How would it have been if particles of matter had no space?
Answers
Answer:
Matter is made of small particles of atoms or molecules. ... The particles in the solid are touching with very little space between them. The particles in a liquid usually are still touching but there are some spaces between them. The gas particles have big distances between them.
Related Questions
If 20g of caco2 and 25g of Hcl are mixed ,what mass of Co2 is produced ?
Answers
Approximately 8.79 grams of CO₂ are produced when 20 grams of CaCO₃ and 25 grams of HCl are mixed.
The balanced chemical equation for the reaction between calcium carbonate (CaCO₃) and hydrochloric acid (HCl) is:
CaCO₃ + 2HCl → CaCl₂ + CO₂ + H₂O
From the equation, we can see that 1 mole of CaCO₃ reacts with 2 moles of HCl to produce 1 mole of CO₂. The molar mass of CaCO₃ is 100.09 g/mol, and the molar mass of HCl is 36.46 g/mol.
To find the mass of CO₂ produced, we need to determine which reactant is limiting. This can be done by calculating the number of moles of each reactant and comparing them to the stoichiometric ratio in the balanced equation.
Number of moles of CaCO₃ = 20 g / 100.09 g/mol = 0.1998 mol
Number of moles of HCl = 25 g / 36.46 g/mol = 0.685 mol
According to the balanced equation, 1 mole of CaCO₃ reacts with 2 moles of HCl. Therefore, the limiting reactant is CaCO₃, since only 0.1998 moles of CaCO₃ are available to react with HCl.
From the balanced equation, we know that 1 mole of CaCO₃ produces 1 mole of CO₂. Therefore, the number of moles of CO₂ produced is also 0.1998 mol.
The molar mass of CO₂ is 44.01 g/mol. Therefore, the mass of CO₂ produced is:
Mass of CO= 0.1998 mol x 44.01 g/mol = 8.79 g
Therefore, approximately 8.79 grams of CO₂ are produced when 20 grams of CaCO₃ and 25 grams of HCl are mixed.
Learn more about molar mass,
https://brainly.com/question/22997914
#SPJ4
Calculate the heat (in calories) released when 115 g of CHCl3 changes from a liquid to a solid at
its freezing point.
Answers
Answer: 8855 Joules energy is released when 115 g of \(CHCl_3\) changes from a liquid to a solid at its freezing point.
Explanation:
Latent heat of freezing is the amount of heat released to convert 1 mole of liquid to solid at atmospheric pressure.
Amount of heat released to freeze 1 gram of \(CHCl_3\) = 77 J/g
Mass of \(CHCl_3\) given = 115 gram
Heat released when 1 g of \(CHCl_3\) is freezed = 77 J
Thus Heat released when 115 g of \(CHCl_3\) is freezed =\(\frac{77}{1}\times 115=8855J\)
Thus 8855 Joules energy is released when 115 g of \(CHCl_3\) changes from a liquid to a solid at its freezing point.
Suppose Earth was tilted much less on its axis. What effect would this have on the planet? Please help me !
Answers
Answer:
If Earth was tilted much less on its axis, it would lead to minor changes in precipitation and temperatures as Earth moves slightly towards and away from the sun.
Explanation:
If a planet tilts more, it leads to colder winters and warmer summers. However, if a planet tilts less, it leads to less severe seasons that is and milder winters and cooler summers.
If Earth was tilted much less on its axis, it would lead to minor changes in precipitation and temperatures as Earth moves slightly towards and away from the sun.
RIGHT ANWSER WILL BE MARKED BRAINLIEST


Answers
Answer:
11460
Explanation:
The carbon atoms in cellulose found in a log originally came from _____ absorbed by the plant. Group of answer choices
Answers
Answer: Your answer is carbon dioxide in the air
Hope this helps!
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
Write four components of a modern detergent and the uses of the components
Answers
The four main components of a modern detergent are builders (50% by weight), surfactants (15%), bleach (7%), and enzymes (2%).
What is the use of the components in the detergent?A detergent can be described as a surfactant or a mixture of surfactants with cleansing properties in dilute solutions. These substances are similar to soap but are more soluble in hard water due to the polar sulfonate is less likely than the polar carboxylate of soap to bind ions in hard water.
Builders are water softeners and their anions react with cations to form insoluble compounds which precipitate onto fabrics and washing machines and which are hard to remove.
Surfactants provide absorption and emulsification of soil into the water and also by reducing the surface tension of water to improve wetting. The main function of bleaches is oxidizable organic stains, which are usually of vegetable origin.
Enzymes are needed to degrade stubborn stains composed of proteins, fats, starch, and cellulose.
Learn more about detergents, here:
https://brainly.com/question/28588910
#SPJ1
7. 25 cm =
what does that equal??
Answers
Answer:
it equals 2.9 rounded and
2.854331 without rounding
Explanation:
formula= divide length value by 2.54
3. Kevin has an unknown element. He wants to find out whether it is a metal or
a non-metal. He heats the substance on one end and the other end gets hot very
quickly. The element is orange in colour and its surface is shiny.
a. Do you think it is a metal or a non-metal?
b. What piece of evidence from the above given information made you decide this?
Explain with respect to the properties of metals and non-metals.
Answers
a) The unknown element that Kevin has is a metal.
Since the unknown element conducts heat, burns with a colored flame, and has a shiny surface- these are the characteristic properties of metals.
b)The piece of evidence that helped to decide was the conduction of heat, orange-colored flame, and shiny surface.
The information provided states that the element is a good conductor of heat as the other end gets heated and burns with an orange-colored flame and also has a shiny surface. These properties confirm that the element is Metal.
Properties of metal-
Metals are good conductors of heat and electricity.Most metals burn with a colored flame.Metals have shiny surfaces.Metals are malleable.Metals are ductile.Properties of non-metal-
Non-metals are poor conductors of heat and electricity.Non-metals do not burn with a colored flame.Non-metals do not have shiny surfaces.Non-metals are not malleable.Non-metals are non-ductile.From the properties stated it is confirmed that the unknown element is a metal.
To learn more differences between metals and non-metals, refer
https://brainly.com/question/13874693
Mrs. Roberts' class won a day at the bowling alley for their good behavior all year. When they
arrived, jason picked the green ball that weighed 6 lbs, and amanda picked the yellow ball that
weighed 12 lbs. Amanda was having a hard time getting her ball to roll down the lane. Jason was
doing great! he was pushing his ball hard, and it was going straight down the center. He even got
a strike twicel
Answers
Amanda’s ball is heavier so it’s harder throw. This was illustrated in the bowling.
How to illustrate the information?Amanda was having such a difficult time because she chose a heavier ball.
Evidence: Jason had no trouble with his 6lb ball, while Amanda struggled with double the weight of Jason's ball, which was 12lb and had more mass.
Reasoning: Jason chose a ball with less mass weighting at 6 pounds, but his ball was more simpler to roll as a result. This was owing to Newton's second law, as having a lighter ball made it easier for him to pass through. Amanda, on the other hand, had a ball that was so heavy that it was difficult to throw.
Learn more about bowling on:
https://brainly.com/question/27885742
#SPJ1
A student is given the equation N₂ + H₂ → NH3
2NH₂-
to balance. She answers with N₂ + 2H₂
Explain why her answer is not correct. Balance
the equation correctly.
Answers
The student's answer N₂ + 2H₂ is not correct because it doesn't balance the charges of the reactants and products. The reactants have no charge, but the product NH3 has a negative charge of 2-.
What are reactants ?In a chemical reaction, reactants are the starting materials that undergo a chemical change or reaction to produce one or more new substances called products. The reactants are written on the left-hand side of the chemical equation, while the products are written on the right-hand side. For example, in the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O), the reactants are H2 and O2, and the product is H2O. The balanced chemical equation for this reaction is:
2H2 + O2 → 2H2O
In this equation, two molecules of hydrogen react with one molecule of oxygen to produce two molecules of water.
To know more about reactants visit :
https://brainly.com/question/17096236
#SPJ1
the pressure inside a 15.0 l gas cylinder at 23.8 °c is 4.26 atm. how many moles of gas are contained in the cylinder? question 2 options: 0.145 moles 32.7 moles 0.381 moles 2.62 moles
Answers
The number of moles of gas contained in the cylinder is approximately 0.381 moles.
To calculate the number of moles of gas contained in the cylinder, we can use the ideal gas law equation:
PV = nRT
Where:
P = Pressure (in atm)
V = Volume (in L)
n = Number of moles
R = Ideal gas constant (0.0821 L·atm/(mol·K))
T = Temperature (in Kelvin)
We need to convert the temperature from Celsius to Kelvin by adding 273.15:
T = 23.8 °C + 273.15 = 296.95 K
Now, we can rearrange the ideal gas law equation to solve for the number of moles (n):
n = PV / RT
Plugging in the values:
P = 4.26 atm
V = 15.0 L
R = 0.0821 L·atm/(mol·K)
T = 296.95 K
n = (4.26 atm)(15.0 L) / (0.0821 L·atm/(mol·K))(296.95 K)
n ≈ 0.381 moles
To know more about moles refer here
https://brainly.com/question/15416344#
#SPJ11
A helium-filled balloon is released into the atmosphere. As the balloon rises, which
would MOST likely increase and cause the balloon to burst?
O the volume of the helium
O the mass of the helium
o the temperature of the helium
O the density of the helium
Answers
How many grams of magnesium hydroxide will precipitate if 25. 0 mL of 0. 235 M magnesium nitrate are combined with 30. 0 mL of 0. 260 M potassium hydroxide?
Answers
The grams of magnesium hydroxide will precipitate if the 25 mL of 0.235 M magnesium nitrate are combined with the 30. 0 mL of 0.260 M potassium hydroxide is 0.227 g.
The reaction is given as :
Mg(NO₃)₂ + 2KOH ----> 2KNO₃(aq) + Mg(OH)₂(s)
moles of Mg(NO₃)₂ = 0.235 × 0.025
= 0.00587 mol
moles of KOH = 0.260 × 0.030
= 0.0078 mol
1 mole of Mg(NO₃)₂ react with 2 mole of KOH
mole of KOH = 0.0078 × 2
= 0.0156 mol
KOH is the limiting reagent.
2 mole of KOH produces 1 mole of Mg(OH)₂
mole of Mg(OH)₂ = 0.0078 / 2 = 0.0039 mol
mass of Mg(OH)₂ = 0.0039 × 58.3
= 0.227 g
To learn more about moles here
https://brainly.com/question/26416088
#SPJ4
What is an Independent Variable (IV)? *
Answers
here's your answer dear friend❤❤

.A person is unable to see distinctly the objects placed at large distances but is able to read a book comfortably. Name the defect of vision he is suffering from
Answers
Answer:
The defect of vision suffered by the person is Myopia
Explanation:
The person can't see farther objects but is able to read a book which is placed closely to the eyes, using these observations the person is suffering from MYOPIA(nearsightedness) in which observer see close objects clearly but farther objects appear blurred.
The light entering the eye isn't correctly bent, the eyeball becomes big , or the eye lens become too converging, which converges the light ray in front of the cornea
This defect can be cured by using concave lenses.
calculate the number of moles in 18.5 g of aluminum thiosulfate, Al2(S2O3)3
Answers
Answer:
0.05 mol
Explanation:
Moles = Mass / Mr (Relative formula mass)
Ar of aluminium = 27
Ar of sulphur = 32
Ar of oxygen = 16
Mr of Al2(S2O3)2 = (27×2)+((32×2)×3)+((16×3)×3)= 390
Moles = 18.5g / 390
Moles = 0.05 mol
the reaction of no(g) cl2(g)nocl(g) cl(g) is found to have an equilibrium constant of 1.1×108 at a particular temperature. \what does this mean?
Answers
The equilibrium constant of 1.1x10^8 for the reaction NO(g) + Cl2(g) ⇌ NOCl(g) + Cl(g) at a particular temperature indicates that the forward reaction (formation of NOCl and Cl) is favored at equilibrium.
In a chemical reaction, the equilibrium constant (K) expresses the ratio of the concentrations (or partial pressures) of the products to the concentrations (or partial pressures) of the reactants, each raised to their respective stoichiometric coefficients. A high equilibrium constant, such as 1.1x10^8, indicates a large concentration of products relative to reactants at equilibrium.
In this case, the high value of the equilibrium constant suggests that the forward reaction (NO + Cl2 → NOCl + Cl) is highly favorable and proceeds to a significant extent, while the reverse reaction (NOCl + Cl → NO + Cl2) is less favored.
This implies that the formation of NOCl and Cl is thermodynamically favored, and the system will tend to reach an equilibrium state where the concentrations of NOCl and Cl are relatively higher compared to NO and Cl2.
The specific value of the equilibrium constant indicates the extent to which the reaction proceeds in the forward direction. In this case, the large value of 1.1x10^8 indicates that the forward reaction is highly favored and the equilibrium mixture will contain a significantly higher concentration of NOCl and Cl compared to NO and Cl2.
Learn more about equilibrium constant from the given link:
https://brainly.com/question/28559466
#SPJ11
16. Students in a chemistry course were asked the following question on a unit exam: "Draw a dia-
gram representing an element using circles as atoms."
a. The following diagrams represent two typical answers given by students. Which drawing is
the best representation of an element? Explain.
b. Imagine that the atom in Drawing B had been removed by physical separation from one
of the substances in Model 1. What substances could have been the source of the atom in
Drawing B?

Answers
If the atom in Drawing B had been removed by physical separation from one of the substances in Model 1, the substance that could have been the source of the atom in Drawing B is an element.
What is the source of the atom?An atom is the smallest part of a substance that can take part in a chemical reaction. We know that elements are composed of atoms. The atoms of an element are discretely arranged. Thus, in an element, there are so many atoms of the substance.
In the image that has been shown in the question, the source of the atom as it has been show is an element. Thus, if the atom in Drawing B had been removed by physical separation from one of the substances in Model 1, the substance that could have been the source of the atom in Drawing B is an element.
Learn more about element:https://brainly.com/question/13794764
#SPJ1
in the movie star wars episode v: the empire strikes back, the character han solo is frozen in the fictional material, carbonite. if carbonite existed, what would be its likely chemical formula?
Answers
If carbonite existed, its likely chemical formula will be CO₂²⁻.
The carbonite is liquid substance that is made from the carbon and the oxygen. the carbonite could change in the solid form through rapid freezing. carbonite is used to freeze the good for preservation is called as the carbon freezing. the carbonite is the blasting explosive. so, the carbon freezing is the process in which the liquid carbonite is frozen into the solid state.
Thus, in the movie star wars episode v: the empire strikes back, the character han solo is frozen in the fictional material, carbonite. if carbonite existed, its likely chemical formula will be CO₂²⁻.
To learn more about carbonite here
https://brainly.com/question/29596527
#SPJ4
An electron cannot have the quantum numbers n = ________, l = ________, ml = ________.
A) 2, 0, 0
B) 2, 1, -1
C) 3, 1, -1
D) 1, 1, 1
E) 3, 2, 1
Answers
An electron cannot have the quantum numbers n = 1, l = 1, and ml = 1. Therefore, option (D) is correct.
What are the quantum numbers?The set of numbers that can describe the position and energy of a particular electron are known as quantum numbers. Four quantum numbers are principal quantum numbers, azimuthal, magnetic, and spin quantum numbers.
Principal quantum numbers (n) can designate the principal electron shell and the most probable distance between electrons and the nucleus. The azimuthal quantum number (l) can designate the shape of an orbital and has a value equal to n -1.
The magnetic quantum number can designate the total number of orbitals in a particular subshell and the orientation of orbitals. It has value -l to l.
Therefore, n = 1 then l = 0, and ml = 0 as well.
Learn more about quantum numbers, here:
brainly.com/question/16977590
#SPJ1
What does a coefficient tell you?
Answers
The coefficient tells you how many molecules of that substance there is.
10 points for this problem that i need help with
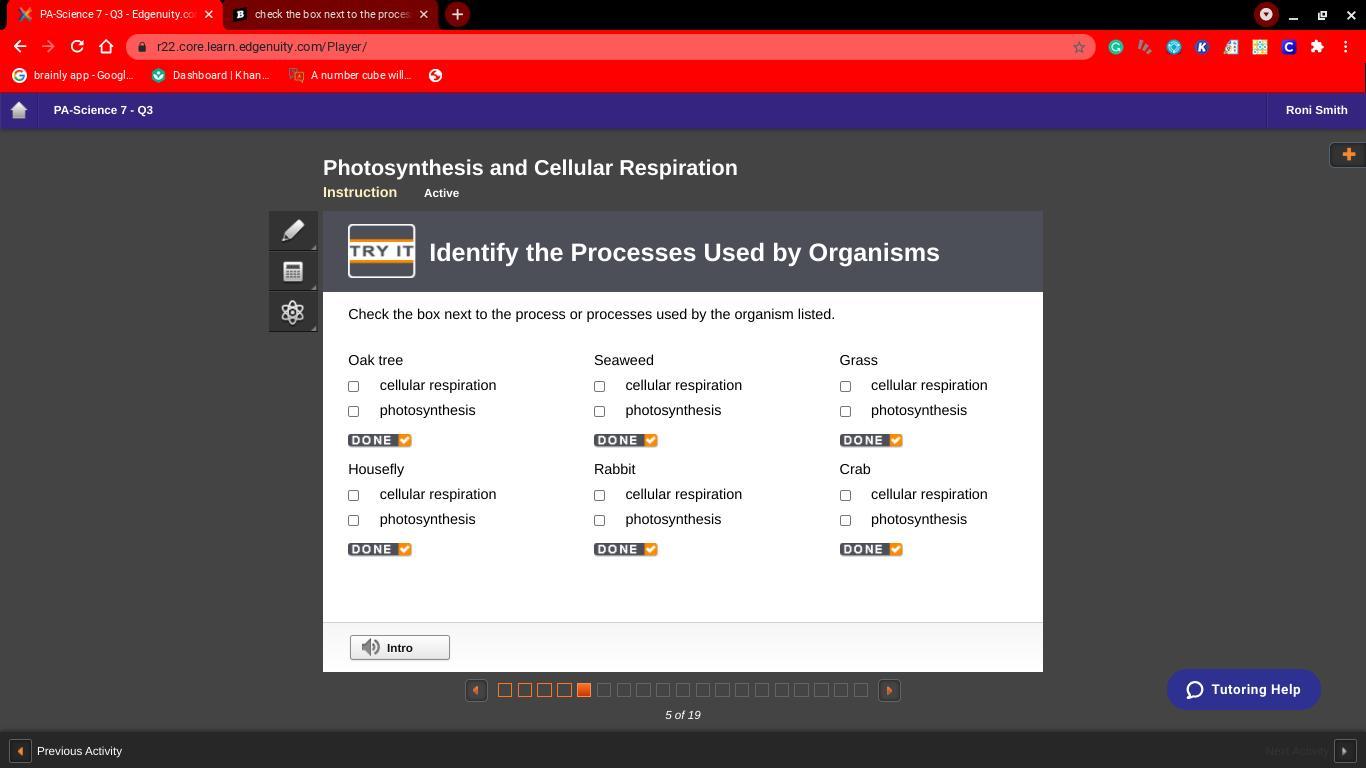
Answers
Answer:
house fly = cellular rabbit = cellular crab = cellular
Explanation:
2NH3 + MgSO4 + 2H20 — Mg(OH)2 + (NH4)2SO4
Using the chemical equation above answer the following questions.
c. If I have 4.6 moles of MgSO, and 5.4 moles of NH, which one is the limiting
reactant? You must show your work for credit.
d. What is the greatest amount of Mg(OH) that can be made with 4.6 moles of
MgSO, and 5.4 moles of NH3? You must show your work for credit
e. How many moles of the excess reactant is left over after the reaction has
been completed? You must show your work for credit
Answers
Based in the equation of the reaction reaction:
NH3 is the limiting reactant.the greatest amount of Mg(OH)2 produced is 2.7 moles.1.9 moles of the excess reactant are left over. Which one of the reactants is the limiting reactant?The equation of the reaction is given below:
2NH3 + MgSO4 + 2H20 — Mg(OH)2 + (NH4)2SO4From the equation of the reaction 2 moles of NH3 reacts with 1 mole of MgSO4
5.4 moles of NH3 will react with 5.4/2 moles of MgSO4 = 2.7 moles of MgSO4.
Therefore, MgSO4 is in excess and NH3 is the limiting reactant.
What is the greatest amount of Mg(OH) that can be made with 4.6 moles of MgSO, and 5.4 moles of NH3?Since NH3 is the limiting reactant:
2 moles of NH3 will produce 1 moles of Mg(OH)2
5.4 moles of NH3 will produce 5.4÷2 moles of Mg(OH)2 = 2.7 moles of Mg(OH)2
Therefore, the greatest amount of Mg(OH)2 produced is 2.7 moles.
How many moles of the excess reactant is left over after the reaction has been completed?MgSO4 is the excess reactant.
2.7 moles of MgSO4 are needed but there are 4.6 moles present.
Moles left over = 4.6 - 2.7 = 1.9 moles
Therefore, 1.9 moles of the excess reactant are left over.
Learn more about about excess reactant at: https://brainly.com/question/25685654
The alpha decay of a radioactive nuclide (X) emits a He-4 nucleus and produces an isotope of Superscript 235 subscript 92 upper U.. What is X?
Answers
Answer: Thus X is Plutonium
Explanation:
Alpha Decay: In this process, a heavier nuclei decays into lighter nuclei by releasing alpha particle. The mass number is reduced by 4 units and atomic number is reduced by 2 units.
General representation of alpha decay :
\(_{Z}^{A}\textrm{X}\rightarrow _{Z-2}^{A-4}\textrm{Y}+_2^4\textrm{He}\)
where Z = atomic number
A= mass number
X and Y = atomic symbol of elements
\(_{Z}^{A}\textrm{X}\rightarrow _{92}^{235}\textrm{U}+_2^4\textrm{He}\)
Thus \(_{94}^{239}\textrm{Pu}\rightarrow _{92}^{235}\textrm{U}+_2^4\textrm{He}\)
Thus X is Plutonium with atomic number 94 and mass number 239
Answer:
the answer is D on edge
Explanation:
What is the net amount of ATP produced in glycolysis?
Answers
By a series of enzyme processes, glucose is transformed into two molecules of pyruvate during the glycolysis process. To start the reaction, two molecules of ATP are used in the process, however
The metabolic process known as glycolysis turns glucose into pyruvate and produces ATP as a source of energy. The initial step of both aerobic and anaerobic respiration, it takes place in the cytoplasm of the majority of living cells. Glucose is first phosphorylated, splitting into two pyruvate molecules with three carbons each. Two ATP molecules are used up along the route, but four are also created, yielding a net gain of two ATP molecules. A vital mechanism in cellular metabolism, glycolysis supplies energy for a variety of cellular functions, including ion transport, protein synthesis, and muscular contraction. It also has a significant impact on conditions like diabetes and cancer, making this an essential topic for
Learn more about glycolysis here:
https://brainly.com/question/14076989
#SPJ4
compare and contrast the arrangements of particles at the atomic level for a liquid and a solid
Answers
The arrangements of particles at the atomic level for a liquid and a solid are compared as the particles in gas do not follow any specific order and are uniformly spaced out.
There is no regular arrangement of liquids next to one another. The solids are regularly arranged and closely packed. Since liquid particles are more dispersed than solid ones, they take on the shape of their container rather than having a distinct shape yet having a distinct volume. Because solid particles are more closely packed together and certain solids even have atomic-level particle patterns, they have a distinct shape that does not alter unless the solid changes state.
The arrangement of the particles in a liquid is more haphazard than that of the particles in a solid. But since they are closely packed, the atoms of solids and liquids are comparable.
Learn to know more about atomic level of liquid on
https://brainly.com/question/12816944
#SPJ1
pq7 a 10.0ml portion of 0.010m hcl is added to 100.0ml of water. what is the ph of he resulting solution?
Answers
The pH of the resulting solution after adding a 10.0 mL portion of 0.010 M HCl to 100.0 mL of water is approximately 2.00.This pH value indicates the high concentration of H+ ions resulting from the complete dissociation of the strong acid, HCl, in water.
To calculate the pH of the resulting solution, we need to consider the dissociation of HCl in water. HCl is a strong acid that completely dissociates into H+ and Cl- ions in aqueous solution.
Given that the initial concentration of HCl is 0.010 M and the volume added is 10.0 mL, we can calculate the moles of HCl added:
Moles of HCl = concentration x volume
= 0.010 M x 0.0100 L
= 0.0001 moles
The total volume of the resulting solution is 100.0 mL + 10.0 mL = 110.0 mL = 0.110 L.
Since HCl is a strong acid, it completely dissociates, and the moles of H+ ions formed will be equal to the moles of HCl added.
Therefore, the concentration of H+ ions in the resulting solution is:
Concentration of H+ ions = moles of H+ ions / total volume
= 0.0001 moles / 0.110 L
= 0.000909 M
To calculate the pH, we use the equation:
pH = -log[H+]
pH = -log(0.000909)
≈ 2.00
Therefore, the pH of the resulting solution is approximately 2.00.
After adding a 10.0 mL portion of 0.010 M HCl to 100.0 mL of water, the resulting solution has a pH of approximately 2.00. This pH value indicates the high concentration of H+ ions resulting from the complete dissociation of the strong acid, HCl, in water.
To know more about solution, visit:
brainly.com/question/15757469
#SPJ11
The photoelectron spectrum for the element nitrogen is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the ato
f the atom?
A. The leftmost peak represents the valence electrons.
B. The two peaks at the right represent a total of three electrons.
C. The electrons in the ls sublevel have the smallest binding energy
D. The electrons in the 2p sublevel have the smallest binding energy

Answers
Answer:
D. Is the correct option.
Explanation:
2p level contains the electrons furthest from the nucleus in the case of Nitrogen thus it's much easier to disperse/remove the electrons from the shell due to low pull of nucleus energy.
The best explanation of how the spectrum is consistent with the electron shell model of the atom is the electrons in the 2p sub level have the smallest binding energy. Thus, option D is correct.
What is photoelectron spectrum?
Photoemission spectroscopy also known as photoelectron spectroscopy which photoelectric effect is the process in which electron are get energy from external source of energy like sunlight deals with energy measurement of energy emission or electrons emitted from solids, gases or liquids by the process of photoelectric effect,
Photoelectric effect is the process in which electron are get energy from external source of energy like sunlight and get excited and comes in excited state from the ground state due to this process continuos flow of electron is take place and after that flow of energy is takes place.
Therefore,The best explanation of how the spectrum is consistent with the electron shell model of the atom is the electrons in the 2p sub level have the smallest binding energy. Thus, option D is correct.
Learn more about electrons here:
https://brainly.com/question/1255220
#SPJ2
Write the orbital diagram for the valence electrons of Ne. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. S d 2 G3 G3 G3 G3 G2 G1 | G2 Gi 11 Write the orbital diagram for the valence electrons of I. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. р 2 G3 G3 G3 63 3 G2G1 G2 G1 5 11 Write the orbital diagram for the valence electrons of Sr. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. S a 2 11 G3 G3 G3 دن 5 G2 G1 11 11 Vrite the orbital diagram for the valence electrons of Ge. rag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. р d 2 G3 G3 G3 G3 G2 G1 G2G1 4 11 1
Answers
electronic configuration of Ge is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2. Thus, the orbital diagram of Ge is: G2 G1 | G2 | G3 G3 G3 G3 4 11 1
Ne:
The electronic configuration of Ne is 1s2 2s2 2p6. Thus, the orbital diagram of Ne is:
G2 G1 | G2 | G3 G3 G3 G3
I:
The electronic configuration of I is 1s2 2s2 2p6 3s2 3p6. Thus, the orbital diagram of I is:
G2 G1 | G2 | G3 G3 G3 G3 5
Sr:
The electronic configuration of Sr is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. Thus, the orbital diagram of Sr is:
G2 G1 | G2 | G3 G3 G3 G3 11
Ge:
The electronic configuration of Ge is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2. Thus, the orbital diagram of Ge is:
G2 G1 | G2 | G3 G3 G3 G3 4 11 1
Br:
The electronic configuration of Br is 1s2 2s2 2p6 3
learn more about electronic configuration here
https://brainly.com/question/29757010
#SPJ4