How would you prepare the following aqueous solutions?
(a) 3.10x10² g of 0.125 m ethylene glycol (C₂H₆O₂) from ethylene glycol and water
Answers
The molarity of the solution is 0.1625M.
What do you meant by MolarityMolarity is the amount of a substance in a certain volume of solution. Molarity is defined as the moles of solute per litres of a solution .
Molarity → no of moles / Volume (L)
SI unit of Molarity is M or mol/ L
We have given here the mass of solution is 3.10×10²g .
molality of the solution is 0.125m
Molality → no of moles / mass in kg
→ 0.125×3.10×10²/ 1000
→ no.of moles = 0.0162
For molarity we can assume volume as 1000 ml .
Molarity = 0.0162×1000/ 100
Molarity →0.162 M.
So, the molarity of solution will be 0.162M.
to Learn more about Molarity click here https://brainly.com/question/8732513
#SPJ4
Answer:
Explanation:vuvlkjbgnkllnuigii
Related Questions
if 100.0 mL of liquid weighs 81.23g what is the density of the liquid
Answers
Answer:
812.3 kilogram/cubic meter
Explanation:
I used an online calculator.
Hope I helped!
choose the ground state electron configuration for ti2+.
Answers
The ground state electron configuration for Ti²⁺ is [Ar] 3d². Here’s how it can be derived: The element with atomic number 22 is Titanium (Ti).
Its full electronic configuration is 1s²2s²2p⁶3s²3p⁶4s²3d². When this atom loses two electrons to form Ti²⁺, it loses two from the 4s sub-shell, and its new configuration is [Ar]3d².
The electron configuration of an atom is the distribution of electrons among its orbitals. It represents the state of an atom's electrons in their ground state or unexcited state. It's important to remember that when an atom becomes an ion, its electron configuration changes.
The process of ionization entails removing or adding electrons from the outermost or valence shell of an atom. The valence shell is the outermost shell in an atom's electron cloud.
To know more about atomic number visit:
https://brainly.com/question/16858932
#SPJ11
In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only available stock solution of the acid has a concentration of 18.0 m. what volume of the stock solution would they use to make the required solution? use m subscript i v subscript i equals m subscript f v subscript f.. 0.900 ml 1.11 ml 6.94 ml 7.20 ml
Answers
The volume of stock solution they use to make the required solution is 6.94mL.
How we calculate the volume?To calculate the value of required volume we will use the below equation:
M₁V₁ = M₂V₂, where
M₁ = molarity of stock solution = 18M
V₁ = volume of stock solution = to find?
M₂ = molarity of H₂SO₄ solution = 2.50M
V₂ = volume of H₂SO₄ solution = 50mL
On putting all these values on the above equation and calculate for the value of V₁ as:
V₁ = (2.50)(50) / (18) = 6.94mL
Hence correct option is (3) i.e. 6.94mL.
To know more about stock solutions, visit the below link:
https://brainly.com/question/3942978
Answer:
C. 6.94 mL
Explanation:
Correct on Edge 2022!!!
Good luck everyone, you got this! Have a great day!
Mass number symbol =
Answers
Answer:
A is the symbol of mass number.
Explanation:
→ Mass number (A) = Protons + Neutrons
It is the required formula. The symbol of the mass number is A.
Answer:
The letter A
Explanation:
In the heating and cooling curves below, identify the letter in the diagram diagram that corresponds to each of the listed processes in the table
I’m so confused if anyone could help (and explain as if I’m a 3 yr old) that would be helpful
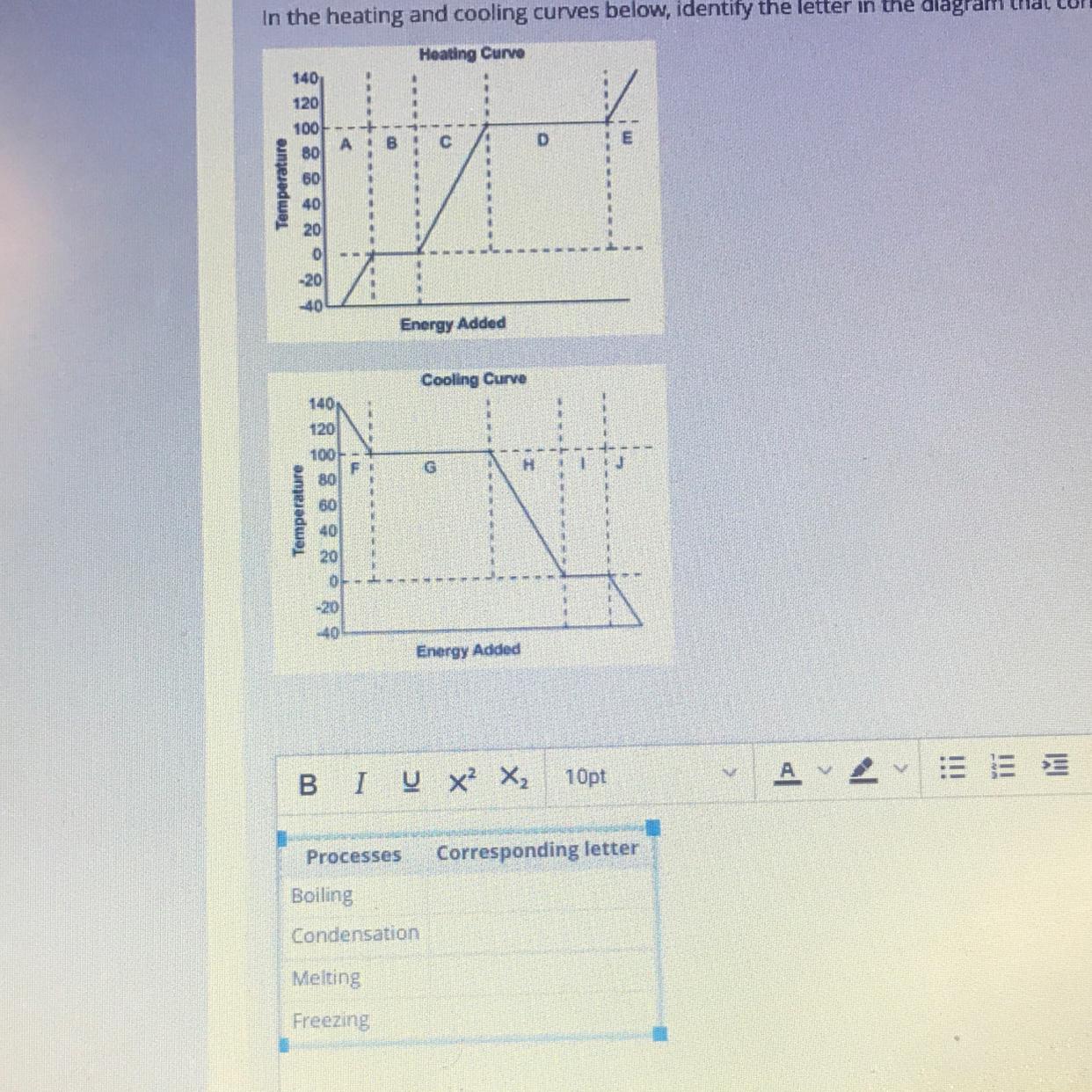
Answers
Answer:
Test for the first one is the best for
Which best describes a chemical reaction that follows the law of conservation of matter?
Group of answer choices
A. The reactants have the same mass as the products.
B. The reactants have the same density as the products.
C. The products conserve all chemical properties of the reactants.
D. The products conserve all physical properties of the reactants.
Answers
Some students believe that teachers are full of hot air. If I inhale 3.5 liters of gas at a temperature of 190 C and it heats to a temperature of 580 C in my lungs, what is the new volume of the gas
Answers
The new volume of the gas in your lungs would be approximately 6.44 liters. The new pressure of the gas would be approximately 2.69 atm. There are approximately 0.218 moles of gas present. The initial volume of the gas would be approximately 16.21 liters.
Let's address each question separately:
Inhaling gas:
To determine the new volume of the gas, we can use Charles's Law, which states that the volume of a gas is directly proportional to its temperature, assuming constant pressure.
Charles's Law equation: V(i)/T(i) = V(f)/T(f)
Solving for V(f):
V(f) = (V(i) × T(f)) / T(i)
V(f) = (3.5 liters × 853 K) / 463 K
V(f) ≈ 6.44 liters
Therefore, the new volume of the gas in your lungs would be approximately 6.44 liters.
Compressing gas:
Since the temperature stays the same, we can use Boyle's Law,
Boyle's Law equation: P(i) × V(i) = P(f) × V(f)
Solving for P(f):
P(f) = (P(i) × V(i)) / V(f)
P(f) = (1 atm × 1.50 liters) / 0.556 liters
P(f) ≈ 2.69 atm
Therefore, the new pressure of the gas would be approximately 2.69 atm.
Inflating potato chip bag:
Similar to question 1, we can use Charles's Law to determine the new volume of the bag.
Charles's Law equation: V(i)/T(i) = V(f)/T(f)
Solving for V(f):
V(f) = (V(i) × T(f)) / T(i)
V(f) = (125 mL × 573 K) / 294 K
V(f) ≈ 243 mL
Therefore, the new volume of the bag would be approximately 243 mL.
Gas at changing pressure, temperature, and volume:
To solve this problem, we can use the combined gas law, which relates the initial and final conditions of pressure, temperature, and volume of a gas.
Combined gas law equation: (P(i) × V(i)) / T(i) = (P(f) × V(f)) / T(f)
Solving for Vi:
V(i) = (P(i) × V(i) × T(f)) / (P(f) × T(i))
V(i) = (1.5 atm × 34 liters × 300 K) / (1.9 atm × 315 K)
V(i) ≈ 16.21 liters
Therefore, the initial volume of the gas would be approximately 16.21 liters.
Size of a bubble:
To find the size of the bubble, we can apply Boyle's Law, which relates the initial and final pressures and volumes of a gas.
Boyle's Law equation: P(i) × V(i) = P(f) × V(f)
Solving for V(f):
V(f) = (P(i) × V(i)) / P(f)
V(f) = (1.5 atm × 17,000 liters) / 245 atm
V(f) ≈ 104.08 liters
Therefore, the size of the bubble would be approximately 104.08 liters.
Moles of gas:
To calculate the number of moles of gas, we can use the ideal gas law, which relates the pressure, volume, temperature, and number of moles of a gas.
Ideal gas law equation: PV = nRT
Solving for n (number of moles):
n = PV / RT
n = (2.4 atm × 8.5 liters) / (0.0821 atm·L/(mol·K) × 395 K)
n ≈ 0.218 moles
Therefore, there are approximately 0.218 moles of gas present.
To know more about Boyle's Law:
https://brainly.com/question/30367133
#SPJ4
--The question is incomplete, the complete question is given:
"Some students believe that teachers are full of hot air. If I inhale 3.5 liters of gas at a temperature of 190 C and it heats to a temperature of 580 C in my lungs, what is the new volume of the gas? If 1.50 L of a gas is at standard temperature and pressure is compressed to 556 mL, what is the new pressure of the gas in atm (hint, temperature stays the same)? On hot days, you may have noticed that potato chip bags seem to “inflate”, even though they have not been opened. If I have a 125 mL bag at a temperature of 21 0C, and I leave it in my car with a temperature of 300 C, what will the new volume, in mL, of the bag be? I have an unknown volume of gas at a pressure of 1.5 atm and a temperature of 315 K. If I raise the pressure to 1.9 atm, decrease the temperature to 300 K, and measure the final volume to be 34 liters, what was the initial volume of the gas? Submarines need to be extremely strong to withstand the extremely high pressure of water pushing down on them. An experimental research submarine with a volume of 17,000 liters has an internal pressure of 1.5 atm. If the pressure of the ocean breaks the submarine forming a bubble with a pressure of 245 atm pushing on it, how big will that bubble be in liters? If I have an unknown quantity of gas held at a temperature of 395 K in a container with a volume of 8.5 liters and a pressure of 2.4 atm, how many moles of gas are present?"--
Explanation:
Those are crazy , ridiculous numbers (as I commented )
P1V1/T1 = P2V2/T2 Where 'T' is in Kelvin
I will assume P is constant..
(...so you don't blow out your lungs in a bloody burnt mess )
V1/T1 = V2/T2 <=====RE ARRANGE TO :
V2 = V1/T1 * T2 PLUG IN THE NUMBERS
V2 = 3.5 / ( 273.15 + 190) * ( 273.15 + 580) = 6 .45 liters
What does it mean if EROEI = 1? a. None of the above b. It's early days of fossil fuel exploration c. It's a perfect return on investment d. The efficiency is 100%
Answers
When EROEI (Energy Return on Energy Investment) is equal to 1, it means that the energy gained from a particular source is equivalent to the energy invested in obtaining that energy. In other words, the energy return is equal to the energy input. This indicates a situation where the energy extraction process is barely breaking even, with no net gain or loss in energy.
EROEI is a metric used to assess the efficiency and viability of energy sources. It measures the amount of usable energy obtained from a particular energy source divided by the amount of energy invested to extract or produce that energy. A value of 1 means that the energy gained is just enough to offset the energy invested.
In practical terms, an EROEI of 1 implies that the energy source being evaluated is not very efficient. It suggests that the amount of energy required to extract, process, or produce the energy is nearly equal to the energy obtained. Therefore, there is little to no surplus energy available for other uses or to sustain the energy extraction process itself.
An EROEI of 1 is often associated with energy sources in their early stages of development or exploration, where the technology or extraction methods may not be fully optimized. It could also indicate energy sources with high production costs or low energy density.
Learn more about EROE
brainly.com/question/32215499
#SPJ11
Information to make scientifically supported judgments by describing the Importance of Ernest Rutherford's gold-foil experiment.
Answers
Answer:
Rutherford's experiment led to the rejection of JJ Thomson's raisin bundle model. In the experiment, a thin gold strip was bombarded with alpha particles. According to Thomson's atomic model, all alpha particles should have passed through the strip, but in this experiment it was found that some of the particles were reflected backwards. Based on his experiment, Rutherford published the result that almost the entire mass of an atom is concentrated in its nucleus. Rutherford's atomic model was later improved by Niels Bohr, who published the Bohr model.
Compare how entropy changes for the following two systems:
System A: Two gases mix when the valve separating two containers is opened.
System B: A solid powder decomposes to form a solid product and a gas product.
Answers
The measure of the randomness of the system is the change in the entropy. The mixing of two gases and decomposition of the solid will increase the entropy.
What is entropy?Entropy is the disorderliness and the randomness of the system when the thermal energy is not present in a sufficient amount to initiate the reaction. In system A, when two gases are mixed then the entropy increases as the number of gaseous molecules increases.
In system B, when a solid powder gets decomposed to form a solid product and a gaseous product the entropy increases as along the solid particles the gas is also produced.
Therefore, in both the systems the entropy increases.
Learn more about entropy here:
https://brainly.com/question/27460189
#SPJ1
how many atoms are in 3.5 moles of arsenic atoms?
Answers
Answer:
1 mol of atoms = 6.022 ×1023 atoms. 3.5mol × 6.022 × 1023 atoms 1mol = 2.11 × 1024 atoms =.........
Explanation:
2160.64 atoms!
The swimmer moves her hand down and to the left and her body goes forward
to the right. *
Which law ??
Answers
Answer:Newton’s first law
Explanation:
You mix two amino acids together: glu and ser. If they reacted, how many different dipeptides would you expect to get? List them.
I'm really struggling with amino acids, so any help is appreciated! I won't be clicking any links, though.
Answers
Answer:
Answers 1. An amide linkage between the carboxyl group of one amino acid and the amine group of the other amino acid. 2. An ester is a compound formed from the reaction of a carboxylic acid and an alcohol. ( I apologise, I cant figure out number 3.. )
If neon gas travels at 402 m/s at a given temperature, estimate the rate of diffusion of butane gas, C4H10, at the same temperature.
Answers
The rate of diffusion of the butane gas would be 592 m/s.
The diffusion rate of gasesThe rate of diffusion of a gas is inversely proportional to the square root of its molar mass, so lighter gases diffuse faster than heavier gases. The molar mass of neon is 20.18 g/mol, while the molar mass of butane is 58.12 g/mol, which means that butane is significantly heavier than neon.
Assuming that the temperature and pressure are constant, we can use Graham's law of diffusion to estimate the rate of diffusion of butane compared to neon:
rate of diffusion of butane / rate of diffusion of neon = square root of (molar mass of neon / molar mass of butane)
Let x be the rate of diffusion of butane. Then:
x / 402 m/s = sqrt(20.18 g/mol / 58.12 g/mol)
Simplifying this equation gives:
x = 402 m/s * sqrt(58.12 g/mol / 20.18 g/mol)
x = 402 m/s * 1.472
x ≈ 592 m/s
Therefore, at the same temperature, the estimated rate of diffusion of butane gas, C4H10, is approximately 592 m/s.
More on the diffusion rate of gases can be found here: https://brainly.com/question/29846633
#SPJ1
Which state of matter can change both shape and volume?
O solid
O liquid
O gas
Answers
Answer:
Gas
Explanation:
Gasses will fill the shape whatever container they are in and can be compressed down into smaller volumes quite easily.
Solids have a definite shape and volume
liquids have an indefinite shape but a definite volume
Organisms typically have more than one form of each gene. If one form can mask the appearance of another form, that form is considered _______ the other form.
A.
better than
B.
dominant over
C.
recessive to
D.
worse than
Answers
If one form of a gene can mask the appearance of another form, that form is considered dominant over the other form. Option B.
What are dominant alleles?According to Mendel, genes are usually made up of 2 alleles. These alleles can be the same or different. When the alleles are the same, the gene is said to be homozygous. If the alleles are different, the gene is said to be heterozygous.
When the two alleles that make up a gene are different, one will be dominant and the other will be recessive. The dominant gene masks the effect of the recessive gene. In other words, the recessive gene cannot be expressed as long as it coexists with the dominant gene. In order for it to be expressed, it has to be in two copies or a homozygous recessive form.
For the dominant allele, however, only one copy is needed for it to be expressed.
In summary, if one form of a gene can mask the appearance of another form, that form is considered dominant over the other form.
More on genes can be found here: https://brainly.com/question/5519888
#SPJ1
HCL is pure covalent compound but soluble in solvent water why
Answers
Answer:
HCl is a polar covalent compound, because of electronegativity difference between Cl(3.5) and hydrogen (2). Hence in this way, the bond between HCl breaks and they formed ions in the polar solvent like water .
If you were to prepare a 3.5% solution of sodium bicarbonate in water that required the total mass of the solution to be 50 g, what would the mass of the sodium bicarbonate be?
Answers
Given data:
- mass % = 3.5%
- mass of solution = 50g
- mass of solute (sodium bicarbonate) = ?
In order to find the mass of solute we must use the next formula
\(mass\text{ \%}=\frac{mass\text{ of solute}}{mass\text{ of solution }}\cdot100\text{ \%}\)Since we need the mass of solute, we must solve the equation for mas of solute
\(\text{mass of solute}=\frac{mass\text{ \%}\cdot mass\text{ of solution}}{100\text{ \%}}\)Now, we must replace the values in the equation
\(\text{mass of solute }=\frac{3.5\text{ \%}\cdot50g}{100\text{ \%}}\)Finally, we must simplify
\(\text{mass of solute}=1.75g\)ANSWER:
The mass of the sodium bicarbonate is 1.75 g
5
volume (cm³)
1. Based on this graph, how does metal B differ from metal A?
2. What is the density of metal B? Show all your work and include appropriate units.
3. What is the mass of 9.0 cm³ of metal B? Find this in two different ways.
a. Mark on the above graph how you might determine this.
b. Show how you could calculate this mathematically.

Answers
Density is an intensive property as it does not depend on the quantity of the substances Whereas mass and volume are extensive property. Therefore, Metal A is lesser denser than metal B.
What is density?Density tells about the compactness of the substances, how much dense is the substances in other words. Object that is more denser than water they just sink in the water.
Mathematically,
Density = Mass of the metal ball ÷volume marked on the graduated cylinder
Since mass of metal a and metal B is same, so now density depend on volume. Volume of metal A is higher than metal B. From formula we can see density is inversely proportional to volume. So, Metal A is lesser denser than metal B.
Therefore, Metal A is lesser denser than metal B.
To know more about density, here:
https://brainly.com/question/16894337
#SPJ1
a certain gas occupies 5 dm³ at 24 °C and 15 cm Hg. Calculate the pressure of the gas if the volume is halved and the temperature is raised to 200°C.
Answers
Explanation:
(P1V1)/T1=(P2V2)/T2
(15X5)/297=(p2x2.5)/473
p2=47.78cmHg
The gas arsine, AsH3, decomposes as follows: In an experiment at a certain temperature, pure AsH3(g) was placed in an empty, rigid, sealed flask at a pressure of 392.0 torr. After 48 hours the pressure in the flask was observed to be constant at 488.0 torr. a. Calculate the equilibrium pressure of H2(g). b. Calculate Kp for this reaction
Answers
The equilibrium pressure of \(H_{2}\) is 288 torr. and \(K_{p}\) for this reaction is 0.786 atm.
from the reaction:- \(2AsH_{3}\) ⇄ \(2As + 3H_{2}\)
initial concentration 392 torr. 0
at equilibrium. 392 - \(2x\). \(3x\)
and the final pressure in the flask = 488 torr.
Hence,
\(( 392 - 2x ) + 3x = 488\\ x = 488-392\\x = 96 torr.\)
The partial pressure of \(H_{2}\) is 3 × 96 = 288 torr.
and \(AsH_{3}\) is 392 - ( 2 × 96 ) = 392 - 192 = 200 torr.
Now, to find \(K_{p}\) for this reaction, we will use \(K_{p} = \frac{(P_{H_{2} })^{2} }{( P_{AsH_{3} } )^{2}}\)
putting all the values, we get,
\(K_{p} = \frac{(288)^{3} }{(200)^{2} }\)
= 597.1968 torr.
= 0.786 atm. ( 1 atm = 760 torr. )
what do you mean by equilibrium?
Equilibrium in chemistry is the phase that exists when a chemical reaction and its opposite reaction happen at the same rates. This word's Latin origin dates back to the prefix aequi-, which means equal, and lbra, that indicates scale or balance.
Learn more about equilibrium reaction here:-
https://brainly.com/question/15118952
#SPJ4
how is it d?explain please?! i do not understand.please dont guess
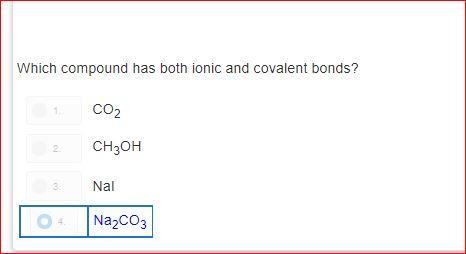
Answers
Answer:
As you can see sodium = ionic and covalent bonds aka NA 2
and CO 3 also has those two bonds the other answers dont have both numbers (bonds) Na2 and CO3 is the only answer choice that has these bonds in simpler terms ( only answer with 2 numbers)
Explanation:
Why should we not taste unknown substances
Answers
Answer:
because it could be harmful and dangerous for our health. like some substances can be acid so if we taste it we can die.
Explanation:
Answer:
Explanation:
There are several reasons why we should not taste unknown substances:
Health risks: The unknown substance may be toxic or harmful to our health, and tasting it can lead to serious health issues or even death.
Allergic reactions: We may be allergic to some substances, and tasting them can cause severe allergic reactions.
Unpleasant taste: The substance may have a terrible taste, and tasting it can cause discomfort or nausea.
Contamination: The unknown substance may be contaminated, and tasting it can lead to the spread of harmful bacteria or viruses.
Legal issues: Tasting unknown substances can be illegal in some cases, such as trying drugs or other illegal substances.
In conclusion, it is always advisable to avoid tasting unknown substances. If you come across something you are unsure about, it is best to seek professional help or advice rather than risking your health or safety.
Blood type in humans is controlled by ____
alleles.
a, three
b. two
c. one
d. four
Answers
Answer:
A: three
Explanation:
Rannan wants to perform a scientific experiment. He writes out a systematic process that involves him making observations and then forming his opinion about those observations. He then collects his opinions together and uses them to show that his findings are supported by evidence.
Answers
He shouldn't present his opinions as evidence.
What is the definition of a hypothesis?
A hypothesis is a tested assertion regarding the relationship between two or more variables or a theory put up to explain an observed occurrence in a scientific setting.
The complete population should be examined in order to ascertain whether a statistical hypothesis is accurate. As it is frequently impractical, researchers usually look at a random sample of the population. The statistical hypothesis is rejected if the sample data do not support it.
Making an experiment is the most typical method of testing a theory. A good experiment employs test subjects or establishes situations where you may determine whether your hypothesis appears to be true by assessing a wide array of of data (test results).
To learn more about hypothesis use link below:
https://brainly.com/question/606806
#SPJ1
Where did the spread of opera start and where did it go?
Answers
With the production of Jacopo Peri's mostly forgotten Dafne in Florence in 1598, opera began in Italy at the end of the 16th century.
Particularly from Claudio Monteverdi's L'Orfeo and quickly spread throughout Europe: Jean-Baptiste Lully in France, Henry Purcell in England, and Heinrich Schütz in Germany
Where was opera popularized?The first nation where opera gained popularity was Italy. Claudio Monteverdi and Jacopo Peri called it home. This exciting form of entertainment eventually spread throughout the remainder of Europe. Italy, France, and Germany are the primary producers of opera.
Learn more about Producer:
brainly.com/question/29391287
#SPJ4
What is the major difference between a for-profit and a not-for-profit organization? goodwill
Answers
The major difference between a for-profit and a not-for-profit organization lies in their primary objectives and the way they handle their finances.
For-profit organizations, as the name suggests, are driven by the goal of making a profit. Their main purpose is to generate revenue and distribute it to the company's shareholders or owners. The profits earned by for-profit organizations are often reinvested back into the business or distributed as dividends to shareholders.
On the other hand, not-for-profit organizations are focused on a mission or cause rather than generating profits. Their goal is to serve the public interest, and any surplus funds generated are reinvested back into the organization to further its mission. Not-for-profit organizations can include charities, educational institutions, and social service agencies, among others.
In terms of finances, for-profit organizations aim to maximize shareholder wealth and financial gains. They are typically funded through equity investments, loans, and sales of goods or services. On the other hand, not-for-profit organizations rely on various sources of funding, such as donations, grants, and government funding, to support their activities and achieve their mission.
One important term related to not-for-profit organizations is "goodwill." Goodwill refers to the intangible value of a not-for-profit organization's reputation, brand, and relationships with its stakeholders. It represents the trust and positive perception that the organization has built within the community. Goodwill can be important for not-for-profit organizations as it helps in attracting donors, volunteers, and supporters who believe in the organization's mission.
In summary, the major difference between for-profit and not-for-profit organizations lies in their primary objectives and financial structures. For-profits focus on making a profit for shareholders, while not-for-profits aim to serve the public interest. Goodwill is an important aspect for not-for-profits as it represents their positive reputation and trust within the community.
Learn more about not-for-profit organization here:-
https://brainly.com/question/33446653
#SPJ11
what makes alkali metals a family?
Answers
Answer:
The metals in this group are lithium, sodium, potassium, rubidium, cesium, and francium. The gas hydrogen is also put in this group because it shares similar reactivity with the alkali metals.
I don't know if this is what you wanted or not sorry if it isn't
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
What is the correct designation for an orbital that has five total nodes, of which two are radial?
a) 5d
b) 3d
c) 6d
d) 3f
e) 4f
f) 6f
g) 5f
Answers
f) 6f is the correct designation for the orbital that has five nodes in total and of which two are radial. Hence, option f) 6f is correct.
As we know umber of radial nodes = n−l−1
where, n is Principal quantum number and l is Azimuthal quantum number.
So, total number of nodes = n−1
n−1 = 5
n=6 and
n−l−1=2
6−l−1 = 2
Now, l=3 which is f - subshell
So, the atomic orbital is 6f.
According to the quantum atomic model, atoms can have many numbers of orbitals and can be categorized on the basis of size, shape or orientation. Smaller sized orbital means there is greater chance of getting any electron near the nucleus and orbital wave function or ϕ is a mathematical function that used for representing the coordinates of the electron.
To know more about orbitals, refer
https://brainly.com/question/28888362
#SPJ11