 moles of electron, and the value for 1 mole of electron is 96485 C.
Thus, 1 mole of Al needs 3 × 96485 C = 289455 C
Now the amount of charge passed is,
T = 2.5 hours
= 2.5 × 3600 s = 9 × 10³ s
Q = Current × Time
= 15A × 9 × 10³ s
= 13.5 × 10⁴ C
The moles of Al plated will be,
= 13.5 × 10⁴ / 289455
= 0.4664 mol
The molecular mass of Al is 26.98 grams per mole
Now the mass of Al will be,
= Number of moles × Molecular mass
= 0.4664 × 26.98
= 12.58 grams
What happens if i don’t pass the chemistry regent?
Answers
Answer:
You will typically get another chance to retake the exam
Answer:
well you either have a chance to retake the test if your teacher or just not let you take it at all. you have to pass 4 regents test including chemistry in order to get a regent diploma. so you can either retake or it you just failed it.
Zoe left her water bottle capped and in her bedroom. She came back some time later to realize that the bottle was “sweating” and left a ring of liquid on her nightstand
Explain thoroughly the science behind why Zoe’s water bottle is sweating
Answers
Answer:
Condensation
Explanation:
Zoe is quite keen to have noticed what we call condensation. Air contains many components, one of those being water vapor. Like how sugar is soluble in water, water can be said to be "soluble" in air. Water will evaporate into the air to a certain extent. The higher the temperature of the air, the more water the air can hold. If the air has more water that it can hold (potentially because of a temperature decrease), the extra water will come out of the air. Zoe's water bottle was cold, and because the air around Zoe's bottle had cooled down, the air can not hold as much water as it could when it was warm, so the air deposited the extra water in the form of liquid water onto the bottle, giving the illusion that her bottle was sweating.
Write down the formulas of the following compounds: magnesium chloride, alumina, chlorine (VII) oxide, sodium sulfide, calcium oxide.
Answers
Answer:
- Magnesium chloride: MgCl₂.
- Alumina: Al₂O₃.
- Chlorine (VII) oxide: Cl₂O₇.
- Sodium sulfide: Na₂S.
- Calcium oxide: CaO.
Explanation:
Hello!
In this case, according to the naming rules for the given compounds, which state that binary salts have a metal and a nonmetal and oxides contain oxygen, we have:
- Magnesium chloride: MgCl₂.
- Alumina: Al₂O₃.
- Chlorine (VII) oxide: Cl₂O₇.
- Sodium sulfide: Na₂S.
- Calcium oxide: CaO.
Best regards!
What are two processes that must occur to form soil?
Question 1 options:
weathering breaks rocks into minerals and plants die and decay
erosion and weathering
Plants produce loam and plants produce humus
erosion transports mineral particles and plants die and decay
Answers
Erosion and weathering are two processes that must occur to form soil.
What is soil formation?Soil formation is the process by which soil is created over time through the physical, chemical, and biological interactions between rocks, minerals, organic matter, water, air, and living organisms.
Soil formation is a slow and complex process that can take centuries or even millennia, and it can be influenced by a variety of factors, including climate, topography, parent material, time, and human activities.
What is erosion and weathering?Weathering refers to the physical and chemical processes that break down rocks and minerals at or near the Earth's surface.
Erosion, on the other hand, refers to the movement and transport of weathered materials, such as soil, rock fragments, and sediment, by water, wind, or glaciers. This can result in the reshaping of landscapes, the creation of new landforms, and the deposition of sediments in new locations.
Learn about Weathering here https://brainly.com/question/829782
#SPJ1
the current wave mechanical model of the atom has electrons in clouds orbitals around the nucleus
Answers
The current wave mechanical model of the atom describes electrons in clouds called orbitals that surround the atomic nucleus is based on principles of quantum mechanics .
This model emphasizes the wave-like properties of electrons. In contrast to the earlier Bohr model, which proposed that electrons move in well-defined paths around the nucleus.
The wave mechanical model suggests that electrons do not possess precise trajectories but instead occupy regions of space with varying probabilities. These regions are mathematically represented by wave functions or orbitals.
This model provides a more precise depiction of electron behavior, facilitating a better understanding of phenomena like electron energy levels, electron-electron interactions, and chemical bonding.
learn more about wave mechanical model :
https://brainly.com/question/15660887
the current wave mechanical model of the atom has electrons in clouds orbitals around the nucleus, on which principle does this phenomenon is based .
Find volume of an object with the mass of 7.9g in the density of 2.28g/ ml
Answers
Answer:
The answer is
3.46 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \)
From the question
mass = 7.9 g
density = 2.28 g/mL
The volume is
\(volume = \frac{7.9}{2.28} \\ = 3.46491228\)
We have the final answer as
3.46 mLHope this helps you
What is the mass percent of a sugar solution when 489 grams of sugar is combined with 877 grams of water?
Answers
The random molecular motion of a substance is greatest when the substance is......a gas.O ...a liquid...frozen...condensed. .
Answers
Answer
The random molecular motion of a substance is greatest when the substance is a gas
Explanation
A matter is something that has mass and occupies space.
Matter can be divided into gas, liquid, and solid. Among these forms of matter, changes can occur. For example:
liquid to gas: evaporate
gas to liquid: condenses
solid to liquid: melting
liquid to solid: freeze.
The increasing order of random molecular motion of all these forms of matter is gas > liquid > solid.
plsss anwer my questions in the picture

Answers
Answer:
17) b
18) c
19) c
20) c
21) b
22) D
23) b
24) b
25) a
26)?
27) D
28) b
29) D
30) c
A pharmacist quizzes a pharmacy intern on the aliquot method in the preparation of 12 capsules each to contain 80 mg of morphine sulfate and 3.2 mg of naltrexone hydrochloride. Lactose is to be used as a diluent, a prescription balance with a sensitivity of 6 mg is proposed, and a 4% error is acceptable. Provide the relevant calculations.
Answers
To prepare 12 capsules each containing 80 mg of morphine sulfate and 3.2 mg of naltrexone hydrochloride, the following calculations can be used:
Calculate the total weight of the morphine sulfate and naltrexone hydrochloride: 80 mg + 3.2 mg = 83.2 mg
Calculate the weight of the diluent required: 12 capsules * 83.2 mg/capsule = 999.6 mg
Calculate the volume of the diluent required based on its density (assume a density of 0.9 g/mL for lactose): 999.6 mg / (0.9 g/mL) = 1.11 mL
Calculate the weight of the diluent required based on its volume: 1.11 mL * 0.9 g/mL = 1 g
Calculate the amount of the diluent required based on the sensitivity of the prescription balance: 1 g / (6 mg/g) = 166.7 mg
The amount of the diluent required according to this calculation is 166.7 mg. This amount should be rounded up to the nearest whole number (167 mg) to account for the 4% error tolerance. The final aliquot would therefore contain 167 mg of lactose, 80 mg of morphine sulfate, and 3.2 mg of naltrexone hydrochloride.
the ph at one-half the equivalence point in an acid base titration was found o be 3.94. what might this unkown acid be
Answers
The pH at one-half the equivalence point in an acid-base titration was found to be 3.94. The pKa of the unknown acid is 3.94.
In an acid-base titration, the pH at the midpoint of the titration is equivalent to the pKa of the weak acid or weak base being titrated. In other words, the pH at the midpoint of a titration is equal to the negative logarithm of the dissociation constant (pKa) of the acid that is being titrated. The pKa value can be used to identify the unknown acid, since every acid has a unique pKa value.
The formula for the pH at the midpoint of a titration is:
pH = pKa + log([A-]/[HA])
where [A-]/[HA] is the ratio of the concentrations of the acid and its conjugate base at the midpoint of the titration. Since the titration is half-complete at the midpoint, the concentration of acid [HA] is equal to the concentration of conjugate base [A-].
Thus, the formula can be simplified as:
pH = pKa + log(1) = pKa .Therefore, in this case, the pKa of the unknown acid is 3.94.
To identify the acid, we can consult a table of pKa values and look for an acid with a pKa value of 3.94. One example of an acid with a pKa of 3.94 is 4-methoxybenzoic acid (pKa = 3.92).
Know more about equivalence point here:
https://brainly.com/question/2496608
#SPJ11
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal
and one mole of water. 5.00 g of the metallic oxide produces 2.32 g of the metal. What is the metallic
oxide? (Use molar masses)
Answers
Answer:
Formulas
3.2 Determining Empirical and Molecular Formulas
Learning Objectives
By the end of this section, you will be able to:
Compute the percent composition of a compound
Determine the empirical formula of a compound
Determine the molecular formula of a compound
The previous section discussed the relationship between the bulk mass of a substance and the number of atoms or molecules it contains (moles). Given the chemical formula of the substance, one may determine the amount of the substance (moles) from its mass, and vice versa. But what if the chemical formula of a substance is unknown? In this section, these same principles will be applied to derive the chemical formulas of unknown substances from experimental mass measurements.
Percent Composition
The elemental makeup of a compound defines its chemical identity, and chemical formulas are the most succinct way of representing this elemental makeup. When a compound’s formula is unknown, measuring the mass of each of its constituent elements is often the first step in the process of determining the formula experimentally. The results of these measurements permit the calculation of the compound’s percent composition, defined as the percentage by mass of each element in the compound. For example, consider a gaseous compound composed solely of carbon and hydrogen. The percent composition of this compound could be represented as follows:
%H=mass Hmass compound×100%
%C=mass Cmass compound×100%
If analysis of a 10.0-g sample of this gas showed it to contain 2.5 g H and 7.5 g C, the percent composition would be calculated to be 25% H and 75% C:
%H=2.5g H10.0g compound×100%=25%
%C=7.5g C10.0g compound×100%=75%
EXAMPLE 3.9
Calculation of Percent Composition
Analysis of a 12.04-g sample of a liquid compound composed of carbon, hydrogen, and nitrogen showed it to contain 7.34 g C, 1.85 g H, and 2.85 g N. What is the percent composition of this compound?
Solution
To calculate percent composition, divide the experimentally derived mass of each element by the overall mass of the compound, and then convert to a percentage:
%C=7.34g C12.04g compound×100%=61.0%%H=1.85g H12.04g compound×100%=15.4%%N=2.85g N12.04g compound×100%=23.7%
The analysis results indicate that the compound is 61.0% C, 15.4% H, and 23.7% N by mass.
Check Your Learning
A 24.81-g sample of a gaseous compound containing only carbon, oxygen, and chlorine is determined to contain 3.01 g C, 4.00 g O, and 17.81 g Cl. What is this compound’s percent composition?
ANSWER:
12.1% C, 16.1% O, 71.8% Cl
Determining Percent Composition from Molecular or Empirical Formulas
Percent composition is also useful for evaluating the relative abundance of a given element in different compounds of known formulas. As one example, consider the common nitrogen-containing fertilizers ammonia (NH3), ammonium nitrate (NH4NO3), and urea (CH4N2O). The element nitrogen is the active ingredient for agricultural purposes, so the mass percentage of nitrogen in the compound is a practical and economic concern for consumers choosing among these fertilizers. For these sorts of applications, the percent composition of a compound is easily derived from its formula mass and the atomic masses of its constituent elements. A molecule of NH3 contains one N atom weighing 14.01 amu and three H atoms weighing a total of (3 × 1.008 amu) = 3.024 amu. The formula mass of ammonia is therefore (14.01 amu + 3.024 amu) = 17.03 amu, and its percent composition is:
%N=14.01amu N17.03amuNH3×100%=82.27%%H=3.024amu H17.03amuNH3×100%=17.76%
This same approach may be taken considering a pair of molecules, a dozen molecules, or a mole of molecules, etc. The latter amount is most convenient and would simply involve the use of molar masses instead of atomic and formula masses, as demonstrated Example 3.10. As long as the molecular or empirical formula of the compound in question is known, the percent composition may be derived from the atomic or molar masses of the
help me in my this plzzz

Answers
Answer:
a is oxidation
b is reduction
c is reduction
d is oxidation
hope it helps you
How can the enthalpy of the
following system be increased?
N2O4 (g) ⇌2NO2 (g)
∆Hrxn = +ve at constant temperature
A.By increasing the pressure of the
system
B.By decreasing the pressure of the
system
C.By increasing the temperature
D.By decreasing the temperature
E.None of the above
Answers
The enthalpy of the following system can be increased by increasing the temperature. So, the correct option is (c).
A thermodynamic system's enthalpy, which is one of its properties, is calculated by adding the system's internal energy to the product of its pressure and volume. It is a state function that is frequently employed in measurements of chemical, biological, and physical systems at constant pressure, which the sizable surrounding environment conveniently provides.
The kinetic and potential energy of the atoms and molecules in a system rise as the system's temperature rises. As a result, the system's internal energy rises, which also raises its enthalpy. This is true whether the system is under constant pressure or volume.
Learn more about enthalpy here;
https://brainly.com/question/3393755
#SPJ9
What happened to the liquid in titans lake?
Answers
The liquid in Titan's lake is believed to be composed of liquid methane and ethane, which are thought to be supplied by geological processes such as cryovolcanism and seepage from the surrounding terrain. Over time, the liquid in Titan's lake can evaporate, change due to geological or meteorological processes, or be affected by the changing seasons on the moon.
If 2.18 grams of magnesium react at 25.0° C and 745 mmHg, how many liters of oxygen will be
used in the balanced chemical reaction below?
2 Mg(s) + O₂(g) → 2 MgO(s)
Answers
The amount of oxygen in liters that will be used in the reaction is approximately 1.22 liters.
As given in the question the balanced reaction is:
2 Mg(s) + O₂(g) → 2 MgO(s)
which means, 2 moles of magnesium react with 1 mole of oxygen to produce 2 moles of magnesium oxide.
The molar mass of magnesium=24.31 g/mol
Given the mass of magnesium = 2.18g
The number of moles of magnesium =2.18g/24.31 g/mol =0.0896mol
Therefore, according to the stoichiometry of the balanced chemical equation:
0.0896 mol of magnesium will react with 0.0896/2= 0.0448 mol of oxygen to form Magnesium oxide.
Now, according to the ideal gas law,
For oxygen, PV=nRT
where P= pressure in atm
V= volume in liters
n= number of moles
R= gas constant(0.08206L atm/mol K)
T= temperature in Kelvin.
Converting the given pressure and temperature units in standard form:
P= 745 mmHg/760 mmHg/atm
=0.979 atm
T= 25.0° C +273.15= 298.15 K
Now putting the values in the ideal gas law:
PV=nRT
Therefore, V =nRT/P
=(0.0448 mol)(0.08206 L atm/mol K)(298.15 K) / (0.979 atm)
=1.22 L(approx)
Hence the volume of oxygen is 1.22 L.
To know about volume-related chemical equations:
https://brainly.com/question/16979981
If the oxide contains 22.24 % O by mass, what is the identity of the metal?
Express your answer as a chemical symbol.
Answers
The oxide contains 22.24% O by mass, so the identity of the metal as a chemical symbol is lead (Pb).
What is metal?A substance with high electrical conductivity, luster, and malleability, which readily loses electrons to form positive ions (cations) are called metals.
Mass percentage of oxygen in the oxide, i.e.,
ω(O) divided by 100 ω(O)
=22.24% ÷ 100%.
Mass percentage of oxygen in the oxide, i.e .ω(O) = 0.2224
Now the mass percentage of oxygen in the oxide multiplied by 100 ten we get the mass of oxygen in the oxide. If we take 100 grams of compound:
1) m(O) = ω(O) * 100 g.
Mass of oxygen in the oxide, i.e. m(O) = 0.2224 * 100 g.
Mass of oxygen in the oxide. i.e., m(O) =22.24g
Amount of oxygen, i.e.,
n(O) = mass of oxygen in the oxide ÷ molar mass of oxygen.
n(O) = m(O) ÷ M(O) amount of oxygen
n(O) = 22.24g ÷ 16 g/mol,
amount of oxygen i.e. n(O) = 1.39 mol
Mass of metal in the oxide, i.e., m(M) = 100 g - 22.24 g.
Mass of metal in the oxide i.e., m(M) = 77.76g
Now we to find the molar mass of metal oxide,
Molar mass of the metal :
= mass of metal in the oxide ÷ amount of oxygen(M)
= m(M) ÷ n(M). M(M)
= 77.76 g ÷ 1.39 mol.
Molar mass of the metal will be 5.94 g/mol.
Therefore, The identity of the metal is lead (Pb).
To know more about metals, refer to below link:
https://brainly.com/question/2874869
#SPJ1
I need help with budgeting someone who works as a Master Business Administrator but it’s entrepreneurship I need help with the Gross annual salary then I have to subtract the federal tax by 19%
Basically multiply gross annual salary by .19 then subtract the amount
Subtract the tax of 11%
Subtract THE fICA tax of 8%
Multiple the gross jayla salary by .08 then subtract the amount
Then I have to find the NEt Annual Salary
then it says mutual the monthly net income then divide the net annual salary by 12
Then I have to find the monthly expense of being a MBA (entrepreneur)
Like mortgage for mortgage I have to go to Zillow and ask for my “debts” this would be any credit card debt , student loans, car payments etc then I have to find utilities, cable , internet , cell phone , car pay , student loans , groceries , car gasoline , health insurance, auto insurance,home insurance, entertainment, hair cuts /nails /beauty , Gym membership , clothes , gifts , vacation fund I have to multiply my month net income by 0.1 to find all these it all depends on how much I make and last but not least I have to find the projected monthly expense
IF YOU can help with me all of these I will sure to mark you BRAINLIEST

Answers
I can give you a general approach to budgeting based on the guidelines you provided.
First, let's assume that the gross annual salary of the MBA entrepreneur is $100,000.
Federal Tax:
19% of $100,000 = $19,000
Net Salary:
$100,000 - $19,000 = $81,000
State Tax:
Assuming the state tax rate is 11%:
11% of $81,000 = $8,910
Net Salary:
$81,000 - $8,910 = $72,090
FICA Tax:
Assuming the FICA tax rate is 8%:
8% of $81,000 = $6,480
Net Salary:
$81,000 - $6,480 = $74,520
Monthly Net Income:
$74,520 divided by 12 = $6,210
Monthly Expenses:
Assuming a debt of $1,000 per month (credit card/student loans/car payments),
Utilities: $150
Cable and Internet: $100
Cell Phone: $50
Car Payment: $400
Groceries: $400
Car Gasoline: $150
Health Insurance: $400
Auto Insurance: $150
Home Insurance: $100
Entertainment: $200
Haircuts/Nails/Beauty: $100
Gym Membership: $50
Clothes: $100
Gifts: $100
Vacation Fund: $200
Total Monthly Expenses:
$2,900
Projected Monthly Expense:
10% of $6,210 = $621
Note that this is just an example, and your budget may differ depending on your specific circumstances and location. It's essential to track your actual expenses carefully to make sure you're sticking to your budget and adjusting it as needed.
Answer:
I apologize if this is not what you wanted! If you want a different answer or would like me to change it please let me know :)
Explanation:
For housing, I should be spending no more than 30% of my monthly net income. This includes rent, mortgage payments, and utilities. For transportation, I should be spending no more than 15% of my monthly net income. This includes car payments, gas, and insurance. For food, I should be spending no more than 10% of my monthly net income. This includes groceries and eating out. For savings, I should be spending no more than 10% of my monthly net income. This includes retirement savings, emergency funds, and other investments. For debt payments, I should be spending no more than 10% of my monthly net income. This includes student loans, credit cards, and other debts. For entertainment, I should be spending no more than 5% of my monthly net income. This includes movies, concerts, and other leisure activities.
In addition to these categories, I may also need to budget for other expenses. This could include medical bills, clothing, and other miscellaneous expenses. It is important to remember that these percentages are just guidelines and may need to be adjusted depending on my individual situation.
Scenario : I would like to save for a new cell phone which variable expense can I cut back ? How much should I save for a month on the phone?
If I would like to save for a new cell phone, I can cut back on my variable expenses. I can reduce my spending on entertainment, clothing, and miscellaneous expenses. I should aim to save at least 5% of my net annual salary for a new cell phone. This would be approximately $1,000 per year, or $83.33 per month.
id k what to do for the other two, but i hope this helps you! :))))))
PLEASE HELP!!!
You have 25.0 L of nitrogen gas at 100°C and 300 kPa. What volume of gas will you have at STP?
Answers
Answer:
59.1L
Explanation:
Convert 300 kPa to atm to get 2.96 atm.
Convert 100 degrees celcius to kelvin to get 373K.
STP is 1 atm at 298K
Plug into this combined gas law:
V2 = (P1*T2*V1)/(P2*T1)
V2 = (2.96atm)(298K)(25.0L)/(1.0atm)(373K)
V2 = 59.1L
Which of the following set of quantum numbers (ordered n, ℓ, mℓ ) are possible for an electron in an atom? Check all that apply
a. 2, 1, 3
b. 5, 3, -3
c. 4, 3, -2
d. -4, 3, 1
e. 2, 1, -2
f. 3, 2, 2
g. 3, 3, 1
Answers
the possible quantum numbers (ordered n, ℓ, mℓ ) are:Option B.5, 3, -3 and Option C. 4, 3, -2
The quantum numbers n, ℓ, mℓ represent respectively the principal quantum number, the orbital angular momentum quantum number and the magnetic quantum number.
These are the three most important quantum numbers. T
here is another quantum number called the spin quantum number, denoted by ms.
Let's see which of the given quantum number sets is possible.2, 1, 3 is not possible because for ℓ = 1, mℓ can only be -1, 0, or 1. 5, 3, -3 is possible.4, 3, -2 is possible. -4, 3, 1 is not possible.
For any value of ℓ, mℓ must be between -ℓ and +ℓ. e. 2, 1, -2 is not possible because for ℓ = 1, mℓ can only be -1, 0, or 1. f. 3, 2, 2 is not possible because for ℓ = 2, mℓ can only be -2, -1, 0, +1, or +2. g. 3, 3, 1 is not possible because for any value of ℓ, mℓ must be between -ℓ and +ℓ.
Therefore, the possible quantum numbers (ordered n, ℓ, mℓ ) are:5, 3, -34, 3, -2
For more questions on quantum numbers
https://brainly.com/question/30881398
#SPJ8
Select all correct statements dealing with electron affinity. a) In general the first electron affinity is: X(g) → X+(g) + e-b) It is the enthalpy change associated with the addition of an electron to a gaseous atom or ion.c) It is the enthalpy change associated with the removal of an electron from a gaseous atom or ion.d) In general the first electron affinity is: X(g) + e- → X-(g)
Answers
Option C and D are correct.
Explanations:What is electron affinity?Electron affinity is dfined as the energy required to remove en electron from a gaseous atom or molecule. An example of a gaseous atom loosing an electron is given as:
\(X(g)+e^-\rightarrow X^-(g)\)From the reaction, you can see that the gaseous element X looses an electron from its outershell to form an ion. This reaction is known as the first electron affinity.
Hence the correct statement dealing with electron affinity are:
• It is the enthalpy change associated with the, removal of an electron, from a gaseous atom or ion.
• In general the, first electron affinity, is:, X(g) + e- → X-(g)
help and will give brainly i promise only if you get it right
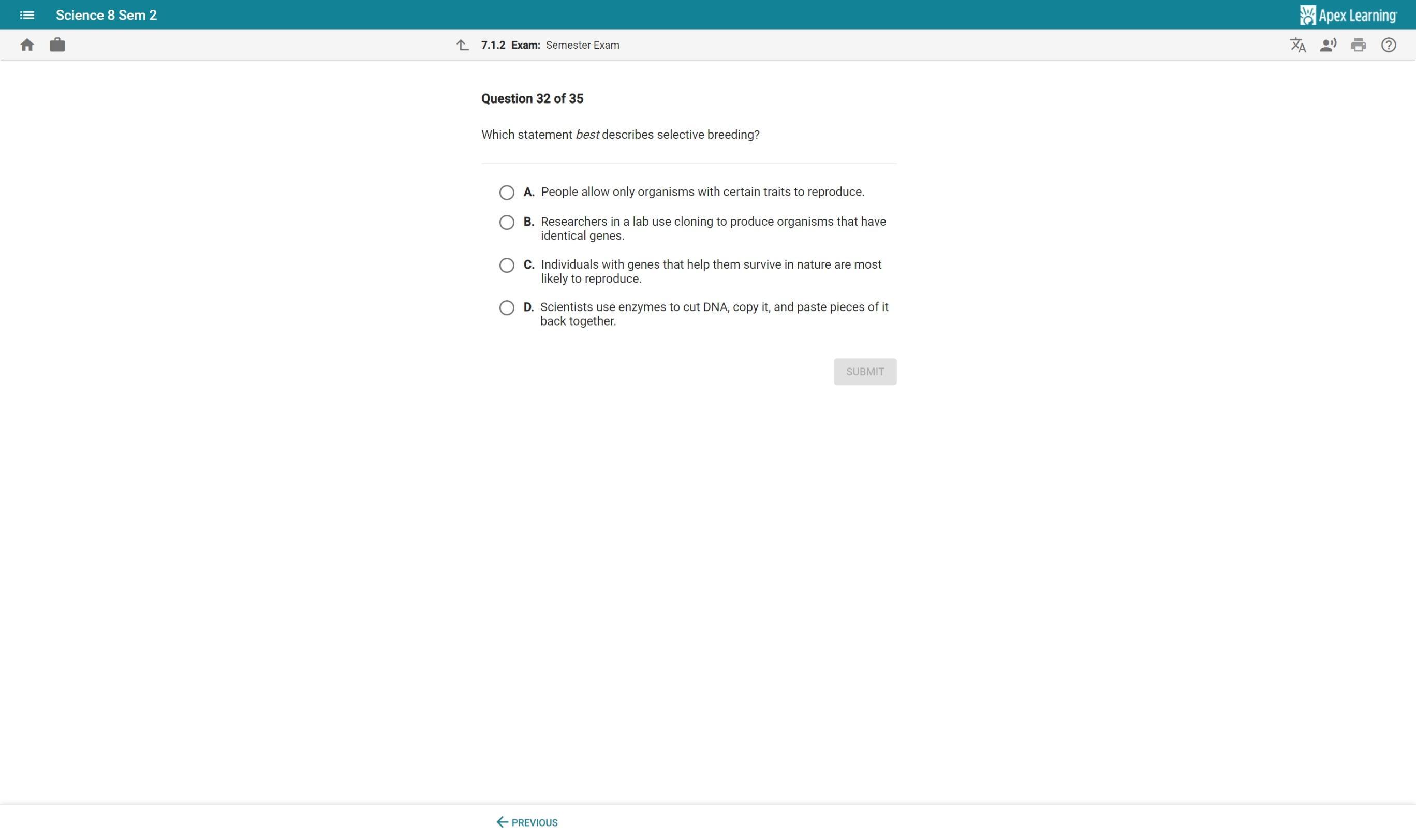
Answers
How many molecules are there in 2.3 grams of NH4SO2?
Answers
Answer:
Hence, there are approximately $1.686\times {{10}^{22}}$ molecules in 2.3 grams of $N{{H}_{4}}S{{O}_{2}}$.