I AM GIVING A LOT OF POINTS SO PLEASE HELP ME!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Can someone please help me with this?
Rules:
Label the section of the roller coaster where the kinetic or potential energy is.

Answers
Answer:c
Explanation:
it’s gained kinetic from the gravitational potential energy at the top
So, potential energy would be: A
And kinetic energy would be: B, C, D
I’m almost sure these would be correct.
Hope this helps!! (:
Related Questions
Please help me I need these answers

Answers
Answer:
Q8) True
Q9) First ionization energy
Q10) Metal oxides
Explanation:
A common property of group one elements (alkali metals) is their combination with water to form basic solutions. E.g 2K(s)+2H2O(l)→2KOH(aq)+H2(g).
The distance of the outermost electron in the atom from the nucleus is inversely related to the first ionization energy. As the distance between the outermost electron and the nucleus in group 17 elements gets larger, the corresponding first ionization energies of elements in the group gets smaller.
Group 1 elements can combine with oxygen to give metal oxides. E.g 4K + O2 --> 2 K2O
A 25.0 mL aliquot was taken from a 500.0 mL sample of tonic water containing an unknown amount of quinine and diluted to a volume of 200.0 mL. At 347.5 nm, the fluorescence intensity of the diluted sample was measured as 73.7 on an arbitrary scale. Under similar conditions, a 25.0 ppm standard quinine solution had a fluorescence intensity of 195 . Calculate the mass of quinine in the original tonic water sample.
Answers
The mass of quinine in the original tonic water sample is 37.8 mg.
What is the concentration of the diluted sample?The concentration of the diluted sample with a fluorescence intensity of 73.7 is calculated using the formula of Lambert-Beer's law as follows:
C₁/C₂ = A₁/A₂
where:
C₁ = 25 ppm or 25 mg/L
C₂ = ?
A₁ = 195
A₂ = 73.7
C₂ = 73.7 * 25 / 195
C₂ = 9.45 mg/L
To concentration of the original sample will be determined from the dilution formula:
C₁V₁ = C₂V₂
where;
C₁ = ?
V₁ = 25. 0 mL
C₂ = 9.45 mg/L
V₂ = 200 mL
C₁ = 9.45 * 200 / 25
C₁ = 75.6 mg/L
The volume of the stock sample was 500 mL or 0.5 L
The mass of quinine = 75.6 mg/L * 0.5 L
mass of quinine = 37.8 mg
Learn more about concentration at: https://brainly.com/question/17206790
#SPJ1
When 127g of copper is reacted with oxygen, 143g of the oxide is formed. What is the empirical formula of this oxide.
Answers
Answer:
CuO
Explanation:
according to the spectrochemical series, which of the following ligands has the strongest splitting field?
Answers
The increasing order of the crystal field splitting- I− < Br− < S2− < SCN− < Cl−< N3 < F−< NCO−< OH−<C2O42−< O2−< H2O < acac− < NCS− < CH3CN <gly <py < NH3 < en < bipy < phen < NO2− < PPh3 < CN− < CO.
Describe the spectrochemical sequence.The ligands (affections to a metal ion) are listed in the spectrochemical series according to the strength of their field. The series was created by superimposing various sequences acquired from spectroscopic research because it is impossible to generate the full series by examining complexes with the single metal ion.
Which ligands in the spectrochemical series are strong field ligands?The ligands cyanide and CO are classified as strong-field ligands, whereas the halides are weak-field ligands. Ligands such as water and ammonia are said to create medium field effects.
To know more about spectrochemical series visit :
https://brainly.com/question/15520506
#SPJ4
The meaning of the word symptom:
Answers
The word "symptom" refers to a specific manifestation or indication of a condition, disease, or disorder that is experienced or observed by an individual.
Symptoms are subjective or objective changes in the body's normal functioning that may be recognized as abnormal, uncomfortable, or problematic. Symptoms can manifest in various ways depending on the nature of the underlying condition. They can be physical, such as pain, rash, cough, fever, or fatigue, indicating an illness or injury affecting the body. Symptoms can also be psychological, such as anxiety, depression, or confusion, reflecting disturbances in mental health.
Symptoms serve as important clues for medical professionals to identify and diagnose diseases or disorders. They provide valuable information about the nature, severity, and progression of an illness, helping healthcare providers formulate appropriate treatment plans. Additionally, symptoms may also be important for individuals to self-assess their own health status and seek appropriate medical attention.
It is essential to note that symptoms alone may not provide a definitive diagnosis, as they can overlap across different conditions. Further evaluation, including medical tests and examinations, is often necessary to confirm a diagnosis and determine the appropriate course of action.
for more such questions on symptom
https://brainly.com/question/21078887
#SPJ8
An element has an atomic number of 36, what element is it? Question 4 options: Kr K Se Es
Answers
Answer:
\(\Huge \boxed{\mathrm{Kr}}\)
Explanation:
Krypton is an element in the periodic table with an atomic number of 36.
The symbol for Krypton is Kr.
Answer:
KR.
Explanation:
Use the periodic table for reference:

How much total energy is released to cool 28.3 g of steam (water vapor) at 100.0°C to liquid water at 25.5°C? Water has a heat of vaporization of ± 40.7 kJ/mol and a specific heat of 4.184 J/g°C.
Answers
Answer:
\(\Delta H=-72870J=-72.9kJ\)
Explanation:
Hello,
In this case, for the computation of the total energy, we must consider two processes:
1. Condensation of steam (heat of condensation is the negative of heat of vaporization).
2. Cooling of hot water (we use the specific heat of water).
Thus, we write:
\(\Delta H=\Delta H_{cond}+\Delta H_{cooling}\)
For each term, we have:
\(\Delta H_{cond}=28.3g*\frac{1mol}{18g} *-40.7\frac{kJ}{mol} *\frac{1000J}{1kJ}= -63989.44J\)
\(\Delta H_{cooling}=28.3g*4.184\frac{J}{g\°C}*(25.5-100.0)\°C =-8880.54J\)
Therefore, the total energy results:
\(\Delta H=-63989.44J-8880.54J\\\\\Delta H=-72870J=-72.9kJ\)
Regards.
Why does the solubility of alkaline earth metal hydroxides in water increase down the group?
Answers
Answer:
The solubility of alkaline earth metal hydroxides in water increases down the group because the size of the metal cation increases as you move down the group. This increase in size results in a decrease in the cation's charge density, which makes it less able to attract and hold onto hydroxide ions. As a result, the hydroxides become more soluble in water as you move down the group. Additionally, the lattice energies of the hydroxides decrease down the group, making it easier to break apart the crystal lattice structure and dissolve the hydroxides in water.
What would be the volume in liters of an 25.15 liter sample of gas at 201 °C and 2.31 atm if conditions were changed to STP?
Answers
The volume of the gas at STP would be 23.93 liters.
The volume of gas at STP (Standard Temperature and Pressure), we need to use the Ideal Gas Law, which states that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature. First, we need to calculate the number of moles of gas in the initial sample. We can use the formula n = PV/RT, where P is the initial pressure, V is the initial volume, R is the gas constant, and T is the initial temperature.
n = (2.31 atm) x (25.15 L) / [(0.0821 L atm/mol K) x (201 + 273.15 K)]
n = 1.067 moles
Now, we can use the molar volume of gas at STP, which is 22.4 L/mol, to calculate the volume of gas at STP.
V = n x 22.4 L/mol
V = 1.067 moles x 22.4 L/mol
V = 23.93 L
Therefore, the volume of the gas at STP would be 23.93 liters.
For more such questions on gas
https://brainly.com/question/25736513
#SPJ11
What is the molar mass
MgCrO4
Answers
The molar mass of MgCrO4 is approximately 140.30 g/mol.
To determine the molar mass of MgCrO4 (magnesium chromate), we need to calculate the sum of the atomic masses of each individual element in the compound.
The chemical formula MgCrO4 indicates that the compound consists of one magnesium atom (Mg), one chromium atom (Cr), and four oxygen atoms (O).
The atomic masses of the elements can be found on the periodic table:
Magnesium (Mg) has an atomic mass of approximately 24.31 g/mol.
Chromium (Cr) has an atomic mass of around 51.99 g/mol.
Oxygen (O) has an atomic mass of about 16.00 g/mol.
Now, we can calculate the molar mass of MgCrO4 by summing up the atomic masses of each element, considering the respective subscripts:
Molar mass = (Atomic mass of Mg) + (Atomic mass of Cr) + 4 × (Atomic mass of O)
Molar mass = (24.31 g/mol) + (51.99 g/mol) + 4 × (16.00 g/mol)
Molar mass = 24.31 g/mol + 51.99 g/mol + 64.00 g/mol
Molar mass ≈ 140.30 g/mol
for more such questions on mass
https://brainly.com/question/24191825
#SPJ8
When some of the sugar added to iced tea remains undissolved at the bottom of the glass, the solution is Most studied answer a) dilute b) unsaturated c) saturated d) polar e) nonpolar
Answers
When some of the sugar added to iced tea remains undissolved at the bottom of the glass, the solution is Saturated.
Solution is saturated which means no more solute can be dissolved in the solvent at the given/present temperature and pressure conditions. To dissolve more sugar in the tea we need to increase the temperature of the tea so that the tea becomes unsaturated, for the given conditions also the solubility of the solids increases with the increase in the temperature, so more of the sugar can be dissolved if the temperature of the solvent is increased.
To learn more about Solubility :
brainly.com/question/13372319
#SPJ4
3. How many grams of oxygen are required to completely react with 240g of C₂H6?
Answers
Approximately 766.08 grams of oxygen are required to completely react with 240g of C₂H₆.
To determine the amount of oxygen required to completely react with 240g of C₂H₆ (ethane), we need to set up a balanced chemical equation for the combustion of ethane.
The balanced equation for the combustion of ethane is as follows:
C₂H₆ + O₂ → CO₂ + H₂O
From the balanced equation, we can see that the stoichiometric ratio between C₂H₆ and O₂ is 1:3. This means that for every one mole of C₂H₆, three moles of O₂ are required for complete combustion.
To calculate the amount of oxygen required, we need to convert the given mass of C₂H₆ to moles using its molar mass, and then use the stoichiometric ratio to determine the moles of O₂ required. Finally, we can convert the moles of O₂ back to grams using the molar mass of oxygen.
The molar mass of C₂H₆ is calculated as follows:
(2 x atomic mass of carbon) + (6 x atomic mass of hydrogen)
(2 x 12.01 g/mol) + (6 x 1.01 g/mol) = 30.07 g/mol
Now, we can proceed with the calculation:
Calculate the moles of C₂H₆:
moles of C₂H₆ = mass of C₂H₆ / molar mass of C₂H₆
moles of C₂H₆ = 240 g / 30.07 g/mol ≈ 7.98 mol
Determine the moles of O₂ using the stoichiometric ratio:
moles of O₂ = moles of C₂H₆ x (3 moles of O₂ / 1 mole of C₂H₆)
moles of O₂ = 7.98 mol x 3 ≈ 23.94 mol
Convert moles of O₂ to grams:
mass of O₂ = moles of O₂ x molar mass of O₂
mass of O₂ = 23.94 mol x 32.00 g/mol = 766.08 g
For more such questions on oxygen visit:
https://brainly.com/question/28009615
#SPJ8
Balance the equation using the
correct coefficient

Answers
2Li + 2H2O____ 2LiaOH + 2H2
Answer:
2 Li + 2 H2O --> 2 LiaOH + 1 H2
HELP!
True or false: When there are zero lone pairs on the central atom, the shape is
symmetrical (assuming all of the bonds are the same).
Answers
What is the pH in a 1.5 M solution of hydrofluoric acid?
Answers
\(HF \rightleftharpoons H^{+} + F^{-}\\\)
We have 1.5 M HF in the beginning and 0 M \(H^{+}\) and \(F^{-}\)
We know that there's gonna be a decrease of x M on \(HF\) and an increase of x M on \(H^{+}\) and \(F^{-}\).
We also find in chemistry tables that \(K_{a}(HF) = 6.8 \cdot 10^{-4}\)
\(K_a = \frac{[H^+] \cdot [F^-]}{[HF]}\\6.8 \cdot 10^{-4} = \frac{x \cdot x}{1.5 - x} \\6.8 \cdot 10^{-4} = \frac{x^2}{1.5}\\6.8 \cdot 10^{-4} \cdot 1.5 = x^2\\x^2 = 1.02 \cdot 10^{-3}\\x = 0.0319\\pH = -\log{0.0319}\\pH = 1.5\)
When you exercise strenuously, your body produces excess heat. Describe what your body does to help prevent your temperature from rising excessively and
explain why your body's response effective.
Answers
Answer:
Your body can cool itself by sweating. When sweat evaporates, it lowers your temperature
Explanation:
To convert m-ethylaniline to m-ethylfluorobenzene, it should be treated with nitrous acid followed by _____________.
Answers
Answer:
\(NaBF_4\)
Explanation:
In this reaction, we must exchange the amino group (\(NH_2\)) for a fluorine atom (\(F\)). Also, the first step in this reaction is the addition of nitrous acid.
We must remember that the amino group in the presence of nitrous acid produces a diazonium salt. The \(N_2\) group is a very good leaving group and many benzene derivatives can be produced from this intermediate (see figure 1).
If what we want is to bond a fluorine atom we must use \(NaBF_4\) to be able to produce m-ethylfluorobenzene (see figure 2).
I hope it helps!

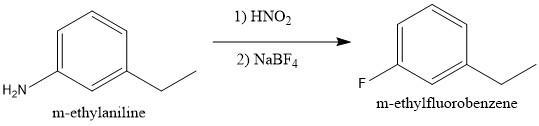
Which of the following represents C3H8?

Answers
Among the given options the compound with the formula C₃H₈ is option C. It is an organic compound under named as propane.
What is propane?
Propane is an organic compound classified as a hydrocarbon with saturated carbon - hydrogen bonds. It's formula is C₃H₈. It contains 3 carbons and 8 hydrogens.
The two end carbons contains 3 hydrogens each and the middle carbon contains 2 hydrogens. They are all bonded through sigma bonding. The compound in A is C₅H₁₂ and in B, it is C₆H₁₄.
The formula of the compound in option D is C₅H₁₀. Hence, the skeleton showing the compound with the formula of C₃H₈ is option C.
To find more on propane, refer here:
https://brainly.com/question/24159489
#SPJ2
Me ayudan con los nombres de los compuestos :D

Answers
If 19mL of alcohol are dissolved in 31mL of water, what is the percentage by volume of alcohol
Answers
Answer:
To find the percentage by volume of alcohol, we need to divide the volume of alcohol by the total volume of the solution, then multiply by 100%:
Volume of alcohol = 19 mL
Total volume of solution = 19 mL + 31 mL = 50 mL
Percentage by volume of alcohol = (19/50) x 100% = 38%
Therefore, the percentage by volume of alcohol in the solution is 38%.
Answer:
20% volume percentage I think
Which type of bonds do polar covalent bonds break down in chemical reactions?
A. polar covalent and ionic bonds
B. nonpolar covalent bonds and polar covalent bonds
C. ionic bonds and nonpolar bonds
D. none of the above
Answers
Answer:
(C) im pretty sure is the answer
Explanation:
The type of bonds does polar covalent bonds break down in chemical reactions is ionic bonds and nonpolar bonds. Therefore, option C is correct.
What is an ionic bond ?Ionic bond is also known as electrovalent bond. The term ionic bond is defined as a chemical bond formed when one atom gives up one or more electrons to another atom.
It is also defined as when positively charged particle produce a bond with negatively charged particle. The chemical molecule sodium chloride is an example of an ionic bond.
In ionic bonding, the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities. The type of bonds does polar covalent bonds break down in chemical reactions is ionic bonds and nonpolar bonds.
Thus, option C is correct.
To learn more about the ionic bond, follow the link;
https://brainly.com/question/11527546
#SPJ2
between ethane, ethene and ethyne which is having shortest bond?
Answers
As you can see the picture, in the three given compounds i.e. ethane, ethene and ethyne, ethyne have shortest bond. Bond length of ethyne is very short when compared to the ethane and ethene. Shorter the bond, bond strength will be more. Hence, our answer is ethyne.

Answer:
ethyne has shortest bond
which property is a property of water
Answers
Answer: Polarity, Boiling and Freezing Points, Specific Heat Capacity, Density, Surface Tension, Heat of Vaporization and Vapor Pressure, Capillary Action, Solid State (Ice), Liquid State (Liquid Water), Gas State (Steam),
Explanation: Water molecules are polar, so they form hydrogen bonds. This gives water unique properties, such as a relatively high boiling point, high specific heat, cohesion, adhesion, and density.
2. What causes a current in an electric circuit?
conductor
voltage
ampere
electric charge
Answers
Answer:
electric charge
Explanation:
Pls help I’ll brainlest and add extra points

Answers
Answer:
a top predator, fighting
what happens to (PH , temperature, conductivity ) of salution when an acid is neutralise
Answers
Answer:
https://edu.rsc.org/cpd/acids-and-bases/2000001.article
Explanation:
Try this website, it may help.
Part A
A sample of ideal gas is in a sealed container. The pressure of the gas is 735 torr, and
the temperature is 29 °C. If the temperature changes to 62 °C with no change in
volume or amount of gas, what is the new pressure, P2, of the gas inside the container?
Express your answer with the appropriate units.
View Available Hint(s)
HÅ
P₂ = Value
Submit
O
Units
?
Answers
Pressure of the gas inside the container is 662.59 torr.
What is ideal gas law?The ideal gas law (PV = nRT) connects the macroscopic characteristics of ideal gases. An ideal gas is one in which the particles are both non-repellent and non-attractive to one another (have no volume).
The general law of ideal gas can be applied here: PV is equal to nRT, where P is the gas pressure in atm.
V is the number of moles of the gas in a mole, and n is the volume of the gas in L. R is the universal gas constant. T is the temperature(Kelvin) of the gas.
If P and T are different values and n and V are constants, then
(P₁T₂) = (P₂T₁).
P₁ = 735 torr, T₁ = 29°C + 273 = 302 K,
P₂ = ??? torr, T₂ = 62°C + 273 = 335 K.
∴ P₂ = (P₁T₂)/(P₁) = (735 torr)(302 K)/(335 K) = 662.59 torr.
To know more about ideal gas law visit:
https://brainly.com/question/29405260
#SPJ1
Which binary molecular compound includes an element with five atoms of one element?
Answers
Answer:
For example, N2O4 is referred to as dinitrogen tetroxide, not dinitrogen tetraoxide, and CO is called carbon monoxide, not carbon monooxide.
...
Binary molecular (covalent) compounds.
Prefixes used in chemical nomenclature
prefix number of atoms
tetra- 4
penta- 5
hexa- 6
Explanation:
Which scientist wrote the Book of Optics?
O A. Warren Washington
O B. In al-Haytham
O C. Christian Doppler
O D. Galileo Galilei
Answers
The scientist who wrote the Book of Optics is In al-Haytham.
option B.
Which scientist wrote the Book of Optics?The Book of Optics presented experimentally founded arguments against the widely held extramission theory of vision (as held by Euclid in his Optica), and proposed the modern intromission theory, the now accepted model that vision takes place by light entering the eye.
The Book of Optics is a seven-volume treatise on optics and other fields of study composed by the medieval Arab scholar Ibn al-Haytham, known in the West as Alhazen or Alhacen.
Thus, the scientist who wrote the Book of Optics is In al-Haytham.
Other options such as Warren Washington, Christian Doppler and Galileo Galilei are not correct.
Learn more about optics here: https://brainly.com/question/12969591
#SPJ1
Identify whether the following are dependent or independent clause
1. Because he cannot pick up our son
2. I am typing on the computer
3. They will have gone to Disney World
4. Before we arrive in Florida
Answers
Answer:
1. dependent clause
2. Independent clause
3. Independent clause
4. Dependent clause
Explanation:
In English language, there are two types of clauses (a group of words that contain subject and predicate) viz; independent clause and dependent clause.
INDEPENDENT CLAUSE is a clause that can stand alone to form a complete sentence, possessing a subject and a predicate.
On the other hand, a DEPENDENT CLAUSE cannot stand alone to form a complete and meaningful sentence, but instead depends on another sentence to make sense. It is often recognizable as they start with subordinating conjunctions such as while, because, when etc.
According to this question, the following are examples of independent and dependent clauses:
1. Because he cannot pick up our son - dependent clause
2. I am typing on the computer - independent clause
3. They will have gone to Disney World - independent clause
4. Before we arrive in Florida - dependent clause