Answers
The Lewis structure is a simplified way of representing how the elements in a molecule and their free valence electrons are bonded. Valence electrons are represented by dots around the element and by lines the bonds (electron pairs) between elements. The Lewis diagram for the compound CH2S will be:

Related Questions
Which statement BEST explains why the Moon is visible from the Earth?
1) The Moon reflects light from the Sun.
2) The Moon is always lit up by the Sun for us to see.
3) The Moon reflects light from the Earth.
4) The Moon produces its own light through radiation.
Answers
Answer: The answer is the ''The moon is always lit up by the sun for us to see.''
Explanation: The moon is directly illuminated by the sun.
Please help me
Phylum ___________ contains organisms that have a cutic|e; a f|exible protective covering.
Answers
Answer:
B Nematoda
Explanation:
While isobaric heat can be measured by using the coffee cup calorimeter, what kind of device would be needed to measure the reaction heat under isochoric condition? Please search literature to answer the question.
To measure the reaction heat more accurately at isobaric condition, what modification(s) would you suggest making on the coffee cup calorimeter? Please justify the suggested change(s).
Answers
To measure reaction heat under isochoric conditions, a bomb calorimeter is needed.
This device is designed to maintain a constant volume (isochoric) during the reaction, allowing for accurate measurement of reaction heat. To improve the accuracy of the coffee cup calorimeter for measuring reaction heat under isobaric conditions, a modification that could be made is to use a stirring device to ensure uniform mixing of the reactants and to minimize heat loss to the surroundings.
Additionally, a lid with a small hole could be placed over the top of the calorimeter to prevent heat loss while still allowing for pressure equalization. These modifications would help to minimize errors in heat measurement and improve the accuracy of the results obtained.
To know more about the Calorimeter, here
https://brainly.com/question/24150308
#SPJ1
a chemical reaction produced 2.50 moles of nitrogen gas. what volume in liters, does this gas sample occupy at STP? (show your work)
Answers
The volume of N₂ at STP=56 L
Further explanationGiven
2.5 moles of N₂
Required
The volume of the gas
Solution
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, the volume per mole of gas or the molar volume-Vm is 22.4 liters/mol.
So for 2.5 moles gas :
\(\tt 2.5\times 22.4=56~L\)
When each piece of ice is placed on a piece of oil it is observed that ice piece 2 melts faster than ice plece 1
Do these results match your hypothesis?
The photo is my hypothesis

Answers
We cannot see it its so small
Also yes it matches your hypothesis
k12 What is the smallest particle representing oxygen
A. diatomic oxygen
B. a proton
C. a compound
D. an atom
Answers
Answer:
d
Explanation:
Answer:
An atom
Explanation:
I took test plz mark brainliest
Based on what you have seen in this gizmo what is the best definition of the doppler effect?
Answers
Answer:
Based on what you have seen in the Doppler Effect Gizmo, what is the best definition of the Doppler effect? A change in the frequency of a wave caused by the motion of the source relative to the observer. The increase in volume of a sound as the source approaches an observer.
Explanation:
A cup of water is warmed from 29 °C to 97 °C. What is the difference between these two temperatures, in kelvins
Answers
Answer:
I believe it is 68
Explanation:
to convert Celsius to kelvins you must add it to 273.15 for example 29 + 273.15 is 302.15
im so very sorry if im wrong.
Predict the chemical formula for the ionic compound formed by the elements K and S
Answers
K is an metal from the first group on the periodic table, while S is a nonmetal from the 16th group.
The K atom can easily lose one electron, so its valence orbital stays with no electrons.
S is similar to O, it only needs two electrons to complete its valence orbital, so it can easily attract two electrons.
Thus, the atom K will form a cation K⁺ while the atom S will form an anion S²⁻.
When putting them together, they will form an ionic compound, and it has to have a neutral charge. Since K is 1+ and S is 2-, we will need 2 K for each S, so the compound formed will be:
\(K_2S\)The cyanide ion (CN-1) is highly toxic, but can be remediated in solution by redox reaction with the hypochlorite ion (the principal component of bleach). The balanced net ionic equation for this process is: 2 CN-1 + 5 ClO-1 + H2O ---> 5 Cl-1 + N2 + 2 HCO3 -1 A 3.5 x 106 L sample of water was found to be contaminated with cyanide ions at a concentration of 25mM. How many grams of cyanide were dissolved in the total sample?
Answers
Answer:
2.275x10⁶g of CN⁻¹ were dissolved in the sample
Explanation:
Molarity is defined as the ratio between moles of solute and liters of solution.
If a CN⁻¹ solution has a concentration of 25mM, there are 0.025 moles of CN⁻¹ per liter of solution.
If the sample has a colume of 3.5x10⁶L, moles of CN⁻¹ are:
3.5x10⁶L × (0.025moles / L) = 8.75x10⁴ moles of CN⁻¹ in the sample of water.
In grams (As molar mass of CN⁻¹ is 26g/mol):
8.75x10⁴ moles CN⁻¹ × (26g / mol) = 2.275x10⁶g of CN⁻¹ were dissolved in the sample
You have two containers at 0°C and 1 atm. One has 22.4 L of hydrogen gas, and the other has 22.4 L of oxygen gas.
Which statement is true?
-))
A)
Both containers contain 6.022x1023 molecules of gas.
B)
B)
The hydrogen gas container has more molecules than the oxygen gas
container
The oxygen gas container has more molecules than the hydrogen gas
container
D)
Both containers have the same number of molecules.
Answers
Answer:
D
Explanation:
According to Avogadro's law, equal volumes of different gases at the same temperature and pressure have equal number of molecules.
It cannot however be a because according to ideal gas equation the number of moles in each container is 0.999moles which translates to 6.019*10^23 molecules
Answer:
Both containers have the same number of molecules.
Explanation:
A scientist studies the effect of adding different amounts of salt on the boiling point of water. He places his results in the
graph below.
What are the independent and dependent variables in this experiment?
Mass is the independent variable, and boiling point is the dependent variable
Bowling point is the independent variable, and mass is the dependent variable
There are two independent variables and no dependent variables
There are two dependent variables and no independent variables
Answers
Answer:
Mass is the independent variable, and boiling point is the dependent variable.
the functional group present in an organic molecule is indicated by the prefix of the name, whereas the suffix of the name indicates the identity, location, and number of substituents attached to the carbon chain. the parent chain name is determined by the number of carbon atoms in the longest continuous carbon chain.
Answers
In organic chemistry, the functional group present in a molecule is identified by the prefix of its name, while the suffix indicates the number, location, and identity of any substituents attached to the parent carbon chain.
The parent chain name is determined by counting the number of carbon atoms in the longest unbranched carbon chain in the molecule. The naming convention helps to identify the type of reaction the molecule can undergo and its reactivity. This information is critical for predicting the chemical behavior of organic compounds.
Find out more about functional group
brainly.com/question/28188353
#SPJ4
When solving a problem it is important to identify your given and needed units, but it is also important to understand the relationship between those units so you will know how to set up your equation in order to solve the problem. Review the data sets below and use the steps of the problem-solving method to determine whether the given measurements would be appropriate for calculating mass, volume, or density.
a. 432 g of table salt occupies 20.0 cm^3 of space
b. 5.00 g 0T balsa wood, density of balsa wood : 0.16 g/cm^3
c. 32 cm^3 sample of gold density of gold 19.3 =g/cm^3
d. 150 g of iron, density of Iron = 79.0 g/cm^3
Answers
Answer:
See Explanation
Explanation:
Given
(a) to (d)
Required
Determine whether the given parameters can calculate the required parameter
To calculate either Density, Mass or Volume, we have
\(Density = \frac{Mass}{Volume}\)
\(Mass = Density * Volume\)
\(Volume = \frac{Mass}{Density}\)
(a) 432 g of table salt occupies 20.0 cm^3 of space
Here, we have:
\(Mass = 432g\)
\(Volume = 20.0cm^3\)
The above can be used to calculate Density as follows;
\(Density = \frac{Mass}{Volume}\)
\(Density = \frac{432g}{20.0cm^3}\)
\(Density = 21.6g/cm^3\)
(b) 5.00 g of balsa wood, density of balsa wood : 0.16 g/cm^3
Here, we have:
\(Mass = 5.00g\)
\(Density = 0.16g/cm^3\)
This can be used to solve for Volume as follows:
\(Volume = \frac{Mass}{Density}\)
\(Volume = \frac{5.00g}{0.16g/cm^3}\)
\(Volume = 31.25cm^3\)
(c) 32 cm^3 sample of gold density of 19.3 g/cm^3
Here, we have:
\(Volume = 32cm^3\)
\(Density = 19.3g/cm^3\)
This can be used to calculate Mass as follows:
\(Mass = Density * Volume\)
\(Mass = 32cm^3 * 19.3g/cm^3\)
\(Mass = 617.6g\)
(d) 150 g of iron, density of Iron = 79.0 g/cm^3
Here, we have
\(Mass = 150g\)
\(Density = 79.0g/cm^3\)
This can be used to calculate volume as follows:
\(Volume = \frac{Mass}{Density}\)
\(Volume = \frac{150g}{79.0g/cm^3}\)
\(Volume = 1.90 cm^3\) Approximated
The gas phase reaction of H2 with CO2 To produce H2O and CO has…
(Refer to the image, please)

Answers
The given reaction has ΔG value -12207KJ. Therefore, the given reaction is a spontaneous reaction as value of ΔG is negative.
A spontaneous process refers to anything that happens by itself, without external energy input. A ball is going to roll down an incline, water will flow downhill, ice will melt into water, radioactive elements will decay, and iron will rust, for instance. It is impossible for a reaction to not be spontaneous if it is exothermic (H negative) and increases the entropy for the system (S positive). The system's overall heat capacity is measured in enthalpy. The system's unpredictability is gauged by entropy.
ΔG=ΔH-T×ΔS
ΔG=11-298×41
= -12207KJ
Since ΔG is negative, reaction is spontaneous
To know more about spontaneous reaction, here:
https://brainly.com/question/31199175
#SPJ1
4-Nitrophenol, NO2C6H4OH (pKa 7.15), is only slightly soluble in water, but its sodium salt, NO2C6H4O-Na+, is quite soluble in water. Describe the solubility of 4-nitrophenol in solutions of sodium hydroxide, sodium bicarbonate (NaHCO3), and sodium carbonate (Na2CO3). The pKa values for the conjugate acids of sodium hydroxide, sodium bicarbonate (NaHCO3), and sodium carbonate (Na2CO3) are 15.7, 6.36, and 10.33, respectively. Aqueous NaOH: _________ Aqueous NaHCO3: _________ Aqueous Na2CO3: _________
Answers
Answer:
Aqueous NaOH: soluble
Aqueous NaHCO₃: insoluble
Aqueous Na₂CO₃: soluble
Explanation:
The organic acid is insoluble. Its salt (ionic) is soluble.
The important principle is:
If you have two acids in a flask, the stronger acid (smaller pKₐ) will protonate the weaker one. The stronger acid will become ionic and therefore more soluble.
1. In NaOH
Let's write the formula for 4-nitrobenzoic acid as HA.
The equation for the reaction is
HA + OH⁻ ⇌ A⁻ + H₂O
pKₐ: 7.15 15.7
HA is the stronger acid. It will protonate the hydroxide ion and be converted to the soluble 4-nitrobenzoate ion.
4-Nitrophenol is soluble in NaOH.
2. In NaHCO₃
HA + HCO₃⁻ ⇌ A⁻ + H₂CO₃
pKₐ: 7.15 6.36
HCO₃⁻ is the stronger acid. It will protonate 4-nitrophenol.
4-Nitrobenzoic acid is insoluble in NaHCO₃.
3. In Na₂CO₃
HA + CO₃²⁻ ⇌ A⁻ + H₂CO₃
pKₐ: 7.15 10.33
HA is the stronger acid. It will protonate the carbonate ion.
4-Nitrophenol is soluble in Na₂CO₃.
3. A Wilkinson’s catalyst is widely used in the hydrogenation of alkenes. Show a catalytic cycle, including: i. chemical structure of the catalyst, with complete stereochemistry ii. molecular geometry of catalyst iii. type of reactions involved iv. the appropriate starting material, reagent and solvent v. major and minor end-products vi. all intermediates, for each reaction stated in (iii)
Answers
We can see here that the catalytic cycle for the hydrogenation of alkenes using Wilkinson's catalyst involves several steps.
What are the steps involved?Here's an overview of the catalytic cycle, including the necessary details:
i. Chemical structure of the catalyst:
Wilkinson's catalyst, also known as chloridotris(triphenylphosphine)rhodium(I), has the following chemical structure: [RhCl(PPh3)3]
ii. Molecular geometry of the catalyst:
The Wilkinson's catalyst has a trigonal bipyramidal geometry around the rhodium center. The three triphenylphosphine (PPh3) ligands occupy equatorial positions, while the chloride (Cl) ligand occupies an axial position.
iii. Type of reactions involved:
The catalytic cycle involves several reactions, including:
Oxidative addition: The rhodium center undergoes oxidative addition, reacting with molecular hydrogen (H2) to form a dihydride intermediate.Alkene coordination: The alkene substrate coordinates to the rhodium center, forming a π-complex.Hydrogenation: The coordinated alkene undergoes hydrogenation, resulting in the addition of hydrogen atoms to the double bond and formation of a metal-alkyl intermediate.Reoxidation: The metal-alkyl intermediate reacts with a hydrogen molecule to regenerate the rhodium dihydride species.iv. Starting material, reagent, and solvent:
The starting material is an alkene, and the reagent is Wilkinson's catalyst ([RhCl(PPh3)3]). The reaction is typically carried out in a suitable solvent, such as dichloromethane (CH2Cl2) or tetrahydrofuran (THF).
v. Major and minor end-products:
The major end-product of the hydrogenation reaction is the fully saturated alkane, resulting from the addition of hydrogen across the double bond. The minor end-product may include cis- or trans-configured alkanes if the original alkene substrate possesses geometric isomers.
vi. Intermediates:
The intermediates in the catalytic cycle include:
Rhodium dihydride complex: [RhH2(PPh3)3]Alkene-Rhodium π-complex: [Rh(η2-alkene)(PPh3)3]Metal-alkyl intermediate: [Rh(alkyl)(PPh3)3]These intermediates play a crucial role in facilitating the hydrogenation reaction and enabling the catalytic cycle to proceed.
Learn more about Wilkinson’s catalyst on https://brainly.com/question/31972308
#SPJ1
How would you prepare ketone from a suitably substituted alkyl bromide containing four carbon atoms in the molecule?
Answers
A combination of -carbon alkylation and -dicarboxylic acid decarboxylation is required to create methyl ketone from an acetoacetic ester or an alkyl halide. The process involves the use of a secondary halide or a methyl halogen.
How is acetoacetate used by the body?
Acetoacetate and -hydroxybutyrate are metabolic byproducts of fatty acid oxidation that are used as fuel in extrahepatic tissues. The production and use rates have an impact on their blood level. Oxidation increases as their serum levels do as well.
Does the brain use acetoacetate?
Glucose serves as the main fuel source for supplying the brain's energy requirements. However, the brain uses ketones, such as acetoacetate or beta-hydroxybutyrate, as energy sources in some circumstances, such as diabetes, famine, during the nursing period, and the state of deep sleep.
To know more about acetoacetate visit:
https://brainly.com/question/28543544
#SPJ1
How many moles of water are required to produce 5.2 moles of iron(III) oxide? (10pts)3Fe(s) + 4H2O(l) ⇨ Fe3O4(s) + 4H2(g)
Answers
Answer
20.8 moles of H₂O
Explanation
Given:
The moles of iron(III) oxide produced = 5.2 mol
Equation: 3Fe(s) + 4H2O(l) ⇨ Fe3O4(s) + 4H2(g)
What to find:
The moles of water required to produce 5.2 moles of iron(III) oxide.
Solution:
Using the mole ratio of water to iron (iii) oxide in the given equation; which is
4 moles of H₂O produced 1 mole of Fe₃O4
So, x moles of H₂O will produce 5.2 moles of Fe₃O4
\(\begin{gathered} x=\frac{5.2mol\text{ }Fe_3O_4}{1mol\text{ }Fe_3O_4}\times4mol\text{ }H_2O \\ \\ x=20.8mol\text{ }H_2O \end{gathered}\)Hence, the moles of water required to produce 5.2 moles of iron(III) oxide is 20.8 moles
The reaction for photosynthesis producing glucose sugar and oxygen gas is:
__CO2(g) + __H2O(l) UV/chlorophyl−→−−−−−−−−−−−−−− __C6H12O6(s) + __O2(g)
What is the volume of oxygen gas at STP produced from 2.20 g of CO2 (44.01 g/mol)?
a. 1.12 L
b. .187 L
c. 4.32 L
d. 6.72 L
e. 1.60 L
Answers
Answer:
a. 1.12 L
Explanation:
Step 1: Write the balanced equation for the photosynthesis
6 CO₂(g) + 6 H₂O(l) ⇒ C₆H₁₂O₆(s) + 6 O₂(g)
Step 2: Calculate the moles corresponding to 2.20 g of CO₂
The molar mass of CO₂ is 44.01 g/mol.
2.20 g × 1 mol/44.01 g = 0.0500 mol
Step 3: Calculate the moles of O₂ produced
The molar ratio of CO₂ to O₂ is 6:6. The moles of O₂ produced are 6/6 × 0.0500 mol = 0.0500 mol
Step 4: Calculate the volume occupied by 0.0500 moles of O₂ at STP
At STP, 1 mole of O₂ occupies 22.4 L.
0.0500 mol × 22.4 L/1 mol = 1.12 L
* Who can help with this lol *
Wegener’s theory of continental drift and the theory of plate tectonics are not the same. Wegener could not identify an explanation for movement of continents. What information was he missing?
Your answer:
fossil and climate change
subduction and seafloor spreading
sea floor spreading and climate changes
subduction and fossils
Answers
Answer: subduction and sea floor spreading
Explanation:
He knew that the continents today were once joined together by fossil records of plants and animals that were found to be on continents far removed from each other. He knew this also by corresponding land forms that matches up as well. What he couldn’t prove is how the land masses would have moved so far away from each other. Subduction and sea floor spreading move the tectonic plates that the continents sit on. That’s what he was missing.
how many moles of each atom in the formula HClO3
Answers
Answer:
H has one mole
Cl has one mole
O has three mole
Explanation:
There is a single Hydrogen, thus there is one mole, this also applies to Cl.
With Oxygen, there are three O's, thus there are three moles of Oxygen.
There are 1 mole of H, 1 mole of Cl, and 3 moles of O.
Number of moles in molecules
The number of moles of an atom present in a molecule is classified as the number located in the lower right corner of that atom in the molecule, for example, in the water molecule below:
\(H_2O\)
you have 2 moles of hydrogen and 1 mole of oxygen.
Thus, in the HClO3 molecule, there are 1 mol of hydrogen, 1 mol of chlorine and 3 mol of oxygen.
Learn more about mole calculation in: brainly.com/question/2845237
Can someone help me over here? I need an negative charge and also another thing is that in a question it asked me to use something that would represent an ion. I’m not sure on what to choose either Hydrogen or Helium but just please send help.

Answers
Answer:
You could use a hydrogen anion which has 2 electrons.
Explanation:
An ion is an atom or molecule with a net electric charge because it has more or less electrons compared to the original atom or molecule.
An anion is a negatively charged ion.
A cation is a positively charged ion.
Normally a hydrogen atom has one proton and one electron.
So a hydrogen anion (H-) means that it has one proton and two electrons, which means the positive charge will be +1 and the negative charge will be -2.
Ultimately, giving the entire molecule a net charge of -1 because -2+1 = -1.
(I hope this helps! I am not 100% sure what's the format of this question. Like is it interactive? online? Did you draw all those electrons on the diagram? or were they given? Are the numbers given as well? or did you type those in? So I just tried my best to give you something that might work.)
What reasoning begins with a prediction based on a general principle
Answers
Answer:
Deductive reasoning (Brainliest please)
Explanation:Deductive reasoning begins with a general principle and a prediction based on this principle; the prediction is then tested, and a specific conclusion can then be drawn. The first step in the process of inductive reasoning is making specific observations.
For a reaction, AH = 206 kJ/mol and A SO = 0.215 kJ/(K•mol). At what
temperatures is this reaction spontaneous?
O A. At all temperatures
O B. At temperatures greater than 958 K
C. At no temperature
O D. At temperatures less than 44 K
SUBMIT
Answers
Determine the name or formula for each polyatomic ion.
formula: PO3−4
name:
name: sulfite ion formula:
name: sulfate ion formula:

Answers
Answer:
See explanation
Explanation:
PO4{3-} is phosphate
Sulfite's formula is SO3{2-}
Sulfate is SO4{2-}
OH- is hydroxide
Note: {x±} signifies the charge of the entire molecule
The polyatomic ions in question are phosphite ion, sulfite ion, and sulfate ion.
Explanation:The formula PO3−4 represents the polyatomic ion called phosphite ion. It is composed of one phosphorus atom bonded to three oxygen atoms. The name of the sulfite ion is SO3−2, and it consists of one sulfur atom bonded to three oxygen atoms. Lastly, the sulfate ion has the formula SO4−2, and it is composed of one sulfur atom bonded to four oxygen atoms.
Learn more about Polyatomic ions here:https://brainly.com/question/35456287
#SPJ6
Which statement is true about sound waves?
They move faster than light.
They make wind.
They vibrate the air.
They move slower in water than in air.
Answers
Answer:
the last one, cuz water has a resistance force which slows it down
Explanation:
Answer: C They vibrate the air
Explanation: Edge 22
A 1.250-g sample of benzoic acid, C7H6O2, was placed in a combustion bomb. The bomb was filled with an excess of oxygen at high pressure, sealed, and immersed in a pail of water which served as a calorimeter. The heat capacity of the entire apparatus (bomb, pail, thermometer, and water) was found to be 10.134 kJ/K. The oxidation of the benzoic acid was triggered by passing an electric spark through the sample. After complete combustion, the thermometer immersed in the water registered a temperature 3.256 K greater than before the combustion. What is DEcombustion per mole of benzoic acid burned
Answers
Answer:
3224 kJ/mol
Explanation:
The combustion of benzoic acid occurs as follows:
C₇H₆O₂ + 13/2O₂ → 7CO₂ + 3H₂O + dE
The change in temperature in the reaction is the change due the energy released, that is:
3.256K * (10.134kJ / K) = 33.00kJ are released when 1.250g reacts
To find the heat released per mole we have to find the moles of benzoic acid:
Moles benzoic acid -Molar mass: 122.12g/mol-:
1.250g * (1mol / 122.12g) = 0.0102 moles
The dE combustion per mole of benzoic acid is:
33.00kJ / 0.0102moles =
3224 kJ/mol
Pls help me asap I don’t know how to do this
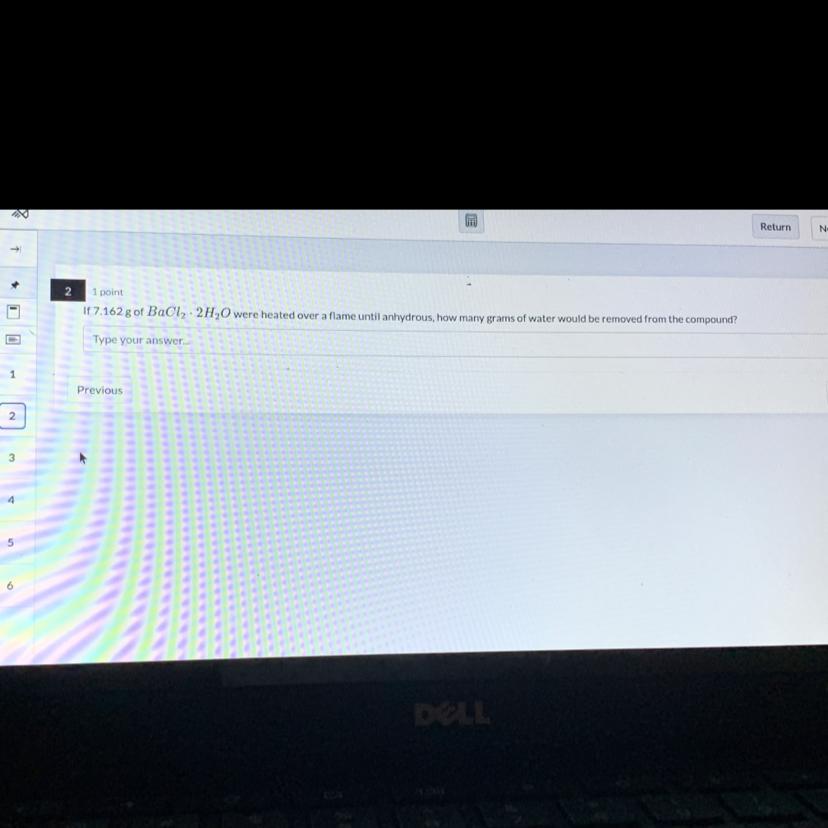
Answers
Answer:
\(1.057\text{ g}\)Explanation:
Here, we want to know the mass of water that would be lost
To get this, we have to divide the molar mass of water, by the molar mass of the hydrate and multiply by the given mass
The molar mass of water in the hydrate is 2(18 g/mol = 36g/mol). 1 mole of water has a mass of 36 g
The molar mass of the anhydrous part is 208 g/mol
Thus, we have the mass as:
\(\frac{36}{36+208}\times\text{ 7.162 =1.057 g}\)prop-1-yne + 2HBr/H2O2 = A;
A + 2H2O = B;
B + K2CO3(aq) = C;
C + heat = D;
D + HBr = E.
find the compounds A, B, C, D and E
Answers
Based on the given reactions, the compounds are as follows:
A: The specific product formed from the reaction between prop-1-yne and either 2HBr or H2O2.
B: The product formed when compound A reacts with 2H2O.
C: The product formed when compound B reacts with K2CO3(aq).
D: The product formed from the heat-induced reaction of compound C.
E: The product formed when compound D reacts with HBr.
Based on the given reactions, let's analyze the compounds involved:
Reaction 1: prop-1-yne + 2HBr/H2O2 = A
The reactant prop-1-yne reacts with either 2HBr or H2O2 to form compound A. The specific product formed will depend on the reaction conditions.
Reaction 2: A + 2H2O = B
Compound A reacts with 2H2O (water) to form compound B.
Reaction 3: B + K2CO3(aq) = C
Compound B reacts with K2CO3(aq) (potassium carbonate dissolved in water) to form compound C.
Reaction 4: C + heat = D
Compound C undergoes a heat-induced reaction to form compound D.
Reaction 5: D + HBr = E
Compound D reacts with HBr (hydrobromic acid) to form compound E.
For more such questions on compounds
https://brainly.com/question/704297
#SPJ8