Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30x10⁻⁸ at 700°C:2H₂S(g) ⇄ 2H₂(g) + S₂(g) If 0.45 mol of H₂S is placed in a 3.0-L container, what is the equilibrium concentration of H₂(g) at 700°C?
Answers
the equilibrium concentration of H₂(g) at 700°C = 0.00193 mol/L
0.00193 mol/L
Given that:
numbers of moles of H₂S = 0.59 moles
Volume = 3.0-L
Equilibrium constant = 9.30 × 10⁻⁸
The equation for the reaction is given as :
2H₂S ⇄ 2H₂(g) + S₂(g)
The initial concentration of H₂S =
The initial concentration of H₂S =
= 0.1966 mol/L
The ICE table is shown be as :
2H₂S ⇄ 2H₂(g) + S₂(g)
Initial 0.9166 0 0
Change -2 x +2 x + x
Equilibrium (0.9166 - 2x) 2x x
(since 2x < 0.1966 if solved through quadratic equation)
The equilibrium concentration for H₂(g) = 2x
∴
= 0.00193 mol/L
Thus, the equilibrium concentration of H₂(g) at 700°C = 0.00193 mol/L
To know more about equilibrium concentration
https://brainly.com/question/13414142
#SPJ4
Related Questions
On the graph:
A represents the .
B represents the .
C represents the .
D represents the .

Answers
On the graph,
Letter A represents reactantsLetter B represents actvation complexLetter C represents productLetter D represents activation energyHow do i know what the letters represents?To know what each letters represents, we must understand what an energy profile diagram is.
An energy profile diagram is a representation of chemical reaction, showing the pathways in which reactants follows to become products.
With the above information, we can say that:
Letter A represents the reactants and letter C represents the productsEvery reaction has a peak energy. This peak energy is referred to as the activation complex. The activation complex is the highest energy of the reaction. Looking at the diagram, we can see that B is at the peak.
Thus, letter B represents the activation complex
Also, every reaction has activation energy. This is the minimum energy required for a reaction to occur. In profile diagram the activation energy is the energy between the peak (i.e activation complex) and the reactant. from the graph, we can see that letter D lies between the letter B and A.
Thus, we can say letter D is the activation energy
Learn more about energy profile diagram:
https://brainly.com/question/28204750
#SPJ1
Calculate the enthalpy of combustion of methane, if the standard enthalpies of formation of methane, carbon dioxide, water are −74.85,−393.5 and −286?
Answers
The enthalpy of combustion of methane is approximately -890.65 kJ/mol.
To calculate the enthalpy of combustion of methane (CH4), we can use the standard enthalpies of formation (ΔH°f) for methane (CH4), carbon dioxide (CO2), and water (H2O).
The combustion reaction of methane can be represented as follows:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
The standard enthalpy change for this reaction, ΔH°comb, can be calculated using the standard enthalpies of formation:
ΔH°comb = Σ(nΔH°f,products) - Σ(mΔH°f,reactants)
where n and m are the stoichiometric coefficients of the products and reactants, respectively.
Given:
ΔH°f(CH4) = -74.85 kJ/mol
ΔH°f(CO2) = -393.5 kJ/mol
ΔH°f(H2O) = -286 kJ/mol
Using the equation above, we can calculate the enthalpy of combustion:
ΔH°comb = [1 × ΔH°f(CO2)] + [2 × ΔH°f(H2O)] - [1 × ΔH°f(CH4)]
= [1 × (-393.5 kJ/mol)] + [2 × (-286 kJ/mol)] - [1 × (-74.85 kJ/mol)]
= -393.5 kJ/mol - 572 kJ/mol + 74.85 kJ/mol
= -890.65 kJ/mol
Therefore, the enthalpy of combustion of methane is approximately -890.65 kJ/mol.
To know more about enthalpy refer here
brainly.com/question/29145818#
#SPJ11
Hello. Can someone help me do these few questions. Thanks

Answers
Answer:
In chemistry, an element is a pure substance consisting only of atoms that all have the same numbers of protons in their atomic nuclei. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by chemical means. Wikipedia
help me plz...........

Answers
Answer:
try use Google search
Explanation:
you need to use it
Why did the changes you made result in more energy storage molecules?
Answers
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
_____ energy has the total amount of
kinetic energy contained in all the
particles of a substance.
A: Thermal
B: Sound
C: Chemical
D: Electric
Answers
Answer: Thermal Energy
Explanation:
Select the correct answer from each drop-down menu.
Much of the mass of an atom is concentrated in its nucleus. The nucleus contains (blank), and the outer part of the atom contains (blank)
Answers
Answer: 1) The answer is: c) protons and neutrons.
2) The answer is: a) electrons.
Explanation:
To see the number of atoms of an element in a given molecule we need to multiply stoichiometry to the number that is written on the foot of the element that is stoichiometry. Therefore, Much of the mass of an atom is concentrated in its nucleus. The nucleus contains protons and neutrons and the outer part of the atom contains electrons.
What is atom?Atom is the smallest particle of any element, molecule or compound. Atom can not be further divided. Atoms contains nucleus in its center and electron that revolve around the atom in fixed orbit. Electron has -1 charge while proton has +1 charge. Neutron is neutral.
Much of the mass of an atom is concentrated in its nucleus. The nucleus contains protons and neutrons and the outer part of the atom contains electrons.
Therefore, much of the mass of an atom is concentrated in its nucleus. The nucleus contains protons and neutrons and the outer part of the atom contains electrons.
To know more about atom, here:
https://brainly.com/question/13518322
#SPJ2
Urgent please please I really need help
I will give you 60 points just please help

Answers
Answer:
1 = -15. 2 = +5
Explanation:
i just did it good luck
A gene or trait that appears or expresses itself over a recessive trait is called the anstwer
Answers
Please help me I don't have time

Answers
Primary carbon - 7
Secondary carbon -2
Tirtiary carbon - 2
quaternary Carbon - 2
For an n-type Silicon wafer that has donor density (Na d
) of 10 17
cm −3
. a) Plot Temperature (K) vs. Intrinsic carrier concentration for the above material. b) Find the temperature, in K, where the intrinsic and extrinsic carrier concentration are equal.
Answers
The plot of temperature (K) vs. intrinsic carrier concentration for an n-type Silicon wafer with a donor density (Na) of \(10^17 cm^−3\) shows an exponential increase in carrier concentration as temperature rises.
How does temperature affect the intrinsic carrier concentration in an n-type Silicon wafer?The intrinsic carrier concentration (ni) in a semiconductor material represents the density of thermally generated carriers (electrons and holes) in the absence of any impurities.
It depends on temperature and the energy bandgap of the material. In silicon, the intrinsic carrier concentration can be described by the following equation:
\(\[n_i = A \cdot T^{3/2} \cdot e^{-E_g/(2kT)}\]\)
Where:
- A is a constant
- T is the temperature in Kelvin
- E_g is the energy bandgap of silicon
- k is the Boltzmann constant
As temperature increases, the intrinsic carrier concentration also increases due to two main factors. Firstly, the exponential term in the equation dominates, causing a significant increase in carrier concentration. Secondly, the temperature dependence of the energy bandgap amplifies the effect.
The exponential increase arises from the increasing number of thermally generated electron-hole pairs. At higher temperatures, more valence band electrons acquire sufficient thermal energy to cross the energy bandgap and become free electrons in the conduction band.
Similarly, more conduction band electrons recombine with valence band holes, leading to an increase in both electron and hole concentrations.
Learn more about intrinsic carrier concentration
brainly.com/question/31970577
#SPJ11
classify each of the following diatomic species as ionic, polar covalent or nonpolar covalent.
Answers
Answer:
1. O2
The oxygen molecule is a non-polar covalent molecule. It is formed by sharing of electrons. As the molecule is symmetric, the electrons are pulled by both the atoms in an equal amount.
2. CaO:
The association of two ions forms calcium oxide. The ions are calcium ion and oxide ion. Such a compound is called an ionic compound.
3. Br2:
Bromine molecule contains a non-polar covalent bond because both the atoms share an equal number of electrons, and it is symmetric.
4. HBr:
Hydrogen bromide is a polar covalent compound. It is an asymmetric charge between hydrogen and bromine. Bromine being more electronegative, tends to pull the electron itself.
which formulas represent compounds that are isomers of each other?

Answers
Answer:
Option D.
Explanation:
Isomerism is a phenomenon where by two or more compounds have the same molecular formula but different structural patterns. The compounds involved are called isomers.
Option A has two different compound with two different molecular formula. Hence they are not isomers.
Option B has two different compound with two different molecular formula. Hence they are not isomers
Option C can not be called isomers because Isomerism can not occur in compound having just 1 carbon atom.
Option D has two different compound with the same molecular formula as C3H8O and their structure are different. Hence they areisomers.
To standardize an H2SO4 solution,you put 10.00 mL of it in a flask with a few drops of indicator and put 0.500 M NaOH in a buret. The buret reads 0.31 mL at the start and 20.36 mL at the end point. Find the molarity of the H2SO4 solution. Create a balanced equation first to solve problem.
Answers
The balanced equation for the reaction between H2SO4 and NaOH is:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
The molarity of the H2SO4 solution is approximately 0.248 M.
From the balanced equation, we can see that the mole ratio between H2SO4 and NaOH is 1:2. This means that for every mole of H2SO4, we need 2 moles of NaOH to react completely.
Given that the volume of NaOH used is 20.36 mL - 0.31 mL = 20.05 mL, which is equal to 0.02005 L.
Using the mole ratio, we can determine the moles of H2SO4:
Moles of H2SO4 = Moles of NaOH / 2
Moles of H2SO4 = (0.500 M) × (0.02005 L) / 2 = 0.005025 mol
Now, we can calculate the molarity of the H2SO4 solution:
Molarity of H2SO4 = Moles of H2SO4 / Volume of H2SO4 (in L)
Molarity of H2SO4 = 0.005025 mol / 0.01000 L = 0.5025 M
Rounding to three significant digits, the molarity of the H2SO4 solution is approximately 0.248 M.
To learn more about molarity visit;
https://brainly.com/question/31545539
#SPJ11
Cellular isozymes of pyruvate kinase are allosterically inhibited by:A) high concentrations of AMP.B) high concentrations of ATP.C) high concentrations of citrate.D) low concentrations of acetyl-CoA.E) low concentrations of ATP.
Answers
High ATP concentrations allosterically block the cellular homologs of pyruvate kinase.
Why does ATP stop phosphofructokinase from working?Phosphofructokinase activity was hindered by low ATP concentrations through lowering the enzyme's affinity for fructose 6-phosphate, the other substrate. Phosphofructokinase was similarly inhibited by citrate and other tricarboxylic acid cycle intermediates.
During gluconeogenesis, which of the these enzymes requires ATP?The reactions of gluconeogenesis, starting with pyruvate, are as follows: Pyruvate carboxylase uses the mitochondrion to carboxylate pyruvate to create oxaloacetate. Pyruvate carboxylase needs biotin as a cofactor and ATP as such an activating molecule.
To know more about concentrations visit :
https://brainly.com/question/10725862
#SPJ4
__________ is the process by which substances are broken down into simpler compounds.
Answers
Decomposition is the process by which substances are broken down into simpler compounds.
It involves the breakdown of complex molecules into smaller, more basic components through chemical or biological processes. These processes can include various mechanisms such as hydrolysis, oxidation, fermentation, or enzymatic reactions. Decomposition is a fundamental part of nutrient cycling and plays a crucial role in the recycling of organic matter in ecosystems.
The decomposition of carbonic acid in soft drinks, which can be represented by the chemical equation:
H₂CO₃ → H₂O + CO₂
To know more about Decomposition Reaction refer to this link
https://brainly.com/question/27300160
Science help me now
Answers
Answer:
but were is the answer
Explanation:
i am so confuesed
What is the wavelength (in nm) of an electron with the following kinetic energies? (a) 20.0 ev (no response) nm (b) 200 ev (no response) nm (c) 2.00 kev (no response) nm (d) 20.0 kev (no response) nm (e) 0.200 mev (no response) nm (f) 2.00 mev (no response) nm which of these energies are most suited for study of the nacl crystal structure? (select all that apply.) 20.0 ev 200 ev 2.00 kev 20.0 kev 0.200 mev 2.00 mev none of these
Answers
The wavelength of an electron can be calculated using the formula: wavelength = h / (mass of electron * velocity). Since kinetic energy is equal to the mass of the electron multiplied by the velocity squared, we can also calculate wavelength by using the formula: wavelength = h / sqrt(2mass of electron kinetic energy).
To convert the kinetic energies given in electron volts (eV) to Joules (J), you can use the formula: 1 eV = 1.6 x 10^-19 J
(a) 20.0 eV = 3.2 x 10^-18 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-18 J) = 2.4 x 10^-12 m or 2.4 pm (picometers)
(b) 200 eV = 3.2 x 10^-17 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-17 J) = 2.4 x 10^-11 m or 24 pm
(c) 2.00 keV = 3.2 x 10^-14 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-14 J) = 2.4 x 10^-8 m or 2.4 nm
(d) 20.0 keV = 3.2 x 10^-13 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-13 J) = 2.4 x 10^-7 m or 24 nm
(e) 0.200 MeV = 3.2 x 10^-11 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-11 J) = 2.4 x 10^-5 m or 0.24 nm
(f) 2.00 MeV = 3.2 x 10^-10 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-10 J) = 2.4 x 10^-4 m or 2.4 nm
A lower energy electron will have a longer wavelength, while a higher energy electron will have a shorter wavelength. To study the crystal structure of NaCl, you would need to use a technique such as X-ray diffraction, which typically uses X-rays with energies in the range of a few keV to a few tens of keV. Based on this, 2.00 keV and 20.0 keV energies are most suited for study of the NaCl crystal structure.
To know more about wavelength of electrons visit :
https://brainly.com/question/17295250?referrer=searchResults
#SPJ4
An atom has 25 protons ,30 neutrons ,and 25 electrons.what is the charge of the atoms
Answers
ok so basically the answer is notijng
Answer:
+25
Explanation:
The charge of the nucleus of an atom with 25 protons is +25 regardless of the number of other particles.
BTW, I believe this is Magnesium... I am not sure, but that ingfo may help you more than I can...
Compare the atomic and ionic radius of chlorine
Answers
Answer:
Explanation:
vdW radius (nm) ionic radius of X- (nm)
Cl 0.175 0.181
Br 0.185 0.196
I 0.198 0.220
The relative number of atoms of a compound can be calculated
by dividing the percentage of an element by the:
Answers
Answer:
Obtain the relative numbers of atoms of each element in the compound by dividing the number of moles of each element in the 100 g sample by the number of moles of the element present in the smallest amount.
Answer:
Obtain the relative numbers of atoms of each element in the compound by dividing the number of moles of each element in the 100 g sample by the number of moles of the element present in the smallest amount.
Which structural formula represents a member of the alkene series?
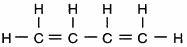
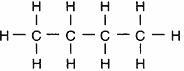
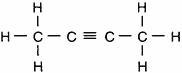
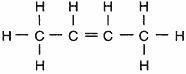
Answers
Answer:
C4H8. butene
the kinetic molecular theory (kmt) is discussed in this lesson, and it helps us understand the behavior of gases. from what we learned, which of these statements are not consistent with kmt?
Answers
The kinetic molecular theory (KMT) is a model used to explain the behavior of gases based on the motion of their individual particles.
Let's analyze the given statements and determine which ones are not consistent with KMT:
1. "Gases consist of small particles called molecules or atoms that are in constant motion." - This statement is consistent with KMT. According to the theory, gases are made up of small particles (atoms or molecules) that are constantly moving and colliding with each other and the walls of their container.
2. "Gas particles are always attracted to each other." - This statement is not consistent with KMT. The theory assumes that gas particles have negligible attractive forces between them. Instead, it states that they only interact through collisions.
3. "Gas particles have definite shapes and volumes." - This statement is not consistent with KMT. According to KMT, gas particles do not have definite shapes or volumes. They take on the shape and volume of their container, as they are highly compressible and can fill the available space.
4. "The average kinetic energy of gas particles is directly proportional to their temperature." - This statement is consistent with KMT. KMT states that the average kinetic energy of gas particles is directly proportional to their temperature. As the temperature increases, the particles move faster and have higher kinetic energy.
In summary, the statements that are not consistent with KMT are:
2. "Gas particles are always attracted to each other."
3. "Gas particles have definite shapes and volumes."
To know more about kinetic molecular theory visit:-
https://brainly.com/question/30648725
#SPJ11
convert 4 hours into seconds (DA practice problems) must solve final answer.
1 & 2

Answers
Answer:
The answer is 14,400 seconds.
Explanation:
Hope it helps man ...

Answer:14400secondsExplanation:

The atmospheric concentration of a gas depends on its emission into the atmosphere and its rates of physical, chemical and biological removal. The time to lower the concentration of the gas to 37% of its original amount is its __________.
Answers
The atmospheric concentration of a gas depends on its emission into the atmosphere and its rates of physical, chemical and biological removal. The time to lower the concentration of the gas to 37% of its original amount is its atmospheric lifetime.
What is atmospheric concentration?The measurements of CO2 equivalents in parts per million CO2 is termed as atmospheric concentration. The pressure exerted by the atmosphere on the gas is called atmospheric pressure.
What is atmospheric lifetime?The atmospheric lifetime of a species mainly measures the time which is require to restore equilibrium in the atmosphere that follows a sudden decrease or increase in the concentration of the species in the atmosphere.
What is Emission?Emission is something which can be released, emitted or discharge.
Types of emission
Direct GHG emissions. Indirect electricity GHG emissions. Other indirect GHG emissions.Thus, we concluded that the atmospheric concentration of a gas depends on its emission into the atmosphere and its rates of physical, chemical and biological removal. The time to lower the concentration of the gas to 37% of its original amount is its atmospheric lifetime.
learn more about atmospheric concentration:
https://brainly.com/question/16657469
#SPJ4
PLEASE HELP ASAP
Volcanoes erupt as a result of
A) Two continental plates converging
B) One oceanic plate converging with one continental plate
C) Two tectonic plates transforming
Answers
Answer:
B.) One oceanic plate converging with one continental plate.
HOPE THIS HELPS!
Answer:
C: Two tectonic plates transforming
PLEASE HELP I WILL GIFT 100 POINTS!!!!!

Answers
Answer:
Liquid A
Explanation:
the least dense will be at the top. like icecubes in water. the ice is less dense than the water so it floats
Answer:
Liquied A
Explanation:
It is at the top and it is the lightest if it is at the bottom then it has the most density
what volume of carbon dioxide is produced when 10.0 L of oxygen is consumed?
balanced equation given is
C2H4(g) + 3 O2(g) -> 2CO2(g) + 2H2O(g)
Answers
The volume of carbon dioxide produced when 10.0 L of oxygen is consumed is 6.67 L of CO2 (rounded to two decimal places).
Given balanced equation: C2H4(g) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)The molar volume of all gases at standard temperature and pressure is 22.4 L/mol.Using the balanced equation, we can find the ratio of the volume of oxygen used to carbon dioxide produced.C2H4(g) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)3 moles of O2 produce 2 moles of CO2So, 1 mole of O2 produces (2/3) moles of CO2 or 1 mole of CO2 produces (3/2) moles of O2.
To find the volume of carbon dioxide produced when 10.0 L of oxygen is consumed, we need to use the mole ratio:3 mol O2 → 2 mol CO22/3 mol CO2 is produced by 3 mol O210.0 L of O2 is consumed, which is equal to 10.0/22.4 = 0.4464 moles of O2Thus, using the mole ratio:0.4464 mol O2 × (2/3) mol CO2/1 mol O2 = 0.2976 mol CO2The volume of 0.2976 mol CO2 is:0.2976 mol CO2 × 22.4 L/mol = 6.67 L of CO2 (rounded to two decimal places).
To know more about carbon dioxide visit:
https://brainly.com/question/3049557
#SPJ11
Round 6.357 to the tenth place 
Answers
Answer:
6.4
Explanation: