Answers
Specific heat capacity of a substance is the amount of heat required to raise the temperature by one degree Celsius of one gram of a substance. Therefore, the total enthalpy of the given reaction is -781.44KJ.
What is Enthalpy?Enthalpy term is basically used in thermodynamics to show the overall energy that a matter have. Mathematically, Enthalpy is directly proportional to specific heat capacity of a substances.
Mathematically,
q = n ×ΔH
where
q = amount of heat
n = no of moles
ΔH = enthalpy
n = w / M.M
w = given mass
M.M = molar mass
4CH\(_3\)NH\(_2\)+2H\(_2\)O\(\rightarrow\)3CH\(_4\)+CO\(_2\)+4NH\(_3\) ΔH=-138.8KJ
number of moles of methyl amine=175/31.06
=5.63moles
enthalpy for reaction of one mole of methyl amine=-138.8KJ
enthalpy for reaction of 5.63 mole of methyl amine=-138.8KJ×5.63
=-781.44KJ
Therefore, the total enthalpy of the given reaction is -781.44KJ.
To know more about enthalpy, here:
brainly.com/question/24170335
#SPJ1
Related Questions
To which homologous series does CH₂CH₂ CH₂CH₂ belong?
Answers
The homologous series that CH₂CH₂ CH₂CH₂ belongs to is alkene.
What is homologous series?Homologous series is any series of aliphatic organic compounds whose members differ only in the addition of a CH₂ group.
The members of the homologous series are as follows;
AlkaneAlkeneAlkyneAlkene is an unsaturated, aliphatic hydrocarbon with one or more carbon–carbon double bonds.
According to this question, the above given compound is butene (a member of alkene) because it has four carbon atoms and 8 hydrogen atoms.
Learn more about homologous series at: https://brainly.com/question/30842881
#SPJ1
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
What is the product of silver nitrate + lead ii nitrate?
Answers
Answer:
Explanation:
In theory, not much of anything. The vast majority of nitrates are water soluble. Aside, not sure what chemistry level you are at but you will probably be asked to know or memorize some solubility rules. This, for lack of a better phrase, Nitrate rule, is near spot on. With one exception—a rare one—all metal cationic nitrates are soluble in water. All of them. So, assuming you are talking about aqueous, water-based solutions of these salts and mixing them together, I expect nothing to occur. Both solutions, I believe are colorless in water and will thus remain so. If you had say a solution of Iron (III) nitrate and copper (II) nitrate, slightly different story. Both are colorful solutions and I would think you might see blending of colors but no reaction; no precipitate will form. You will probably learn about markers of a chemical reaction. One of these is a color change. Note, you should read this as a change of color from what you previously had. Going from red to blue or colorless to colored (or vice versa) is a strong indication of a reaction (e. g. evidence of bond-breaking and bond-formation). The mere mixing of colors does not constitute a chemical reaction.
What is the pH of the solution obtained when 135 mL of 0.616 M NaOH is diluted to 16.0 L with water?
Express your answer using two decimal places.
Answers
The pH of a solution obtained when 135mL of 0.616 M NaOH is diluted to 16.0 L with water is 2.28.
How to calculate pH?The pH of a solution is the degree of acidity or alkalinity of a solution.
The pH of a solution can be calculated by using the following equation;
pH = - log {H+}
However, the molarity or concentration of the NaOH solution must first be calculated as follows:
C₁V₁ = C₂V₂
Where;
C₁ = initial concentrationV₁ = initial volumeC₂ = final concentrationV₂ = final volume135 × 0.616 = C × 16000
83.16 = 16000C
C = 0.0052M
pH of NaOH = - log {0.0052}
pH = 2.28
Therefore, the pH of the NaOH solution is 2.28.
Learn more about pH at: https://brainly.com/question/11300720
#SPJ1
Can anyone help me understand how to calculate the moles of H+ and OH-?

Answers
To calculate the moles of H+ and OH-, you need to know the concentration of the solution in terms of its pH or pOH value.
How to calculate the molesWhen you get the pH of the solution, you can use this formula to calculate the concentration of H+ ions: [H+] = 10^(-pH)
Also, if you know the pOH of the solution, you can use this formula to calculate the concentration of OH- ions: [OH-] = 10^(-pOH)
Having determined the concentration of H+ and OH- ions, the molarity formula can be used to calculate the number of moles of each ion as follows: moles = concentration (in mol/L) x volume (in L)
Learn more about moles calculation here:
https://brainly.com/question/14357742
#SPJ1
Task:
For each "station", click on the link. You should describe the initial appearances and observations of the
reaction during and after. Using your observations, determine if the change is a physical or chemical change.
Station #1: Lead Nitrate and Potassium lodide solutions. Shower of yellow
QUESTION/OBSERVATION
INITIAL APPEARANCE (what does the
substance look like in the beginning)
Answers
The expected observations for the chemical reaction involving lead nitrate and potassium iodide are as follows as per theory.
INITIAL APPEARANCE:Before the reaction, you'd have two separate solutions:
Lead Nitrate solution: This is typically a clear, colorless solution.
Potassium Iodide solution: This is also usually a clear, colorless solution.
REACTION OBSERVATIONS:
As soon as you combine these two solutions, a chemical response takes place, resulting in the almost instantaneous development of a yellow precipitate. Lead iodide is a substance that cannot be dissolved in water.
FINAL APPEARANCE:
The final mixture would have a yellow precipitate (lead iodide) suspended in the solution.
The reaction leads to the formation of lead iodide, a substance with distinctive properties, suggesting a chemical change. The presence of this novel compound is indicated by the yellow hue of the precipitate.
Read more about chemical change here:
https://brainly.com/question/1222323
#SPJ1
What is the molar mass
MgCrO4
Answers
The molar mass of MgCrO4 is approximately 140.30 g/mol.
To determine the molar mass of MgCrO4 (magnesium chromate), we need to calculate the sum of the atomic masses of each individual element in the compound.
The chemical formula MgCrO4 indicates that the compound consists of one magnesium atom (Mg), one chromium atom (Cr), and four oxygen atoms (O).
The atomic masses of the elements can be found on the periodic table:
Magnesium (Mg) has an atomic mass of approximately 24.31 g/mol.
Chromium (Cr) has an atomic mass of around 51.99 g/mol.
Oxygen (O) has an atomic mass of about 16.00 g/mol.
Now, we can calculate the molar mass of MgCrO4 by summing up the atomic masses of each element, considering the respective subscripts:
Molar mass = (Atomic mass of Mg) + (Atomic mass of Cr) + 4 × (Atomic mass of O)
Molar mass = (24.31 g/mol) + (51.99 g/mol) + 4 × (16.00 g/mol)
Molar mass = 24.31 g/mol + 51.99 g/mol + 64.00 g/mol
Molar mass ≈ 140.30 g/mol
for more such questions on mass
https://brainly.com/question/24191825
#SPJ8
•) At 741 torr and 44°c, 7.10gol a gas occupy a volume of 5.40ml
what is the moler mass of the gas?
Answers
3.5 × 10^6 g
according to ideal gas equation, PV = nRT
where, p is pressure v is volume, n is mole of gas, r is gas onstant and t is temperature.
Pressure (p) = 741 Torr = 1 bar
Volume (V) = 5.40 ml= 0.0054litre
Amount of substance (n) mol = ?
Temperature (T) 44°C= 371kelvin
so by putting all values in equation. PV = nRT
= 1 × 0.0054 =n ×8.31 ×371
n ≈ 0.000002 = 2.0 × 10^-6
now, n is mole of gase,
mole = given mass ÷ molecular mass
given mass = 7.10g
molecular mass = given mass ÷ mole
7.10 ÷ 2.0 × 10^-6
= 3.5 × 10^6 g
To know more about ideal gas equation visit :
https://brainly.com/question/3637553
#SPJ9
Determine the mass of 15 mol N.
Answer in units of g.
Answers
Answer:
210.15 g
Explanation:
The molar mass of nitrogen is approximately 14.01 g/mol.
14.01*15=210.15
a molecule is the ___________particle of a substance that can normally exist independenty
Answers
Answer:
a molecule is the smallest particle of a substance that can normally exist independenty
combustion always result in to formation of water. what other type of reactions may result into formation of water? examples of these reactions
Answers
As combustion always result into the formation of water, the other type of reactions that may result into formation of water are Acid-Base Neutralization Reactions and Hydrogen and Oxygen Reaction.
Acid-Base Neutralization Reactions:
A neutralisation reaction is a chemical process in which an acid and a base combine to produce salt and water as the end products.
H⁺ ions and OH⁻ ions combine to generate water during a neutralisation reaction. Acid-base neutralisation is the most common type of neutralisation reaction.
Example: Formation of Sodium Chloride (Common Salt):
HCl + NaOH → NaCl + H₂O
Hydrogen and Oxygen Reaction:
Water vapour is created when hydrogen gas (H₂) and oxygen gas (O₂) are combined directly. This reaction produces a lot of heat and releases a lot of energy.
Example: 2 H₂ + O₂ → 2 H₂O
Learn more about reactions:
https://brainly.com/question/25769000
PREDICT How do you think the atoms in metal elements are different from those in
nonmetals or metalloids? How might the atoms of different metals vary from one another?
Answers
Answer:
See explanation
Explanation:
The atoms of metals have fewer valence electrons than the atoms of metals and metalloids.
Atoms of metals have only very few valence electrons in their outermost shells hence they donate electrons during bonding. However, atoms of nonmetals have more electrons in their outermost shells and rather accept electrons during bonding. The atoms of metalloids just have a number of valence electrons that are intermediate between those of metals and nonmetals and mostly share electrons in covalent bonds.
Similarly, atoms of metallic elements differ from each other in the number of valence electrons present in the valence shell of the atom of each element. For instance, sodium has one electron in the valence shell of its atom while aluminium has three electrons in the valence shell of its atom.
The atoms of metallic elements are different from the atoms of non metals or metalloids base on the outer electron/ valency electrons and the its bonding pattern.
The atoms of different metals varies in it ability to bond quickly.
The atoms of metallic elements are different from the atoms of non metals or metalloids base on the outer electron/ valency electrons and how it bonds.
Metallic atoms have very few electrons in the outermost shell. The valency electrons of this metallic atoms are few and are easily lost during bonding. They have the ability to release there valency electrons easily. Example of this metals are sodium, potassium , calcium etc.
On the other hand non metallic elements have numerous electron in the outermost shell and easily receive electron during bonding. Example are chlorine, fluorine, oxygen etc.
The metalloid atoms like silicon and germanium have an average number of electron in their outermost shell. They are in between.
The atoms of different metals varies in it ability to bond quickly. For example the group 1 metals are very reactive than the group 2 metals. This simply means the group 1 metals(alkali metals) goes into bonding more easily than the group 2 metals(alkali earth metals).
read more: https://brainly.com/question/1903992?referrer=searchResults
Analyze how elements and compounds in a balanced equation relate to the law of conservation of mass.
Answers
Answer:
Balanced equation is related to the law of conservation of mass as it states that mass can neither be created nor be destroyed in a chemical reaction so the total no. of atoms in a reaction should remain same. in a balanced chemical equation also total no. of atoms of each elements remain same.
Can you identify some techniques in separating mixtures? In what
ways can you separate the different raptures krund at home as Ilustrated
in Figare 2 below? This lesson will help you to learn more!
Answers
Answer:
Boiling
Filtration
Distillation
Evaporation
How many moles are in a 62.5-g sample of potassium nitrate (KNO3)?
Answers
Answer:25.3 g of KNO₃ contain 0.25 moles.
Explanation:
The drawing below shows a mixture of molecules:
carbon
nitrogen
oxygen
hydrogen
sulfur
chlorine
Suppose the following chemical reaction can take place in this mixture:
CS₂(g)+30₂(g) → CO₂(g) +2 SO₂(g)
Of which reactant are there the most initial moles? Enter its chemical formula:
Of which reactant are there the least initial moles? Enter its chemical formula:
Which reactant is the limiting reactant? Enter its chemical formula:

Answers
The most reactants in the system are the oxygen molecules while the least reactants in the system is carbon sulfide. As such carbon sulfide is the limiting reactant.
What is the reaction?We know that when we talk about a chemical reaction, what we are referring to is the interaction that is able to occur between the reactants and the products that are found in the system. We know that when we write down a chemical reaction, the species that are combined are the species that we can find on the left hand side and they are called the reactants. The species that we obtain in the reaction are the species that we can find on the right hand side and they are called the products of the reactions.
If we look at the reaction as it has been shown, we can see that there are ten oxygen molecules and three carbon sulfide molecules that were initially present in the system as we can see here. The entire reaction equation is given as; CS₂(g)+30₂(g) → CO₂(g) +2 SO₂(g).
Learn more about reaction equation:https://brainly.com/question/3588292
#SPJ1
I NEED HELP!!OMG OMG OMG

Answers
Winter is purple, spring is blue, summer is orange, fall is green.
Zeros between nonzero digits are significant
Answers
Answer:
Explanation:
If a zero is found between significant digits, it is significant
Conservation of Mass
In the following reaction, H2 + F2 → 2HF, a student reacts 8g of H2 with 20g of F2. Assuming no mass is lost (due to the Law of Conservation of Mass), how many grams of HF is produced?
You can use the following Sentence Stem to formulate your answer.
Conservation of mass is mass _________ be _______ or _____________. In this reaction 8 _____ of ___________ + ____ grams of F2 produce ___ moles of ____________, the _____________. Since there is no _________ of ____________ due to the law of _________________ of ___________. There will be _____ grams of HF _______________.
Answers
The two main postulates that was given by Antoine Lavoisier are, oxygen play an important role in combustion and the other is mass of the reactant and product is conserved. Therefore the mass of HF is 28grams.
What is law of conservation of mass?According to Law of conservation of mass, mass can neither be created nor be destroyed. Mass can only be transformed from one form to another. The law of conservation of mass was given by Antoine Lavoisier. Every reaction in nature follow the law given by Antoine Lavoisier that is mass is always conserved.
H\(_2\) + F\(_2\) → 2HF
Mass of H\(_2\)=8Grams
mass of F\(_2\)= 20grams
According to law of conservation f mass
mass of H\(_2\) + mass of F\(_2\) = mass of HF
Substituting all the given values, we get
8grams + 20grams =mass of HF
mass of HF=28grams
Therefore the mass of HF is 28grams.
To know more about law of conservation of mass, here:
https://brainly.com/question/28711001
#SPJ1
What creates a weather front?
Answers
A weather front is a transition zone between two different air masses at the Earth's surface. Each air mass has unique temperature and humidity characteristics. Often there is turbulence at a front, which is the borderline where two different air masses come together. The turbulence can cause clouds and storms.
a cube of iron pyrite is 0.31 cm on each side and has a mass of 0.040g. what is the density of the sample?
Answers
The density of the iron pyrite cube is 1.343 g/cm³.
Given,
Side of iron pyrite cube = 0.31 cm
Mass of iron pyrite = 0.040 g
The volume of iron pyrite cube = s³ cm³
Or, volume = 0.029791 cm³
We have to find the density of the sample.
Density is defined as the mass per unit volume. Or, it is the ratio of mass to the volume of the substance.
Using the formula for density, we get,
Density = mass/volume
Or, density = 0.40/0.029791
Or, density = 1.343 g/cm³
Hence, the density of the iron pyrite cube is 1.343 g/cm³.
To learn more about density, visit: https://brainly.com/question/15164682
#SPJ9
Which sample would you expect to have the smallest amount of kinetic energy?
THIS IS THE ANSWER. NOT A QUESTION
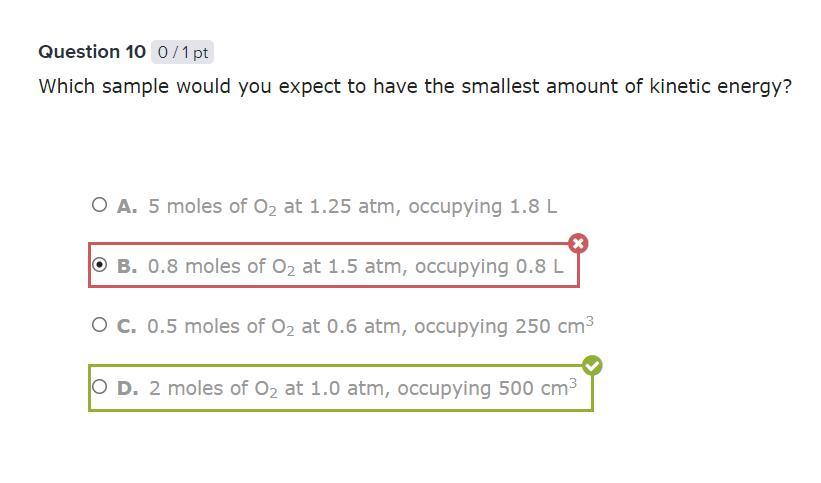
Answers
Answer:
ok so what do i do now that you posted the answer?
Answer: D
Explanation:
definitely didn't copy you
the accepted density of a certain material is 7.44 g/cm^3. A student measures the density of the same material as 7.30 g/cm3. What is the percentage error of the measurement?
Answers
The percentage error of the measurement, given that the accepted measurement is 7.44 g/cm³, is 1.88%
How do I determine the percentage error?First, we shall determine the absolut error. This can be obtained as follow:
Accepted value = 7.44 g/cm³Measured value = 7.30 g/cm³Absolute error = ?Absolute error = accepted value - measured value
Absolute error = 7.44 - 7.30
Absolute error = 0.14 g/cm³
Haven obtained the absolute error, we shall determine the percentage error. Details below:
Absolute error = 0.14 g/cm³Accepted value = 7.44 g/cm³Percentage error =?Percentage error = (Absolute error / accepted value) × 100
Percentage error = (0.14 / 7.44) × 100
Percentage error = 1.88%
Thus, we can conclude that the percentage error is 1.88%
Learn more about percentage error:
https://brainly.com/question/17880579
#SPJ1
Explain why phosphorus pentafluoride, PF5 is a stable well known molecule but the very similar molecule NF5 where the phosphorus is replaced by nitrogen doe not exist
Answers
There are vacant orbitals on the phosphorus atom that allows it to expand its octet.
Why is it that the phosphorus pentafluoride is stable?There is a concept that we would need to consider as we are answering the question that we here and that is the idea of the octet rule. The octet rule states that it is only about eight electrons that can be found on the outermost shell of an atom and as such all of the compounds can be formed in obedience to this rule.
Now we should know that there are no vacant d orbitals that are present on the nitrogen atom and this stems from the fact that it does not have a 3d level as such there are no orbitals that can be able to help the Nitrogen atom so as to be able to expand its octet. This is the reason why its pentafluoride can be easily formed.
Learn more about octet rule:https://brainly.com/question/865531
#SPJ1
What kind of change forms a new substance?
A physical change
Answers
Answer:
Chemical change
Explanation: Chemical changes occur when a substance combines with another to form a new substance, called chemical synthesis or, alternatively, chemical decomposition into two or more different substances.
Which formula represents a polar molecule?
C12
N2
CC14
HCI
Answers
Answer:
H-Cl reperesents polar bond.
Explanation:
Because H and Cl atoms have diffrent dipole moment so they cannot cancel out each other.
The pressure inside the cabin of an airplane decreases to 12.5 psi during a high altitude flight. Convert this pressure value to units of …. (1 psi= 0.068 atm: This is another common pressure)
a. ... millimeters of mercury (mm Hg):
b. Submit
c. ... atmospheres (atm):
d. Submit
e. ... kilopascals (kPa):
The new helium gas cylinders have just arrived at the loading dock of a local party store. In the cool January air, the 20.0-L cylinders have a pressure of 55.7 atm. Once brought into the store, the cylinder’s temperatures increases to 31.9°C and the pressure adjusts to 61.3 atm. What is the outdoor temperature in °C? (Assume a constant volume.)
A sample of oxygen gas at 68.5°C and 3.88 atm is heated until its pressure has increased to 6.10 atm. What is the new temperature (in °C)?
Will B. Sellabraten purchases a set of Helium balloons at the local card shop. Each balloon occupies a volume of 15.8 liters when in the store; but when taken outside, they are observed to shrink. If the indoor temperature is 26.6°C and the outside air temperature is -2.4°C, then what is the new volume of the balloons when taken outside?
A sample of helium gas at 61.8°C has a volume of 342 mL. What is the volume of this gas at -71.7°C?
Answers
1 Which of the following statements is true about a magnetic field?
A It’s found in all metalsincorrect answer
B It's an invisible area of forceincorrect answer
C It makes metals permanent magnetsincorrect answer
D It exists within a magnet
Answers
Option D is correct It is present inside a magnet Outside of the magnet, magnetic field lines travel from north to south.
A magnet's North pole is where magnetic field lines always start and run down to the South pole. Because field lines act as though they are repelling one another, they never intersect as they travel back to the North pole from the South pole inside the magnet.
The magnetic field's characteristic is that it always seeks the route with the least amount of resistance between magnetic poles. Never do they cross each other. They are all equal in strength.
At the poles, the magnetic field is strongest. Magnetic field lines have the following characteristics: they start at the North Pole and converge at the South Pole.
Learn more about magnetic field from here;
https://brainly.com/question/12947677
#SPJ1
Balance the following half eqn. in alkaline medium. Mno-4___ Mno2
Answers
MnO4- + 4e- → MnO2 + 2H2O Now the half-equation is balanced in alkaline medium.
To balance the half-equation MnO4- → MnO2 in alkaline medium, we need to follow the steps for balancing redox reactions in basic solution. The goal is to balance the number of atoms and charges on both sides of the equation.
Start by balancing the atoms other than oxygen and hydrogen. In this case, we only have manganese (Mn) atoms. There is one Mn atom on both sides, so the Mn atoms are already balanced.
Balance the oxygen atoms by adding water (H2O) molecules to the side that lacks oxygen. Since there are four oxygen atoms on the left side (MnO4-) and only two on the right side (MnO2), we need to add two water molecules to the right side:
MnO4- → MnO2 + 2H2O
Next, balance the hydrogen atoms by adding hydrogen ions (H+) to the side that lacks hydrogen. In this case, the left side (MnO4-) already has sufficient hydrogen atoms, so no hydrogen ions need to be added.
Balance the charges by adding electrons (e-) to the side that has a higher charge. MnO4- has a charge of -1, while MnO2 has no charge. Since the left side has a higher charge, we need to add electrons to the right side:
MnO4- + 4e- → MnO2 + 2H2O
Now the half-equation is balanced in alkaline medium. The Mn atoms, oxygen atoms, hydrogen atoms, and charges are all balanced. The addition of water and hydrogen ions helps balance the oxygen and hydrogen atoms, while the addition of electrons balances the charges.
For more such questions on alkaline medium. visit:
https://brainly.com/question/27960992
#SPJ8
a chemical reaction experiment was carried out with the objective of comparing if a new catalyst b would give higher yields than the old catalyst a. the experiment was run on five different batches of raw material which were known to be quite different from one another. each batch was divided into two portions to which a or b was applied at random. the data collected are given in the following table: Catalyst 10 30 28 18 23 22 21 12 a). Explain the experimental design (b)_ Carry out the appropriate t-test.
Answers
According to the problem, we need to determine whether new catalyst B would give higher yields than old catalyst A. The given data is a paired sample, because both the catalysts were experimented on 6 different batches of raw materials.
(a) Explain the experimental design.
Here the data collected is from 6 different raw materials, which were divided into two portions. Therefore, two data were obtained for each of the 6 raw materials, which implies in scientific terms that repeated data was obtained for each of the 6 raw materials. Thus, this type of design is known as Repeated-Measures Design.
(b) Carry out the appropriate t test.
For testing whether the new catalyst B will give higher yields than old catalyst A, a Paired-Sample t-test needs to be performed. The test will be performed using R Studio. The R codes and output are as below.
R CODE
# Load the Data A <- c(9,19,28,22,18,8) B <- c(10,22,30,21,23,12) # Paired Sample t-test t.test(A,B,paired = TRUE,alternative = "less")
R OUTPUT
# Paired Sample t-test > t.test(A, B, paired = TRUE, alternative = less) Paired t-test = data: A and B t = -2.6458, df = ">
The decision rule for this test is: "If the p-value < 0.05, then reject the null hypothesis, under 0.05 level of significance; otherwise accept the null hypothesis".
Decision: As p-value (= 0.02283) < 0.05, so we decide to REJECT the null hypothesis, under 0.05 level of significance.
Conclusion: As the null hypothesis is to be rejected, so we can conclude that "there is sufficient evidence conclude that the new catalyst B gives a higher yield than old catalyst A".
(c) Construct a 95% confidence interval for the difference between catalysts A and B.
From part (b),
we have obtained the R output about the 95% confidence interval for the difference between catalysts A and B as:
Therefore, the 95% confidence interval for the difference between catalysts A and B is (-∞, -0.56).
In frequency statistics, the confidence interval (CI) is the range of estimated values for an unknown parameter. Confidence intervals are computed at the specified confidence level. A 95% confidence level is the most common, but other levels such as 90% and 99% are sometimes used . The confidence level represents the proportion of corresponding CIs over time that contain the true value of the parameter. For example, 95% of all intervals computed at the 95% level must contain the true value of the parameter.
Factors that affect the width of the CI include confidence level, sample size, and within-sample variability. All other things being equal, the larger the sample, the narrower the confidence interval. Similarly, the more varied the sample, the wider the confidence interval, and the higher the confidence level, the wider the confidence interval.
Learn more about confidence interval here : https://brainly.com/question/16742675
#SPJ4
