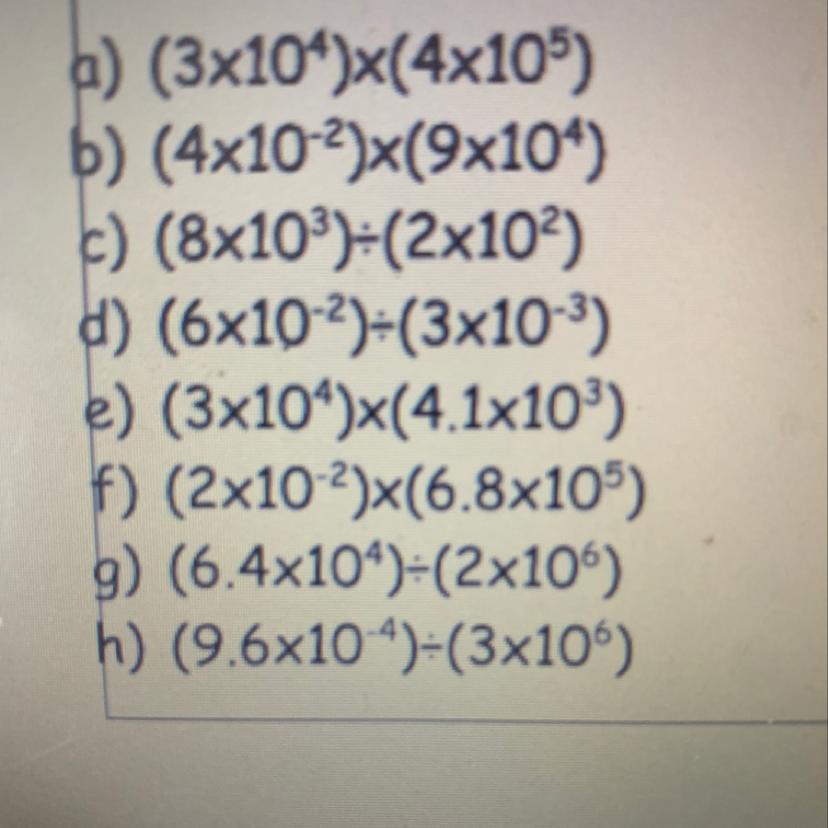Answers
Answer:
just use calculator mannn
Related Questions
two atoms are isotopes of the same element, the atoms must have
Answers
Answer:
the answer is isotopes
I hope this will help you
If anyone can help with this thank you

Answers
= 8.96 g/ml
Assume that the following reaction occurs at constant pressure: 2Al(s)+3Cl2(g)â¶2AlCl3(s). (a) If you are given ÎH for the reaction, what additional information ...
Answers
If we are given the enthalpy change (∆H) for the reaction 2Al(s) + 3Cl2(g) → 2AlCl3(s), we can use it to determine the amount of heat released or absorbed during the reaction under different conditions.
(a) To determine the additional information needed to calculate the standard entropy change (∆S°) for the reaction, we can use the Gibbs free energy change (∆G) equation:
∆G = ∆H - T∆S where T is the temperature in Kelvin.
At constant pressure, the change in Gibbs free energy (∆G) is equal to the maximum amount of work that can be obtained from the system, so for a spontaneous reaction (∆G < 0), the reaction will release energy in the form of heat.
At constant temperature, we can use the equation:
∆G° = -RT ln K where R is the gas constant, T is the temperature in Kelvin, and K is the equilibrium constant for the reaction.
We can use the relationship between ∆G and ∆G° to calculate ∆S°:
∆G° = ∆H° - T∆S°
Since ∆G° and ∆H° can be determined experimentally, we can use the above equation to solve for ∆S°:
∆S° = (∆H° - ∆G°) / T
Therefore, we need to know the temperature at which the reaction occurs and the equilibrium constant (K) at that temperature in order to calculate ∆S° for the reaction.
Learn more about enthalpy change here:
#SPJ11
298 g of BaSO4
express your answer using three significant figures
Answers
298 g of BaSO4 is the mass of Barium Sulfate which is expressed using three significant figures.
Explain about Barium Sulfate:Significant figures are the digits in a measurement that are known with certainty, plus one last digit that is uncertain. In this case, the number 298 is made up of three significant figures, which are 2, 9, and 8.The use of significant figures helps to communicate the level of precision or uncertainty in a measurement. In scientific or technical work, it is important to use the correct number of significant figures in order to indicate the precision of a measurement and to avoid errors in calculations.When working with measurements, it is important to be consistent with the number of significant figures used throughout your calculations. When you perform mathematical operations on measurements, the result should have the same number of significant figures as the measurement with the least significant figures.In this case, 298 g of BaSO4 is a precise measurement, with three significant figures. It is important to keep this same number of significant figures in any further calculation that involves it.To learn more about significant figures refer to:
https://brainly.com/question/24491627
#SPJ1
The protocol for a given lab experiment specifies that you should prepare 100 mL of a 0.10 M (mol/L) aqueous solution of NaNO3. You use a tared balance to deliver a given mass of solid into a weighing boat. To which piece of laboratory glassware should you transfer the solid to finish preparing the solution
Answers
Answer:
It should be transferred to a Beaker to finish preparing the solution
Explanation:
NaNO₃ is sodium Nitrate. It is a salt that is soluble in water hence it's aqueous solution can be prepared by dissolving it in water.
A beaker is a laboratory glassware that is used for various functions; one of which includes the preparation of reagents or aqueous solutions of a compound. In the example narrated in the question, the measured NaNO₃ solid can be transferred to a 100 mL or 200 mL beaker and then added to it is the desired volume of water to make the solution 100 mL. This solution can then be stirred with a "glass rod"
Perform the following operation
and express the answer in
scientific notation.
9.2x10^7 ÷ 3.7x10^9

Answers
Answer:
coefficient green : 2.4865
exponent (yellow) : 16
Explanation:
Bonded Atoms: 2
Lone Pairs: 1
Electron Domains: 3
Ideal Bond Angle?
Hybridization?
Polar or NonPolar?
Answers
The Ideal bond angle is120 degrees.
The Hybridization of the molecule is sp2.
The polarity of the compound depends on the atoms and their electronegativity.
The molecule has two bonded atoms and one lone pair, which gives a total of three electron domains around the central atom.
The ideal bond angle for a molecule with three electron domains is 120 degrees.
The hybridization of the central atom can be determined using the formula:
Hybridization = (number of valence electrons on central atom + number of surrounding atoms - charge)/2
Assuming the central atom has five valence electrons, the number of surrounding atoms is two, and there is no charge on the molecule, the hybridization would be:
Hybridization = (5 + 2 - 0)/2 = 3
Therefore, the central atom is sp2 hybridized.
To determine if the molecule is polar or nonpolar, we need to look at the molecular geometry and the polarity of the bonds. In this case, since there are only two bonded atoms and one lone pair, the molecular geometry is bent or V-shaped.
The polarity of the molecule depends on the polarity of the bonds and the molecular geometry. If the polar bonds are symmetrically arranged, then the molecule will be nonpolar. However, if the polar bonds are asymmetrically arranged, then the molecule will be polar.
Click the below link, to learn more about Hybridisation and polarisation:
https://brainly.com/question/29290058
#SPJ11
Identify the gas from it calculated molar mass. P = 103.2 kPa V = 225 mL T = 25.00 *C m= 0.375 g N2 Ar CO2 Kr O2 H2 Og Xe H2O He Ne
Answers
Answer:
Not sure yet but I will get back
Explanation:
Which of the following is a property of metals?
1. Olow melting point
2. O good electrical conductor
3. O good thermal insulator
4. O cannot bend without breaking
Answers
In each row check off the boxes that apply to the highlighted reactant. The highlighted reactant acts as a... (check all that apply) reaction Bransted-Lowry acid Bransted-Lowry base Lewis acid HINO) 2(aq) + C2H,NH 2(aq) (aq) NO2 (aq) + C2H,NH; Lewis base Bransted-Lowry acid Zn2+(aq) + 6CH3CN(aa) → Zn(CH3CN)2+(aa) -sted-Lowry base Lewis acid Lewis base Brensted-Lowry acid Brensted-Lowry base Lewis acid Lewis base 3 + Al3t(aq) + 6H2O() → Al(H20),(aq)
Answers
For the first reaction: - \(C_2H_5NH_2\) is a reactant and acts as a Bransted-Lowry base since it accepts a proton from\(HNO_2\).
For the second reaction:- \(CH_3CN\) is a reactant and acts as a Lewis base since it donates electron pairs to \(Zn^2^+\).
For the third reaction: - \(H_2O\) is a reactant and acts as a Lewis base since it donates electron pairs to \(Al^3^+\).
For the first reaction: \(HNO_2(aq) + C_2H_5NH_2(aq) → NO_2-(aq) + C_2H_5NH_3+(aq)\)
- \(HNO_2\) is a reactant and acts as a Bransted-Lowry acid since it donates a proton to \(C_2H_5NH_2\).
- \(C_2H_5NH_2\) is a reactant and acts as a Bransted-Lowry base since it accepts a proton from \(HNO_2\).
For the second reaction: Zn2+(aq) + 6CH3CN(aq) → Zn(CH3CN)2+(aq)
- Zn2+ is a reactant and acts as a Lewis acid since it accepts electron pairs from CH3CN.
- CH3CN is a reactant and acts as a Lewis base since it donates electron pairs to Zn2+.
For the third reaction: Al3+(aq) + 6H2O(l) → Al(H2O)63+(aq)
- Al3+ is a reactant and acts as a Lewis acid since it accepts electron pairs from H2O.
- H2O is a reactant and acts as a Lewis base since it donates electron pairs to Al3+.
Learn more about Bransted-Lowry base here
https://brainly.com/question/21736327
#SPJ11
What is the vol., in mL, of a sample of glycerol with a density of 1.20 g/mL and a mass of 43.7 g?
36.4
Answers
The volume of the glycerol sample is 36.4 mL.
To calculate the volume of a substance, we can use the formula:
Volume = Mass / Density.
Given that the mass of the glycerol sample is 43.7 g and the density is 1.20 g/mL, we can substitute these values into the formula:
Volume = Mass / Density
Volume = 43.7 g / 1.20 g/mL
Volume = 36.4 mL
In this calculation, we use the formula Volume = Mass / Density, where the mass is given in grams and the density is expressed in grams per milliliter. Dividing the mass by the density gives us the volume in milliliters, as density is defined as mass per unit volume.
learn more about Density here:
https://brainly.com/question/952755
#SPJ4
6th grade help me pleaseee

Answers
Answer:
D hope this helps
Explanation:
Answer:
d. what will happen to the freezing point of the water if salt is added?
You were given a 100. G wine sample to verify its age. Using tritium dating you observe that the sample has 0. 688 decay events per minute. Tritium has a half life of 12. 3 and fresh water exhibits 5. 5 decay events per minute per 100g. What year was the wine produced?.
Answers
Wine was produced 37 years ago (1984 as usual year 15,2021) that is shown in the calculations below.
Reaction rate is calculated using the formula rate = Δ[C]/Δt, where Δ[C] is the change in product concentration during time period Δt. The rate of reaction can be observed by watching the disappearance of a reactant or the appearance of a product over time.
The time can be represented as follows:
t= 2.303\∧ log A0/A
∧= 0.693/t 1/2
The rate of a reaction is proportional to the reciprocal of the time taken. Rate α 1 time Rate is inversely proportional to time. Units: s-1, min-1 etc.
The given parameters are as follows:
t1/2=12.3
A0=5.5
A=0.688
t= 2.303/(0.693/12.3) log (5.5/0.688)
t=36.9
t=37 years
Thus, wine was produced 37 years ago (1984 as usual year 15,2021)
To learn more about rate of reaction check the link below:
https://brainly.com/question/24795637
#SPJ4
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
Tony is trying to find the average atomic mass of an unknown element. He has a sample where 34.7% of the atoms have a mass of 15.97 amu, while the rest have a mass of 17.85 amu. What is the average atomic mass of Tony's sample?
Answers
Answer:
17.20 amu
Explanation:
Depending on the number of isotopes, the average atomic mass of an isotopic element can be calculated using the expression:
Average atomic mass = (mass of isotope1 × decimal abundance of isotope1) + (mass of isotope2 × decimal abundance of isotope2)...........
In this case, Tony is trying to find the average atomic mass of an unknown element.
- The first isotope of the element has mass= 15.97 amu, and percent abundance= 34.7%.
- The other isotope has mass= 17.85 amu, and percent abundance= (100-34.7) = 65.3%.
Decimal abundance of isotope1 = 34.7/100 = 0.347
Decimal abundance of isotope2 = 65.3/100 = 0.653
Therefore, the average atomic mass =
(15.97 × 0.347) + (17.85 × 0.653)
= 5.54159 + 11.65605
= 17.19764
= 17.20 amu
Arrange these gases in order of solubility, NH3, N2 CO2
Answers
The correct increasing order of solubility for the given gases is: NH₃ < N₂ < CO₂
What does solubility mean?A chemical's solubility is the maximum amount of that substance that will dissolve in a specific amount of solvent at a specific temperature. Solubility has several practical applications, including water filtering, beverage manufacturing, and vitamin storage.
Why is solubility important?The ability of a medicine to dissolve is essential to its effectiveness. A drug substance could be absorbed without it, which results in limited bioavailability. Drugs with poor solubility can potentially cause problems with metabolism or permeability, interaction with other medications, or the requirement for prolonged drug release.
To know more about Solubility visit:
https://brainly.com/question/8591226
#SPJ1
Carbon tetrafluoride, CF, contains four identical C-F bonds. Fluorine is more
electronegative than carbon. How will this impact each of the bonds between carbon and
fluorine?
a. The electrons in each C-F bond will be more attracted to the carbon atom.
b. The electrons in each C-F bond will be attracted equally to both atoms.
C. The electrons in each C-F bond will be more attracted to the fluorine atom.
d. The electrons in each C-F bond will separate easily.
Answers
Answer:
C. The electrons in each C-F bond will be more attracted to the fluorine atom.
What is the goal of cellular respiration? A. To produce food for the plant B. To produce ATP energy C. To create water D. To facilitate photosynthesis
Answers
Answer:
B
Explanation:
The goal of cellular respiration is to produce energy (in the form of ATP) for the cell.
During cellular respiration, food materials are broken down metabolically and the energy locked-up in them is extracted for day-to-day usage by the cell. This energy is usually in the form of the energy currency of the cell - Adenosine Tri Phosphate. The cellular respiration could either be in the presence of oxygen (aerobic) or in the absence of it (anaerobic).
Correct option: B.
What do all types of waves transfer from place to place? A. air B. energy C. matter D. water
Answers
Answer:
Water is the answer
mark me brainlist
in the cno cycle, carbon is used as a catalyst for the fusion of hydrogen into helium. this means that
Answers
CNO cycle uses carbon as catalyst in which carbon is used in the reaction but in the end the carbon is returned to be used again
What is CNO cycle?
CNO cycle full form carbon-nitrogen-oxygen cycle sequence of thermonuclear reactions which provides most of the energy radiated by the hotter stars. It is a process of stellar nucleosynthesis in which stars on Main Sequence fuse hydrogen into helium via a six-stage sequence of reactionsThe sequence is as follows:
Carbon-12 nucleus captures a proton and emits gamma ray, producing nitrogen-13.Nitrogen-13 is unstable and emits beta particle decaying to carbon-13.Carbon-13 captures a proton and becomes nitrogen-14 via emission of gamma-ray.Nitrogen-14 captures another proton and becomes oxygen-15 by emitting gamma-ray.Oxygen-15 becomes nitrogen-15 via a beta decay.Nitrogen-15 captures a proton and produces a helium nucleus and carbon-12 which is where the cycle started.¹²C + ¹H → ¹³N + γ
¹³N → ¹³C + e⁺ + ν
¹³C + ¹H →¹⁴N + γ
¹⁴N + ¹H → ¹⁵O + γ
¹⁵O →¹⁵N + e⁺ + ν
¹⁵N + ¹H → ¹²C + ⁴He
The carbon-12 nucleus used in initial reaction is regenerated in the final one and hence acts as catalyst for the whole cycle
Learn more about CNO cycle at https://brainly.com/question/13549590
#SPJ4
Which one is a single replacement reaction? (Whoever gets it correct first I’ll mark)

Answers
The equation that represents a single replacement reaction given the various options is 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
What is a single replacement reaction?A single replacement reaction, also known as single displacement reaction is a reaction in which elements higher in the electro-chemical series displace or replace elements lower in the electro-chemical series displace from a solution.
The following example illustrates single replacement reaction:
A + BC -> AC + B
From the above reaction, we can see that A has replace/displace B to from AC.
With the above information, we can determine the equation that represents single replacement reaction. Details below:
Equation from the questions:
2Al + 3Cl₂ -> 2AlCl₃2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g)2AlCl₃(aq) -> 2Al + 3Cl₂ AlCl₃ + 3KOH -> Al(OH)₃ + 3KClFrom the above, we can see that only 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) conform to single replacement reaction.
Thus, the correct answer to the question is: 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
Learn more about single replacement reaction:
https://brainly.com/question/29662825
#SPJ1
1.5 mol of the complex substance carbon dioxide (CO2) is just a few oxygen atoms:
Answers
Answer:
9.03 x 10²³atoms
Explanation:
Given parameters:
Number of moles of CO₂ = 1.5mol
Unknown:
Number of atoms = ?
Solution:
In a mole of a substance, we have the Avogadro's number of particles which is 6.02 x 10²³atoms.
Using this number, we can determine the amount of atoms in any substance.
If;
1 mole of CO₂ contains 6.02 x 10²³ atoms;
1.5mole of CO₂ will contain 1.5 x 6.02 x 10²³ atoms
= 9.03 x 10²³atoms
A milkman boils milk before distributing it in pots. He raises the temperature of the milk from 10 degrees Celsius to 130 degrees Celsius with the thermal energy of 120000 J. If the mass of the milk Is 25000 g. the specific heat capacity of the milk would be
Answers
Answer: The specific heat capacity of the milk is 0.24 J/g°C.
Explanation:
The specific heat capacity (C) of a substance is defined as the amount of heat energy required to raise the temperature of a unit mass of the substance by one degree Celsius. To find the specific heat capacity of the milk in this case, we need to divide the total heat energy added to the milk (120000 J) by the change in temperature of the milk (120 degrees Celsius) and the mass of the milk (25000 g).
Using this formula, the specific heat capacity of the milk can be calculated as follows:
C = (120000 J) / (25000 g * 120°C)
C = (120000 J) / (25000 g * 120 K) (converting Celsius to Kelvin)
C = 0.24 J/g°C
So, the specific heat capacity of the milk is 0.24 J/g°C.
Plz help me with this plz pt.2

Answers
What mass of calcium bromide is needed to prepare 150.0 mL of a 3.50 M solution? (Assume that the molecular weight of CaBr2 is 200.618 g/mol)
Answers
Answer:
105g
Use the following formula to calculate the mass of calcium bromide that is needed:
volume of solution × molar concentration × molar mass = mass of solute
The result is 105 g, which has three significant figures, because the term with the least precision (3.50 M) has three significant figures.
Explanation:
If the temperature of a fixed amount of an ideal gas is increased, it NECESSARILY follows that?
a. the pressure of the gas will increase.
b. the volume of the gas will increase.
c. the average kinetic energy will increase
d. All of the above statements are correct.
Answers
The volume of an ideal gas must obviously rise if the temperatures of a fixed sum of the gas is raised.
Explain what an ideal gas is.The phrase "ideal gas" describes a fictitious gas made up of molecules that adhere to the following principles: No attraction or repellence exists between the molecules of ideal gases. Only an unbalanced force upon impact would occur between molecules of a perfect gas.
What is the ideal gas worth?Because when pressure is expressed in kPa, the real gases characteristic is found to be 8.314J/Kmol. The pressure, area, temperature, & number of moles of an ideal gas are all related by a single equation known as the ideal gas law. Pressure, density, & temperature of a gas are related by the combined gas law.
To know more about ideal gas visit:
https://brainly.com/question/28257995
#SPJ4
How many photons are produced in a laser pulse of 0.528 J at 679 nm?
Answers
To calculate the number of photons produced in a laser pulse of 0.528 J at 679 nm, we can use the following formula:
Number of photons = (Energy of laser pulse) / (Energy of a single photon)
Therefore, there are approximately 1.81 x 10^18 photons produced in a laser pulse of 0.528 J at 679 nm.
In a laser pulse of 0.528 J at 679 nm, the number of photons produced can be calculated using the equation E = hc/λ, where E is the energy of the laser pulse, h is Planck's constant, c is the speed of light, and λ is the wavelength of the laser. Plugging in the given values, we get:
E = 0.528 J
h = 6.626 x 10^-34 J s
c = 3.00 x 10^8 m/s
λ = 679 nm = 6.79 x 10^-7 m
E = hc/λ
Number of photons = E/ (hc/λ)
Substituting the values, we get:
Number of photons = 0.528 / (6.626 x 10^-34 x 3.00 x 10^8 / 6.79 x 10^-7)
Number of photons = 1.95 x 10^18 photons
Therefore, there are approximately 1.95 x 10^18 photons produced in a laser pulse of 0.528 J at 679 nm.
To know more about photons visit:
https://brainly.com/question/29409292
#SPJ11
Feel the heat Part 2: Why does the temperature change when a powder is dissolved in water
Answers
When a powder is dissolved in water, it changes the temperature because the chemical reaction that occurs between the particles of the solute and the solvent when they mix. The heat energy that is released or absorbed when two particles come together is known as enthalpy of solution.
In order to understand the temperature change in detail, we need to look at the two types of enthalpies that are involved when a powder is dissolved in water.
The first type is called the lattice enthalpy which is the energy that is needed to break the bonds between the particles in the solid. The second type is the hydration enthalpy which is the energy that is released when the particles of the solute are surrounded by the solvent particles.
When a powder is dissolved in water, the lattice enthalpy is broken and the particles of the solute are dispersed throughout the solvent. This requires energy, so the temperature of the solution decreases. Then the solvent particles surround the particles of the solute and release energy, so the temperature of the solution increases. The overall temperature change depends on the difference between the lattice enthalpy and the hydration enthalpy.
When a powder is dissolved in water, it changes the temperature because of the chemical reaction that occurs between the particles of the solute and the solvent when they mix. When two particles come together, the heat energy that is released or absorbed is known as enthalpy of solution. There are two types of enthalpies involved when a powder is dissolved in water.
The first type is lattice enthalpy, which is the energy that is needed to break the bonds between the particles in the solid. The second type is hydration enthalpy, which is the energy that is released when the particles of the solute are surrounded by the solvent particles.
When a powder is dissolved in water, the lattice enthalpy is broken, and the particles of the solute are dispersed throughout the solvent. This requires energy, so the temperature of the solution decreases. Then, the solvent particles surround the particles of the solute and release energy, so the temperature of the solution increases. The overall temperature change depends on the difference between the lattice enthalpy and the hydration enthalpy.
If the lattice enthalpy is higher than the hydration enthalpy, the solution will be cold because more energy is required to break the bonds in the solid than is released when the particles are surrounded by solvent particles. Conversely, if the hydration enthalpy is higher than the lattice enthalpy, the solution will be hot because more energy is released when the particles are surrounded by solvent particles than is required to break the bonds in the solid.
When a powder is dissolved in water, the temperature of the solution changes because of the chemical reaction between the particles of the solute and the solvent when they mix. The overall temperature change depends on the difference between the lattice enthalpy and the hydration enthalpy. If the lattice enthalpy is higher than the hydration enthalpy, the solution will be cold, and if the hydration enthalpy is higher than the lattice enthalpy, the solution will be hot.
To know more about hydration enthalpy :
brainly.com/question/31180293
#SPJ11
when was the last time a third-party candidate won any electoral votes?
Answers
The last third-party candidate to win one or more states was George Wallace of the American Independent Party in 1968.
Who was George Wallace?
Born in Clio, Wallace attended the University of Alabama School of Law and served in the United States Army Air Corps during World War II. After the war, he won election to the Alabama House of Representatives and served as a state judge.He first sought the Democratic nomination in the 1958 Alabama gubernatorial election. Initially a moderate on racial issues, Wallace adopted a hardline segregationist stance after losing the 1958 nomination.To know more about George Wallace, click the link given below:
https://brainly.com/question/480072
#SPJ4
Antimony has two stable isotopes. The first isotope 121Sb has a mass of 120.9038 amu and a natural
abundance of 57.2%. Calculate the percent abundance for the other stable isotope of antimony.
Answers
The percent abundance for the other stable isotope of antimony is 121Sb = 57.2% and 123Sb = 42.8%.
Any of two or more species of atoms of a chemical element with the equal atomic quantity and nearly identical chemical conduct however with differing atomic mass or mass number and exceptional bodily isotope.
Given,
121.76amu is the average mass of all antimony isotopes.
x+y=1⇒y=1−x(both abundances add up to 100%),
=120.904x+122.904y=121.76
Using alzebra,
= 120.904x+122.904⋅(1−x)=121.76
∴x=0.572,
x+y=1
∴y≈0.428
changing into percentage = 0.428 *100 = 42.8%
Hence, 121Sb has a relative abundance of approximately 57.2%, and 123Sb has a relative abundance of approximately 42.8%.
Learn more about isotopes here:-https://brainly.com/question/14220416
#SPJ9