Answers
Answer:
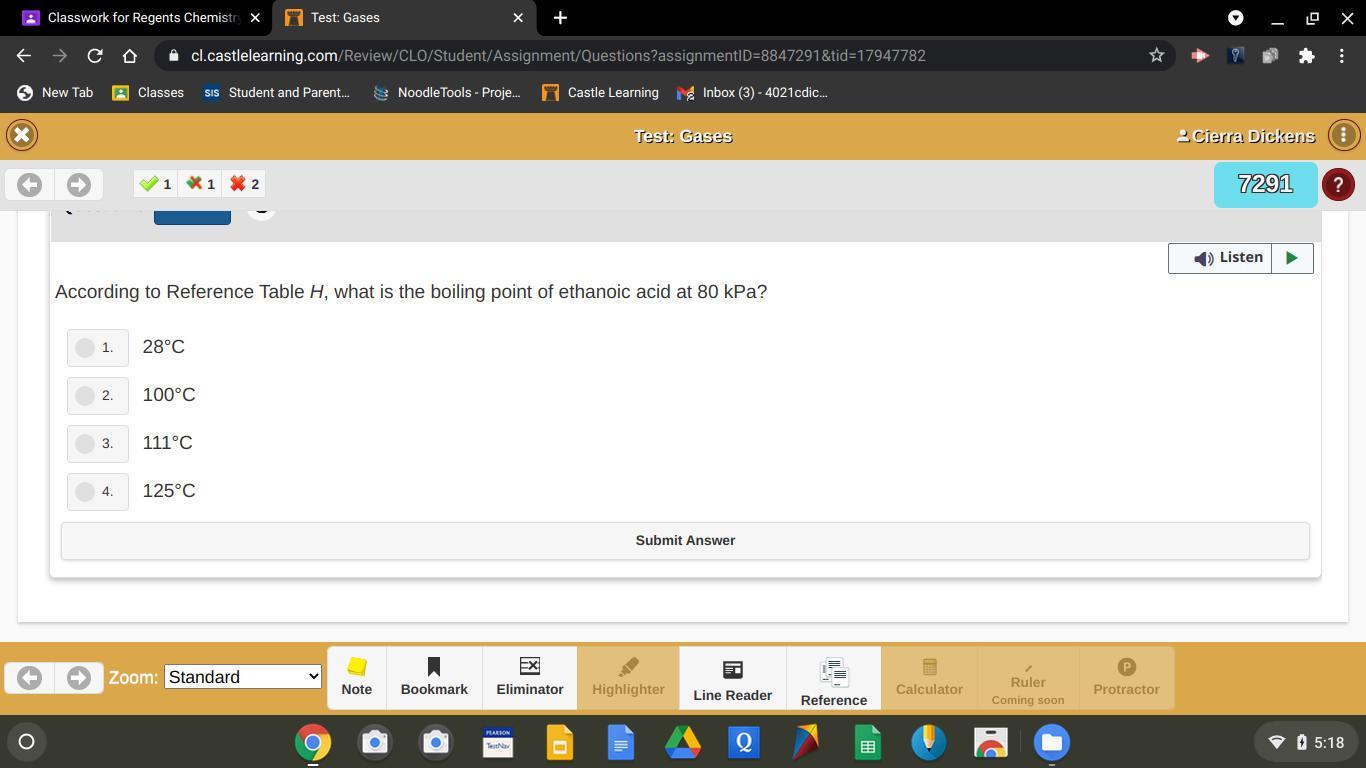
4. 125°C
Explanation:
I hope this helps you :D
Answer:
Explanation:can you take a pic of it a little up so i can see the h please??
Related Questions
aqueous solutions of fe 2(so 4) 3 and sncl 4 are prepared at 1 m. how many moles of each ion are formed in each solution?
Answers
Using the concepts of balanced equations, we got that Fe³⁺ has 2 ions,
SO₄²⁻ has 1 ions, Sn\(4^{+}\) has 3 ions, Cl⁻ has 1 ions when aqueous solution is formed
A balanced equation is a equation in which the number of atoms for each element in the reaction and the total charge is same for both the reactants and the products. Means, we can say that the mass and the charge are balanced on both sides of the reaction.
The balanced chemical equation is
4Fe₂(SO₄)₃ + 6SnCl₄------->6Sn(SO₄)₂+8FeCl₃
Hence, after observing the above balanced chemical equations we get when we prepare aqueous solutions of fe2(so 4) 3 and Sncl4 in 1 M, then
2 ions as Fe³⁺ ,1 ions as SO₄²⁻ ,3 ions as Sn\(4^{+}\),1 ions as Cl⁻ will be formed.
To know more about balanced equations, visit here:
https://brainly.com/question/28294176
#SPJ4
an element with an electronegativity of 0.9 bonds with an element with an electronegativity of 3.1. which phrase best describes the bond between these elements?
Answers
The bond between the elements with electronegativities of 0.9 and 3.1 can be described as polar covalent.
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. When two atoms with different electronegativities form a bond, the shared electrons are pulled more towards the atom with higher electronegativity, creating a polar covalent bond.
In this case, the element with an electronegativity of 3.1 is significantly more electronegative than the element with an electronegativity of 0.9. The difference in electronegativity values suggests that the shared electrons are more strongly attracted to the more electronegative atom, creating a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom.
Therefore, the bond between these elements can be described as polar covalent due to the unequal sharing of electron density resulting from the difference in electronegativity.
To learn more about Electronegativity click here: brainly.com/question/3393418
#SPJ11
lier's principle, which of the following statements (a-c) is false? select one: a. increasing the temperature of an endothermic reaction results in a shift towards the products. b. when adding a solid to a solution in a test tube, the solution is considered to be saturated when additional solid will no longer dissolve and there is a presence of undissolved solid at the bottom of the test tube. c. when a product (solute or gas) concentration is increased, the reaction shifts left to reestablish equilibrium.
Answers
The false statement among the three given options is option c. According to Le Chatelier's principle, when the concentration of a product is increased, the equilibrium of the reaction shifts to the left to balance the increased concentration.
This is because the reaction tries to reduce the excess concentration of the product by forming more reactants. Therefore, option c stating that the reaction shifts left to reestablish equilibrium when the product concentration is increased is false. Instead, the reaction shifts to the right when the reactant concentration is increased, and it shifts to the left when the product concentration is increased. Options a and b are correct statements based on Le Chatelier's principle. Increasing the temperature of an endothermic reaction provides energy to the reactants, which causes the reaction to shift towards the products to absorb the extra energy. Similarly, when a solid is added to a solution, the solution becomes saturated when no more solid can dissolve, and there is undissolved solid present at the bottom. Therefore, option c is the false statement, and the correct statement is that the reaction shifts right when the reactant concentration is increased and shifts left when the product concentration is increased.
learn more about concentration
https://brainly.com/question/14228116
#SPJ11
I also really need help on this one.

Answers
Answer:
I would say ether the first one or the third one because on those questions you always want to choose the one that gives the most information or sound more believe
Hope this helped
A 4.80g piece of magnesium displaces 2.76 mL of water when it is placed in a graduated cylinder. What is the density of magnesium?
Answers
Answer:
6
What do AIA, SAG, AFP and AMA all stand for? Select four options.
American Institute of Architects
American Society of Mechanical Engineers
American Medical Association
Screen Actors Guild
Association for Finance Professionals
Professional Colleges
How many valence electrons does Z have?
Answers
Answer: 12 if you mean Zirconium
hope it helps
Answer:
12
Explanation:
hope it helps
Help!! I will gladly take your help

Answers
The statements that are true are the statements that have been marked 1 - 4 in the options. Statement 5 is not true. Options A,B,C,D.
What is thermal energy?The term thermal energy has to do with the energy that is possessed by a substance whose particles are in motion. This is why we can say that the thermal energy does derive from the total kinetic energy of the molecules that are present in a substance.
It then follows that when we hit the object up, there is an increase in the total kinetic energy of the molecules as well as an increase in thermal energy of the substance. The cold substances also has thermal energy as long as its molecules are still in motion.
Learn more about thermal energy:https://brainly.com/question/11278589
#SPJ1
Which of the following have the thickest layer of peptidoglycan in the cell wall?
mycoplasma
gram-positive bacteria
gram-negative bacteria
L-forms
Answers
The following have thickest layer of peptidoglycan in the cell wall : gram-positive bacteria
What is the thick layer of peptidoglycan?Thickened peptidoglycan layer in Gram positive cells allows them to retain the stain ( and hence remaining 'stain positive' or 'Gram positive) where as thin layer seen in Gram negative cells cannot prevent the stain from leeching out (and hence stain and Gram negative).
Gram-positive organisms have thicker peptidoglycan cell wall as compared to gram-negative bacteria. It is 20 to 80 nm thick polymer whereas peptidoglycan layer of the gram-negative cell wall is 2 to 3 nm thick and covered with outer lipid bilayer membrane.
To know more about peptidoglycan, refer
https://brainly.com/question/8670727
#SPJ1
What form of chemical weathering is responsible for breaking the serpentinite down on Ruby Jones Hall
Answers
The form of chemical weathering that is responsible for breaking the serpentinite down on Ruby Jones Hall is hydrolysis.
Hydrolysis is a type of chemical weathering that occurs when minerals in rocks react with water and create new compounds as a result. It is particularly important in the weathering of silicate minerals, including the serpentinite found on Ruby Jones Hall. During hydrolysis, water molecules split into hydrogen and hydroxide ions and then react with the minerals. This reaction alters the minerals and creates new ones, often resulting in a softer, weaker rock that is more easily eroded. The process of hydrolysis breaks down the serpentinite on Ruby Jones Hall. Serpentinite is a rock made primarily of the mineral serpentine.
Serpentine is a magnesium-rich mineral that is susceptible to hydrolysis because it reacts readily with water to form other minerals. When water reacts with serpentine, it breaks down the mineral and produces new minerals, including clay minerals like kaolinite and smectite. These new minerals are much softer and more easily eroded than the original serpentine, which is why serpentinite is often found in areas with high rates of weathering and erosion.
To know more about hydrolysis refer to:
https://brainly.com/question/30718479
#SPJ11
True or False: Uncouplers "short circuit" the proton gradient, thereby dissipating the proton motive force as heat
Answers
True. Uncouplers, such as dinitrophenol, work by disrupting the coupling between electron transport and ATP synthesis, leading to a decrease in the proton motive force and an increase in mitochondrial respiration.
True. Uncouplers, such as dinitrophenol, work by disrupting the coupling between electron transport and ATP synthesis, leading to a decrease in the proton motive force and an increase in mitochondrial respiration. This "short circuits" the proton gradient, leading to the dissipation of the proton motive force as heat instead of being used to drive ATP synthesis.
Uncouplers "short circuit" the proton gradient, thereby dissipating the proton motive force as heat. They interfere with the normal functioning of the electron transport chain, leading to a decrease in ATP production and an increase in heat generation.
learn more about ATP synthesis,
https://brainly.com/question/30051393
#SPJ11
the ________ of a solution is the negative logarithm of the hydrogen ion concentration expressed in moles per liter.
Answers
The pH of a solution is the negative logarithm of the hydrogen ion concentration expressed in moles per liter.
pH is a measure of the acidity or alkalinity of a solution. It quantifies the concentration of hydrogen ions (H+) present in the solution. The pH scale ranges from 0 to 14, where a pH of 7 is considered neutral, pH values below 7 indicate acidity, and pH values above 7 indicate alkalinity.
The pH value is determined by taking the negative logarithm (base 10) of the hydrogen ion concentration. Mathematically, it can be expressed as pH = -log[H+], where [H+] represents the concentration of hydrogen ions in moles per liter.
By taking the negative logarithm, the pH scale becomes a convenient way to represent the concentration of hydrogen ions on a logarithmic scale, making it easier to compare the acidity or alkalinity of different solutions. Lower pH values indicate higher concentrations of hydrogen ions and stronger acidity, while higher pH values indicate lower concentrations of hydrogen ions and greater alkalinity.
learn more about hydrogen here:
https://brainly.com/question/31485826
#SPJ11
complete the initial count for the balancing chart

Answers
3 Ca + 2 DPO4 ⇒ 2 D + Ca3(PO4)2 is balanced equation.
What are Chemical reactions?
Chemical reactions have two components: the breaking of chemical bonds between reactant molecules (particles) and the formation of new atomic connections in product particles (molecules). The number of molecules will vary even though the number of atoms is the same before and after the chemical change.
A chemical equation that is balanced conserves mass and has the same number of atoms of each element on both sides of the equation. Equations can be balanced independently if you follow these fundamental rules: Check that all of the numbers in the equation are correct. Just pay attention to one thing at a moment. Huge sums are added during adjusting.
To learn more about chemical reactions use link:
brainly.com/question/11231920
#SPJ1
How many grams of NaCl are produced when 15.0 kilograms of magnesium chloride react with 50.0 kilograms of sodium?
Answers
The mass of sodium chloride, NaCl produced from the reaction is 18474 g
Balanced equationMgCl₂ + 2Na —> 2NaCl + Mg
Molar mass of MgCl₂ = 24 + (35.5 × 2) = 95 g/mole
Mass of MgCl₂ from the balanced equation = 1 × 95 = 95 g = 0.095 Kg
Molar mass of Na = 23 g/mole
Mass of Na from the balanced equation = 2 × 23 = 46 g = 0.046 Kg
Molar mass of NaCl = 23 + 35.5 = 58.5 g/mole
Mass of NaCl from the balanced equation = 2 × 58.5 = 117 g = 0.117 Kg
SUMMARY
From the balanced equation above,
0.095 Kg of MgCl₂ reacted with 0.046 Kg of Na to produce 0.117 Kg of NaCl
How to determine the limiting reactantFrom the balanced equation above,
0.095 Kg of MgCl₂ reacted with 0.046 Kg of Na
Therefore,
15 Kg of MgCl₂ will react with = (15 × 0.046) / 0.095 = 7.26 Kg of Na
From the above calculation, only 7.26 Kg of Na out of 50 Kg given is needed to react completely with 15 Kg of MgCl₂.
Thus, MgCl₂ is the limiting reactant
How to determine the mass of NaCL producedFrom the balanced equation above,
0.095 Kg of MgCl₂ reacted to produce 0.117 Kg of NaCl
Therefore,
15 Kg of MgCl₂ will react to produce = (15 × 0.117) / 0.095 = 18.474 Kg = 18474 g of NaCl
Thus, 18474 g of NaCl were obtained from the reaction
Learn more about stoichiometry:
https://brainly.com/question/14735801
#SPJ1
7. Ammonia can be formed by reacting
nitrogen and nydrogen gases.
N₂(g) + 3H₂(g) → 2NH3
If the rate of disappearance of hydrogen
-2.7 x 10-² what is the rate of formation of ammonia
is
Answers
The rate of formation of ammonia is approximately -1.8 x 10⁻² units (per unit time) based on the given rate of disappearance of hydrogen.
What is the rate of the formation of ammonia?The balanced equation for the reaction is:
N₂(g) + 3H₂(g) → 2NH₃(g)
According to the stoichiometry of the reaction, for every 3 moles of hydrogen (H₂) consumed, 2 moles of ammonia (NH₃) are formed.
Given that the rate of disappearance of hydrogen (-2.7 x 10⁻² is negative, indicating its consumption, we can determine the rate of formation of ammonia using the stoichiometric ratio.
Rate of formation of ammonia = (Rate of disappearance of hydrogen) × (2/3)
Rate of formation of ammonia = (-2.7 x 10^(-2)) × (2/3)
Rate of formation of ammonia ≈ -1.8 x 10^(-2) (units depend on the units of the rate given)
Learn more about rate of reactions at: https://brainly.com/question/12904152
#SPJ1
HELP PLEASE!!!
How are both stability and change seen in properties of elements?
Answers
Answer:
In both natural and built systems, stability and change are an important focus of study for both scientists and engineers. Stability refers to a system that is unchanging. ... A dynamic equilibrium exists when chemical reactions or physical movements occur at rates that balance out, creating no net change in a system.
Answer:
In both natural and built systems, stability and change are an important focus of study for both scientists and engineers. Stability refers to a system that is unchanging. ... A dynamic equilibrium exists when chemical reactions or physical movements occur at rates that balance out, creating no net change in a system.
Explanation:
Oxygen's atomic number is 8. This means that an oxygen atom has
*
2 poin
8 neutrons in its nucleus
a total of 8 protons and neutrons
8 protons in its nucleus
a total of 8 neutrons and electrons
Answers
Answer:
8 protons in its nucleus
what is ε of the following cell reaction at 25°c? ε°cell = 0.460 v. cu(s) | cu2 (0.018 m) || ag (0.17 m) | ag(s)
Answers
To calculate the standard cell potential, we use the formula:
ε°cell = ε°(reduction at cathode) - ε°(oxidation at anode)
From the given cell notation, we can see that the reduction half-reaction occurs at the cathode (Ag+ + e- → Ag), and the oxidation half-reaction occurs at the anode (Cu → Cu2+ + 2e-).
The standard reduction potential of the Ag+|Ag half-cell is +0.800 V, and the standard reduction potential of the Cu2+|Cu half-cell is +0.340 V.
So,
ε°cell = ε°(reduction at cathode) - ε°(oxidation at anode)
= +0.800 V - (+0.340 V)
= +0.460 V
This is the given standard cell potential (ε°cell).
To calculate the cell potential (ε) at 25°C under non-standard conditions, we use the Nernst equation:
ε = ε°cell - (RT/nF) ln(Q)
Where R is the gas constant (8.314 J/mol*K), T is the temperature in Kelvin (298 K), n is the number of moles of electrons transferred in the balanced cell reaction (2 in this case), F is the Faraday constant (96,485 C/mol), and Q is the reaction quotient.
The reaction quotient (Q) is calculated using the concentrations of the species involved in the cell reaction.
Q = ([Ag+] / [Cu2+]) = (0.17 M / 0.018 M) = 9.44
Plugging in all the values, we get:
ε = ε°cell - (RT/nF) ln(Q)
= 0.460 V - (8.314 J/mol*K * 298 K / (2 * 96,485 C/mol) * ln(9.44))
= 0.356 V
Therefore, the cell potential (ε) at 25°C under the given non-standard conditions is 0.356 V.
To know more about standard cell potential refer here
https://brainly.com/question/29797132#
#SPJ11
question 5(multiple choice worth 5 points) (03.05 mc) how will the concentration of h and oh− ions change when a substance with a ph 3.2 is added to water? both h and oh− will increase both h and oh− will decrease h will increase and oh− will decrease h will decrease and oh− will increase
Answers
The concentration of H+ (hydrogen) ions will increase, and the concentration of OH- (hydroxide) ions will decrease.
To understand why this happens, it's important to know that pH measures the acidity or alkalinity of a substance. A pH value less than 7 indicates acidity, and a pH value greater than 7 indicates alkalinity. A pH of 7 is considered neutral.
In this case, a substance with a pH of 3.2 is acidic. When it is added to water, it releases H+ ions into the solution. These H+ ions increase the concentration of H+ ions in the water, making it more acidic.
On the other hand, the concentration of OH- ions will decrease because the substance with a pH of 3.2 is acidic. Acids have a higher concentration of H+ ions compared to OH- ions. So, when the acidic substance is added to water, the H+ ions will combine with the OH- ions already present in the water, forming water molecules (H2O) and reducing the concentration of OH- ions.
In summary, when a substance with a pH of 3.2 is added to water, the concentration of H+ ions will increase, making the solution more acidic, while the concentration of OH- ions will decrease due to the combination with the H+ ions.
To know more about acidity visit:-
https://brainly.com/question/29796621
#SPJ11
Explain in a three-paragraph essay the mechanics of how a battery works. How does the choice of metals used in a battery affect its performance? what specific metals work best?
Answers
A battery is a device that converts chemical energy into electrical energy through a process known as an electrochemical reaction.
How does a battery work ?When a battery is connected to a circuit, the electrochemical reaction causes a flow of electrons from the anode to the cathode, generating an electric current that can power a device.
The metal chosen for the anode must be capable of losing electrons easily, while the metal chosen for the cathode must be capable of accepting electrons. The choice of metals can also affect the voltage and capacity of the battery, as well as its overall efficiency.
In general, the metals used in a battery should have a large difference in their electronegativity values, which determines how easily an atom can attract electrons. Common metals used in batteries include zinc, lithium, nickel, and cadmium.
Find out more on batteries at https://brainly.com/question/16553902
#SPJ1
How many formulas units are in 3.11 mole of Ca(NO3)2 can you show your work by step by step correct answer
Answers
Answer:
18.73× 10²³ formula units
Explanation:
Given data:
Number of moles of Ca(NO₃)₂ = 3.11 mol
Number of formula units = ?
Solution:
Avogadro number:
"It is the number of atoms , ions and molecules in one gram atom of element, one gram molecules of compound and one gram ions of a substance"
For example,
18 g of water = 1 mole = 6.022 × 10²³ molecules of water
1.008 g of hydrogen = 1 mole = 6.022 × 10²³ atoms of hydrogen
Number of formula units of Ca(NO₃)₂:
1 mole contain 6.022 × 10²³ formula units
3.11 mol × 6.022 × 10²³ formula units / 1 mol
18.73× 10²³ formula units
Thank you lord lord please thank you lord lord for that you lor
A solution of 0.600m hcl is used to titrate 15.00 ml of koh solution. the endpoint of
titration is reached after the addition of 27.13 ml of hci. what is the concentration of
the koh solution?
9.000m
o 1.09m
o 0.332m
0 0.0163m
Answers
The final concentration is 0.332 M.
Calculation:Here, dilution formula is used,
The concentration of a solution is lowered by the process of dilution. This is commonly accomplished by increasing the solvent content of the solution, which lowers the moles per liter.
M₁V₁ = M₂V₂
where,
M₁ = Initial concentration
V₁ = Initial volume
M₂ = Final concentration
V₂ = Final volume
Given,
M₁ = 0.600 M
V₁ = 15.00 mL
V₂ = 27.13
To calculate,
M₂ =?
Put the given values in the above formula,
M₁V₁ = M₂V₂
0.600 × 15.00 = M₂ × 27.13
9 = M₂ × 27.13
M₂ = 9/27.13
M₂ = 0.332 M
Therefore the final concentration is 0.332 M.
Learn more about dilution formula here:
https://brainly.com/question/7208546
#SPJ4
What is the mass number of an element?
A. Mass number is the mass of the protons in the nucleus.
B. Mass number is the number of protons in the nucleus.
• C. Mass number is the mass of the most abundant isotope.
D. Mass number is the atomic mass of a particular isotope.
Answers
Answer:
D. Mass number is the atomic mass of a particular isotope.
Explanation:
Suppose 500.0 mL of 0.150 M NaOH is added to 525 mL of 0.200 M weak acid (Ka=8.59×10−5) What is the pH of the resulting buffer?
HA(aq)+OH−(aq)→H2O(l)+A−(aq)
Answers
The pH of the resulting buffer is 4.446.
To find the pH of the resulting buffer, we need to first determine the moles of weak acid and moles of NaOH that have been added to the solution.
Moles of weak acid = (0.200 mol/L) x (0.525 L) = 0.105 mol
Moles of NaOH = (0.150 mol/L) x (0.500 L) = 0.075 mol
Next, we need to determine the moles of weak acid and moles of conjugate base (A-) in the buffer solution.
Since the weak acid is in excess, all of the NaOH will be neutralized by the weak acid to form its conjugate base.
Moles of A- = 0.075 mol
Moles of HA remaining = 0.105 mol - 0.075 mol = 0.03 mol
The initial concentration of weak acid was 0.200 M, so its initial moles were:
Initial moles of HA = (0.200 mol/L) x (0.525 L) = 0.105 mol
The final volume of the solution after mixing the two solutions together is:
Final volume = 0.500 L + 0.525 L = 1.025 L
Using the Henderson-Hasselbalch equation, we can find the pH of the resulting buffer:
pH = pKa + log([A-]/[HA])
pKa = -log(Ka) = -log(8.59×10−5) = 4.066
[A-]/[HA] = 0.075 mol/0.03 mol = 2.5
pH = 4.066 + log(2.5) = 4.446
To learn more about buffer solution click here
brainly.com/question/24262133
#SPJ11
When a more complex compound breaks down into two simpler ones
Answers
Answer:
Displacement reaction
Explanation:
Select all the correct answers.
Which of the following are considered pure substances?
element
compound
homogeneous mixture
heterogeneous mixture
Answers
Element and compound both are considered as pure substances.
Thus, both option 1 and 2 are correct.
What is a pure substance?Pure substances are defined as materials with a consistent chemical composition that cannot be physically separated through the use of techniques like filtration, evaporation, etc.
Anything that is made up of the same atom or molecule throughout is a pure substance. Everything else is a mixture.
Since elements are not physically or chemically broken down to generate simple, tiny entities like zinc, elements and compounds are pure substances. Furthermore, pure substances like water are combined to create compounds.
Both homogeneous and heterogeneous mixtures are not pure substances since they lack predetermined ratios and proportions, making it difficult to identify their exact composition.
Pure substances include sodium, water, a copper wire, an aluminum foil, etc.
Thus, elements and compounds both are categorised as pure substances.
To learn more about pure substances, refer to the below link:
https://brainly.com/question/11134780
#SPJ2
Write the condense structural formula of the following compounds.
1. The alkene 2,7 Undecadiene with the molecular formula C11H20 (Note the number of double/triple bonds present)
2. 8 Nonene with the molecular formula C9H18
3. Write the condense structural formula of 5 Decyne with the molecular formula C10H18
Answers
A condensed structural formula is a simple way of representing organic compounds.
The given organic compounds and condensed structural formula is presented below;
The condense structural formula of alkene 2,7 Undecadiene with molecular formula C₁₁H₂₀ is in the image uploaded.The condense structural formula of 2,8 Nonene with the molecular formula C₉H₁₈ is in the image uploaded.The condense structural formula of 5 Decyne with the molecular formula C₁₀H₁₈ is in the image uploaded.Learn more about structural formula here: https://brainly.com/question/514499

What happens to a liquid when you keep on cooling it until it changes?
Answers
Answer:
it turns into a solid
Magnetism is an example of a property of what?
A) behavior
B)chemistry
C)flammability
D) mass
Answers
Answer:
A) behavior
Explanation:
Does Na2 gas posses metallic character? Explain your answer..
Answers
Explanation:
It contains Na2 molecules and the atoms in this molecule are held together by a purely covalent bond because the electronegativity of the two atoms is identical.Metallic bonding would not kick in until you make clusters of quite a few atoms. Such clusters would likely not be very stable because thermodynamically the larger the clump of material the more stable it gets. So they tend to coalesce until you have chunk of metal.Metallic bonding is in a sense a form of covalent bonding, but it is very collective (delocalized over a great many atoms) and electron deficient (there are more states than electrons to fill them up with, leading to conductive properties. This means that “a metallic bond” is a bit of an oxymoron like a forest with only one tree.Reply me in commentsYes, Na2 gas possesses a metallic character.
Does NA contain metallic bonds?In the stable state, metal sodium functions as an array of Na+ ions which can be surrounded by way of a sea of 3s electrons. However, it would be wrong to consider metal sodium as an ion when you consider that the ocean of electrons is shared by using all of the sodium cations, quenching the nice fee.
Sodium most effective has one valence electron. So, in metallic bonding, it is able to only donate one electron to be delocalized at some point of the structure. In steel bonding, the real bonding is the electrostatic force between the effective cations and the delocalized electrons.
Learn more about metallic bonds here: https://brainly.com/question/20536777
#SPJ2
the antibiotic prontosil, developed by bayer pharmaceuticals in 1932, was used in saving the life of president roosevelt's son from severe strep throat in 1936. prontosil is an azo dye, which can be prepared from an aryldiazonium ion and an aromatic compound. draw the structures of the diazonium ion and the coupling component for this drug. draw the diazonium ion. draw hydrogens on the nitrogen atom, where applicable. draw the coupling component.
Answers
The diazonium ion used in the synthesis of prontosil is an aryldiazonium ion, which is a cationic species containing a nitrogen-nitrogen double bond and an aryl group attached to one of the nitrogen atoms.
To draw the structure of the diazonium ion, we first need to select an aryl group that can be attached to the nitrogen atom. A common aryl group used in the synthesis of azo dyes is phenyl (C6H5-), so we will use that in our structure. The diazonium ion is formed by the reaction of an aromatic amine with nitrous acid (HNO2), which converts the amino group (-NH2) to a diazonium group (-N2+). The structure of the diazonium ion can be represented as follows: N≡N⁺ - C6H5.
The antibiotic prontosil is an azo dye that can be prepared from an aryldiazonium ion and an aromatic compound containing a reactive functional group. The diazonium ion used in the synthesis of prontosil is an aryldiazonium ion containing a nitrogen-nitrogen double bond and an aryl group attached to one of the nitrogen atoms. The coupling component used to prepare the azo dye is an aromatic compound that contains a reactive functional group, such as an amino or hydroxyl group, that can undergo coupling with the diazonium ion to form the azo bond.
To know more about nitrogen visit:
https://brainly.com/question/16711904
#SPJ11