Answers
Answer:
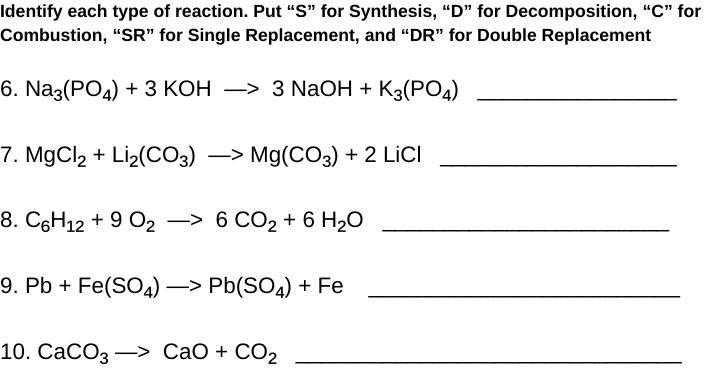
6. Double replacement "DR"
7. Double replacement "DR"
8. Combustion "C"
9. Single replacement "SR"
10. Decomposition "D"
Explanation:
- number 6 and 7 are double replacements because if you look at the compounds in the reactants vs the products, it is evident that there has been a replacement between Na & K in number 6 and Mg & Li in number 7, a "DR" occurs when the anions and cations in 2 molecules switch places to form 2 new compounds.
- number 8 is a combustion reaction because a combustion reaction occurs when oxygen is combined with another compound to form water and carbon dioxide (H2O & CO2).
- number 9 is a single replacement reaction because the Pb and Fe have simply switched places, "SR" are when an element trades with another to form a new compound and the element that was swapped.
- number 10 is a decomposition because it can be observed that the compound in the products is being broken down into the molecules or compounds that it is made up of, started with one and finished with 2
Related Questions
but please I need this answer fast and if you don't know it don't answer it D.

Answers
Answer:
its just a chromosome getting ready to split in a cell, Its called the telophase and its copying itself for the mother and daughter cell
how many moles of (NH4)2Fe(SO4)2 are in 94.00 mL of 2.50 M (NH4)2Fe(SO4)2 solution.
Answers
Answer:
\(n_{solute}=0.235mol\)
Explanation:
Hello.
In this case, we are talking about molarity which is an unit of concentration relating the moles of the solute and the volume of the solution in liters only:
\(M=\frac{n_{solute}}{V_{solution}}\)
Since the solute is the (NH4)2Fe(SO4)2 and the volume in liters:
\(V=94.00mL*\frac{1L}{1000mL} =0.094L\)
Thus, for the given 2.50-M solution, the moles of solute result:
\(n_{solute}=M*V=2.50mol/L*0.094L\\\\n_{solute}=0.235mol\)
Best regards.
A self-aldol or crossed aldol reaction REQUIRES a(n) ___________________. Group of answer choices strong acid only a very, very strong base, such as LDA (lithium diisopropyl amide), NaOH or LiOH won't work acid catalyst a strong base such as LiOH or NaOH
Answers
Answer:
\(\mathbf{a \ strong \ base \ such \ as\ LiOH \ or \ NaOH.}\)
Explanation:
In the condensation reactions of carbonyl compounds, it is essential to establish the order of events in advance to minimize or suppress the possibilities of self-condensation and the occurrence of cross-condensation, which unfortunately are an obvious threat in these reactions.
Self-condensation:
Any carbonyl compound that has one or more alpha hydrogens, on the carbons adjacent to the carbonyl group, runs the risk of undergoing a self-condensation reaction if the corresponding rigor is not carried out.
Cross-condensation:
Ideally, in the condensation reactions of carbonyl compounds, one of the reacting molecules should quickly enolise, while the other preferably should not have Hα, to ensure that no other by-products are formed.
\(\text{To achieve this process;}\) \(\text{A self-aldol or crossed aldol requires a strong base such as LiOH or NaOH.}\)
A solution containing 113. g of KCl in 270. g of H2O at 50 ∘C is cooled to 20 ∘C . It goes to 34 degrees Celsius when cooled down.
Answers
The solution of KCl in water will undergo precipitation of KCl crystals as the temperature decreases from 50 °C to 20 °C and then to 34 °C due to the decreasing solubility of KCl in water.
What happens to a solution of KCl in water when cooled from 50 °C to 20 °C and then to 34 °C?
When the solution of KCl in water is cooled from 50 °C to 20 °C, the solubility of KCl in water decreases. As a result, some of the KCl will start to come out of the solution and form solid crystals.
Then, when the temperature of the solution is further decreased from 20 °C to 34 °C, the solubility of KCl in water decreases even further, causing more KCl to come out of the solution and form solid crystals. This is known as precipitation.
The amount of KCl that will come out of the solution depends on the solubility of KCl at the respective temperatures. The solubility of KCl in water is about 34 g/100 mL at 20 °C and about 42 g/100 mL at 34 °C. Therefore, when the solution is cooled from 50 °C to 20 °C, some KCl will come out of solution until the concentration in solution reaches about 34 g/100 mL. Further cooling to 34 °C will cause more KCl to come out of solution until the concentration in solution reaches about 42 g/100 mL.
The exact amount of KCl that will come out of solution can be calculated using thermodynamic models and experimental data on the solubility of KCl in water at different temperatures.
To learn more about precipitation, visit: https://brainly.com/question/14160641
#SPJ1
Heat is added to ice at 0 °C. Explain why the temperature of the ice does not change. What does change?
Answers
Heat is added to ice at 0 °C. Explain why the temperature of the ice does not change. What does change?When heat is added to ice at 0°C, the temperature of the ice does not change. This happens because all the heat energy is used up in overcoming the intermolecular forces of attraction (hydrogen bonds) that exist between the water molecules in ice.
As a result, the ice undergoes a phase change, from a solid to a liquid. This process is called melting. During melting, the temperature of the ice remains constant at 0°C because all the heat energy is used up in overcoming the intermolecular forces of attraction.The energy required to melt ice is known as the heat of fusion. The heat of fusion is the amount of heat energy required to change 1 kilogram of a solid into a liquid at its melting point. For water, the heat of fusion is 334 kJ/kg. This means that 334 kJ of heat energy is required to melt 1 kg of ice at 0°C. Therefore, during the melting of ice, the temperature of the ice does not change, but the internal energy of the ice does change, and this is manifested in the change of phase from a solid to a liquid.In summary, when heat is added to ice at 0°C, the temperature of the ice does not change, and all the heat energy is used up in overcoming the intermolecular forces of attraction between the water molecules in ice. This results in the melting of ice without any change in temperature.For such more question on molecules
https://brainly.com/question/475709
#SPJ8
How many grams of sulfur dioxide will exert a pressure of 0.705 atm in a 2.50L tank at 0 °C?
Answers
The mass of sulfur dioxide required is approximately 6.36 grams.
To determine the number of grams of sulfur dioxide (SO2) required to exert a pressure of 0.705 atm in a 2.50L tank at 0 °C, we can use the ideal gas law equation: PV = nRT.
First, we need to convert the temperature from Celsius to Kelvin by adding 273.15, so the temperature becomes 273.15 K. The ideal gas constant (R) is 0.0821 L·atm/(mol·K).Rearranging the ideal gas law equation to solve for the number of moles (n), we get n = PV / RT.
Plugging in the given values, n = (0.705 atm) * (2.50 L) / [(0.0821 L·atm/(mol·K)) * (273.15 K)]. Calculating this expression, we find that n is approximately 0.0993 moles.The molar mass of sulfur dioxide is 64.06 g/mol (32.06 g/mol for sulfur + 2 * 16.00 g/mol for oxygen).
Finally, we can calculate the mass of sulfur dioxide using the formula: mass = n * molar mass = 0.0993 moles * 64.06 g/mol. Thus, the mass of sulfur dioxide required is approximately 6.36 grams.
For more such questions on sulfur dioxide
https://brainly.com/question/30352862
#SPJ11
At 25∘C, the Henry's law constant for CO2 is 0.034Matm. What pressure of carbon dioxide is needed to maintain a CO2 concentration of 0.80 M?
Answers
Answer:
P = (Henry's law constant) * (CO2 concentration)
P = (0.034 M/atm) * (0.80 M) = 0.027 atm.
In order to maintain a CO_2 concentration of 0.80 M at 25 degrees Celsius, a pressure of about 23.53 atm would be needed according to Henry's Law.
In the field of Chemistry, Henry's law is used to describe the solubility of gases in liquids. This law states that at a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with the liquid.
Using the given values from the problem, where the Henry's Law constant (KH) is 0.034 M⋅atm and the desired CO_2 concentration (C) is 0.80 M, we can insert these into the formula for Henry's law: P = C/KH.
By substituting the relevant values, you get P = 0.80 M / 0.034 M⋅atm which equates to approximately 23.53 atm. Therefore, a pressure of about 23.53 atm of carbon dioxide would be needed to maintain a CO_2 concentration of 0.80 M at 25 degrees Celsius.
Learn more about Henry's Law here:
https://brainly.com/question/35540348
#SPJ2
a compound has an empirical formula of NaO and a molar mass of 78g/mol. what is its molecular formula
Answers
Answer:
Na2O2
Explanation:
Molecular Formula= Molar Mass/Empirical formula mass
To find empirical formula it is atomic weight of each element times the subscript added together. so since no subscript just mass of the elements added together
Na=23
O=16
23+16=39
78/39 = 2
2 times each element and thats the subscript
What physical property of matter can be measured using the triple beam balance?
A
volume
B
mass
C
height
D
length
SUBMIT ANSWER
Answers
Answer:
B) Mass- I searched it up
Which of the following statements is true?
A:Transverse waves move particles parallel to the direction of energy.
B:Longitudinal waves move particles at right angles to the direction of energy.
C:Transverse waves usually occur in solids, while longitudinal waves occur in liquids and gases.
D:All of the above.
Answers
Answer:B
Explanation:
What is paper made of?
Answers
Paper used as a writing material is made of pulp (wood).
What is paper?Paper is a sheet material used for writing on or printing on (or as a non-waterproof container), usually made by draining cellulose fibres from a suspension in water.
Paper is made from cellulose found in trees, which are the main source of cellulose fibre (or woodpulp). Besides woodpulp, paper can be made from other materials such as cotton, flax, esparto, straw, hemp, manilla and jute.
Wood pulp is usually a softwood, used for pulping to make paper.
Learn more about pulp at: https://brainly.com/question/23590026
#SPJ1
The
collected in scientific
investigations is often in the
form of measurements.
Answers
The collected data/information in scientific investigations is often in the form of continuous measurements for quantitative variables.
What are continuous quantitative variables?Quantitative variables, also known as continuous variables, are those variables expressed in the form of interval range values and therefore do not have a specific value.
Conversely to quantitative variables, discrete variables are those expressed as a single value, but both type of variables can be used as empirical evidence.
In conclusion, the collected data/information in scientific investigations is often in the form of continuous (range value) measurements for quantitative variables.
Learn more about quantitative variables here:
https://brainly.com/question/14037311
#SPJ1
Hello, I just need someone help, just to check it if I got it right please thank you

Answers
Answer:
yes your really smart Believe I'm yourself
Why is an indicator used in a titration?To help reactants react successfully.To bind to the acid to form products.To show when the reaction has reached or past the equivalence point.To provide a surface for the reaction to occur.
Answers
Explanation:
The use of pH indicators in the titration process is useful to determine the end point of the titration, indicating that the entire sample is reacted.
Answer: To show when the reaction has reached or past the equivalence point.
What are the law of 10 in energy Transfer
Answers
Answer:
When the energy is passed on from one trophic level to another, only 10 percent of the energy is passed on to the next trophic level.
Explanation:
A sample of gas contains 0.1700 mol of OF2(g) and 0.1700 mol of H2O(g) and occupies a volume of 19.5 L. The following reaction takes place: OF2(g) + H2O(g)O2(g) + 2HF(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant. L
Answers
Answer: The volume of the sample after the reaction takes place is 29.25 L.
Explanation:
The given reaction equation is as follows.
\(OF_{2}(g) + H_{2}O(g) \rightarrow O_{2}(g) + 2HF(g)\)
So, moles of product formed are calculated as follows.
\(\frac{3}{2} \times 0.17 mol \\= 0.255 mol\)
Hence, the given data is as follows.
\(n_{1}\) = 0.17 mol, \(n_{2}\) = 0.255 mol
\(V_{1}\) = 19.5 L, \(V_{2} = ?\)
As the temperature and pressure are constant. Hence, formula used to calculate the volume of sample after the reaction is as follows.
\(\frac{V_{1}}{n_{1}} = \frac{V_{2}}{n_{2}}\)
Substitute the values into above formula as follows.
\(\frac{V_{1}}{n_{1}} = \frac{V_{2}}{n_{2}}\\\frac{19.5 L}{0.17 mol} = \frac{V_{2}}{0.255 mol}\\V_{2} = \frac{19.5 L \times 0.255 mol}{0.17 mol}\\= \frac{4.9725}{0.17} L\\= 29.25 L\)
Thus, we can conclude that the volume of the sample after the reaction takes place is 29.25 L.
Determine the total kilojoules in two tablespoons
Answers
The total kilojoules in two tablespoons is 836.8 kJ.
To determine the total kilojoules in two tablespoons of a substance, we need to know the specific substance and its energy content per tablespoon. Different substances have different energy values, so without this information, it is not possible to provide an accurate calculation.
The energy content of food or substances is typically measured in kilocalories (kcal) or kilojoules (kJ). 1 kilocalorie is equal to 4.184 kilojoules. The energy content of a substance is often listed on food labels or in nutritional databases.
For example, if we have the energy content of a substance as 100 kilocalories (kcal) per tablespoon, we can convert it to kilojoules by multiplying it by 4.184:
100 kcal * 4.184 kJ/kcal = 418.4 kJ
So, if we have two tablespoons of this substance, the total energy would be:
418.4 kJ/tablespoon * 2 tablespoons = 836.8 kJ
It's important to note that the energy content of a substance can vary depending on its composition, density, and other factors. Therefore, it is always recommended to refer to reliable sources such as food labels, nutritional databases, or consult a qualified professional to obtain accurate information regarding the energy content of specific substances.
For more such information on: kilojoules
https://brainly.com/question/29497478
#SPJ8
Assuming that protein synthesis was under way when the radioactive amino acids were added, which of the following best describes how the radioactivity was distributed in one of the first molecules of Protein X that was completely translated?
A.Radioactive amino acids were randomly located throughout the molecule.
B.Radioactive amino acids were located only at one end of the molecule.
C.Radioactive amino acids were located at both ends, but not in the middle, of the molecule.
D.Radioactive amino acids were located in the middle, but not at either end, of the molecule.
Answers
The statement that best describes how the radioactivity was distributed in one of the first molecules of Protein X that was completely translated is B. Radioactive amino acids were located only at one end of the molecule.
Protein X was one of the first molecules to be completely translated, and the distribution of its radioactivity was unique. Radioactive amino acids were not dispersed throughout the molecule, but instead were located only at one end of the molecule.
This means that the statement B best describes how the radioactivity was distributed in one of the first molecules of Protein X that was completely translated.
With this knowledge, we can be sure that the radioactivity was not evenly distributed throughout the molecule, but was instead concentrated at one end.
This information provides us with a better understanding of the structure of Protein X, and how it is different from other molecules.
To learn more about amino acids, click here:
https://brainly.com/question/28362783
#SPJ4
PLZ HELP I NEED THIS RN!
when the balls reach the bottom of the ramp, which statement will describe their energy?
A. they will have no kinetic energy
B. they will have 100% potential energy
C. they will have 100% kinetic energy
C. they will have different amounts of potential energy
Answers
Answer:
they will have 100% kinetic energy
Explanation:
all the potential energy has been used when it is at the bottom of the ramp
Which drawing best accounts for the polarity of methanol, CH3OH, and the bond polarities that make a major contribution to the overall molecular polarity
Answers
The question is incomplete, the question is;
Which drawing best accounts for the polarity of methanol, CH3OH, and the bond polarities that make a major contribution to the overall molecular polarity?
A) drawing (1) B) drawing (2)
D) drawing (4) C) drawing (3)
Answer:
B) drawing (2)
Explanation:
In Chemistry, the direction of dipole is shown from positive end to negative end.
The image that contains the options in the question asked has been attached.
We can see in image 2 that the oxygen atom was correctly designated as the negative end of the dipole while the carbon and hydrogen atoms were each designated as positive ends of the dipole in accordance with the magnitude of electronegativity difference between the two atoms. The net dipole moment is now taken in the direction shown in image 2. This is the correct answer.

Drawing 2 best accounts for the polarity of methanol, CH3OH. Therefore, the correct option is B.
A carbon atom (C) joins with three hydrogen atoms (H), a hydroxyl group (OH), and three other atoms to form methanol (CH3OH). An oxygen atom (O) is attached to a hydrogen atom to form a hydroxyl group. The three hydrogen atoms and hydroxyl groups are arranged in three dimensions, giving the molecular structure of methanol a tetrahedral form around the core carbon atom.
A polar covalent bond is formed between oxygen and hydrogen because the oxygen atom in the hydroxyl group is more electronegative than the carbon and hydrogen atoms. Due to the unequal sharing of electrons, oxygen has a partial negative charge (-) and hydrogen has a partial positive charge (+), resulting in this polarity.
So, the correct option is B.
Learn more about Methanol here:
https://brainly.com/question/18725375
#SPJ6
Your question is incomplete, most probably the complete question is:
Which drawing best accounts for the polarity of methanol, CH3OH, and the bond polarities that make a major contribution to the overall molecular polarity?
A) drawing (1) B) drawing (2)
D) drawing (4) C) drawing (3)
Which is a valid velocity reading for an object? 45 m/s 45 m/s north 0 m/s south 0 m/s
Answers
Answer:
45 m/s north
Explanation:
Edge 2020
Answer: (B) 45 m/s north
Explanation: right on edge 2020 (so basically the same reason as person above)
What type of reaction?
HCN,Na2So4
Mg3N2
Co2, H2O
Cu,Zn(NO3)2
Na,N2
Answers
HCN, Na2SO4: Combination of compounds.
Mg3N2: Chemical compound.
CO2, H2O: Dissolution or hydration reaction.
Cu, Zn(NO3)2: Single-replacement reaction.
Na, N2: Combination of elements.
Let's analyze each chemical combination to determine the type of reaction involved:
HCN, Na2SO4:
The combination of hydrogen cyanide (HCN) and sodium sulfate (Na2SO4) does not represent a specific chemical reaction. It is simply the combination of two compounds.
Mg3N2:
Mg3N2 represents a chemical compound, magnesium nitride. It does not indicate a specific reaction.
CO2, H2O:
The combination of carbon dioxide (CO2) and water (H2O) represents a chemical reaction known as hydration or dissolution. When carbon dioxide dissolves in water, it forms carbonic acid (H2CO3), which can further dissociate into hydrogen ions (H+) and bicarbonate ions (HCO3-).
Cu, Zn(NO3)2:
The combination of copper (Cu) and zinc nitrate (Zn(NO3)2) represents a single-replacement reaction. Copper displaces zinc from the compound, resulting in the formation of copper nitrate (Cu(NO3)2) and zinc metal (Zn).
Na, N2:
The combination of sodium (Na) and nitrogen gas (N2) does not represent a specific reaction. It is simply the combination of two elements.
For more question on Combination click on
https://brainly.com/question/28304510
#SPJ11
What is parallax?
O A The distance to nearby stars
O
B. The apparent change in position of a nearby star when looked at from different plac
O C. The apparent change in position of a nearby star over time
O
D. The unit of measurement that describes the change in position of a star over time
Answers
Answer:
B
Explanation:
Because it is viewed in a different place
Answer:
Answer is B.the apparent change in position of a nearby star when looked at from different place.
Explanation:
I hope this helps!
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
22. What is the mass in grams of each of the following?
a. 3.011 x 1023 atoms F
b. 1.50 x 1023 atoms Mg
c. 4.50 x 1012 atoms Cl
d. 8.42 x 1018 atoms Br
e. 25 atoms W
f. 1 atom Au
Answers
The mass in grams of 3.011 x 10²³ atoms of F is 9.5 g.
The mass in grams of 1.50 x 10²³ atoms of Mg is 5.98 g.
The mass in grams of 4.50 x 10¹² atoms of Cl is 2.65 x 10⁻¹⁰ g.
The mass in grams of 8.42 x 10¹⁸ atoms of Br is 1.12 x 10⁻³ g.
The mass in grams of 25 atoms of W is 3.1 x 10⁻²¹ g.
The mass in grams of 1 atom of Au is 3.27 x 10⁻²² g.
What is the mass in grams of 3.011 x 10²³ atoms F?
The mass in grams of 3.011 x 10²³ atoms of F is calculated as follows;
6.023 x 10²³ atoms = 19 g of F
3.011 x 10²³ atoms F = ?
= (3.011 x 10²³ x 19 g)/(6.023 x 10²³)
= 9.5 g
The mass in grams of 1.50 x 10²³ atoms of Mg is calculated as follows;
6.023 x 10²³ atoms = 24g of Mg
1.5 x 10²³ atoms F = ?
= (1.5 x 10²³ x 24 g)/(6.023 x 10²³)
= 5.98 g
The mass in grams of 4.50 x 10¹² atoms of Cl is calculated as follows;
6.023 x 10²³ atoms = 35.5 g of Cl
4.5 x 10²³ atoms Cl = ?
= (4.5 x 10¹² x 35.5 g)/(6.023 x 10²³)
= 2.65 x 10⁻¹⁰ g
The mass in grams of 8.42 x 10¹⁸ atoms of Br is calculated as follows;
6.023 x 10²³ atoms = 80 g of Br
8.42 x 10¹⁸ atoms Br = ?
= (8.42 x 10¹⁸ x 80 g)/(6.023 x 10²³)
= 1.12 x 10⁻³ g
The mass in grams of 25 atoms of W is calculated as follows;
6.023 x 10²³ atoms = 74 g of W
25 atoms W = ?
= (25 x 74 g)/(6.023 x 10²³)
= 3.1 x 10⁻²¹ g
The mass in grams of 1 atom of Au is calculated as follows;
6.023 x 10²³ atoms = 197 g of Au
1 atom of Au = ?
= (1 x 197 g)/(6.023 x 10²³)
= 3.27 x 10⁻²² g
Learn more about atomic mass here: https://brainly.com/question/338808
#SPJ1
Which metalloid has three valence electrons?
boron
arsenic
silicon
lithium
Answers
Answer:
the correct answer is baron
Metalloid which is having 3 electrons in the valance shell is boron. option A is correct.
What are metalloids?
Metalloids are those which show the property of both the metals and nonmetals in other words we can say that they will always show intermediate properties of both the metal and nonmetal
They are also known as semiconductors and their conduction can be increased by doping in which we add some impurities to increase their conductivity. By adding some metals to increase the conductivity or by adding some nonmetals to decrease the conductivity.
Boron is mostly in a crystalline solid state and some other major components of it’s are boric powder etc. They are situated in the 13th group of the periodic table and have 3 electrons in their outermost shell and are highly reactive and unstable due to partially filled s orbital
Therefore, they have 3 electrons in their outermost shell. Option A is correct.
Learn more about metalloids, here:
https://brainly.com/question/1566231
#SPJ6
what is the answer to this question and how do i figure it out?

Answers
The m/z that can be seen in propan - 1 ol but not propan - 2 ol is m/z = 15
What is the mass spectrum?The distribution of ions produced by a sample in a mass spectrometer is depicted graphically as the mass spectrum. A sample is ionized in a mass spectrometer, where the resultant ions are sorted according to their m/z ratio.
Each peak in the mass spectrum corresponds to a certain m/z ratio and represents one of the several ions that the sample produced. Thus the propan - 1 ol has the m/z = 15
Learn more about the mass spectrum:https://brainly.com/question/26500669
#SPJ1
Why does a red object appear red?
O A. It reflects light of wavelengths other than red.
OB. It absorbs light of red wavelengths.
O C. It absorbs light of wavelengths other than red.
O D. It reflects infrared radiation.
Right answers only for brainly
Answers
Answer:
Objects appear different colours because they absorb some colours (wavelengths) and reflected or transmit other colours. ... For example, a red shirt looks red because the dye molecules in the fabric have absorbed the wavelengths of light from the violet/blue end of the spectrum
A red object appear red because it absorbs light of wavelengths other than red. Therefore, option C is correct.
What is wavelength ?The distance between identical points (adjacent crests) in adjacent cycles of a waveform signal propagated in space or along a wire is defined as the wavelength. This length is typically specified in wireless systems in meters (m), centimeters (cm), or millimeters (mm) (mm).
A transverse wave's wavelength is defined as the distance between two adjacent crests. A longitudinal wave's wavelength can be calculated as the distance between two adjacent compressions.
The wavelengths that are reflected or transmitted are what we see as colors. A red shirt, for example, appears red because the dye molecules in the fabric have absorbed light wavelengths from the violet/blue end of the spectrum. The only light reflected from the shirt is red light.
Thus, option C is correct.
To learn more about the wavelength, follow the link;
https://brainly.com/question/13533093
#SPJ6
What is my formula? Silver l acetate
Answers
Answer:
Ag(I)C2H3O2 ....................
Silver only has a single valence value (+1) so you really don't need to put the (I) in the chemical formula.
Organelles carry out specif functions within the cell. True False
Answers
Answer:
true
Explanation: