Answers
Answer:
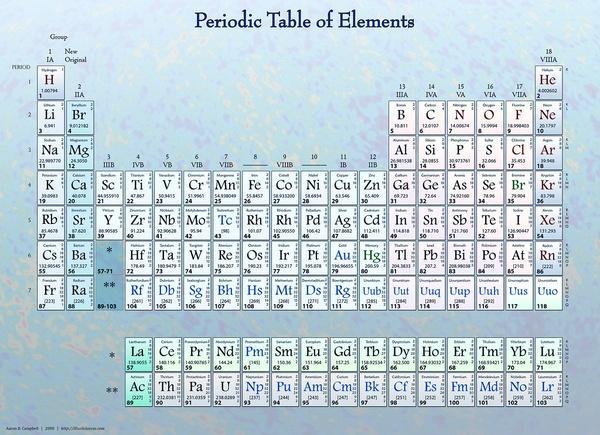
This is element 8 which is Oxygen.
Oxygen has two shell
Where L = 2
M = 8
I. E. 8 - 2 =>6
.: it has 6 valence electrons with 0 unpaired electrons.
Related Questions
Prioritise the type of hammer you would use to drive or shape material that could easily be damaged by hammers such as the following:
Answers
Some common types of hammers and their uses are
Claw hammer - used for driving nails and removing themSledgehammer - used for heavy-duty jobs like breaking concrete or driving stakesFraming hammer - used for framing houses and other construction workWhich hammers are used for delicate shpaes?When working with delicate shapes, the following types of hammers can be used
Rubber Mallet - It has a rubber head that is used to avoid damage to the surface of the material while striking.
Soft-Face Hammer - This hammer has replaceable soft tips made of materials like nylon, brass, or plastic that prevent damage to the surface.
To mention a few.
Learn more about Hammer:
https://brainly.com/question/31604480
#SPJ1
What is an example of a physical change?
A. having less ash than the paper that was burned to form it
B. ice forming and then melting back into water
C. rust having a different identity than the iron it forms on
D. the irreversible reaction of vinegar and baking soda
What one would you guys choose?
Answers
Answer:
B is correct answer
Explanation:
Answer:
B.
Explanation:
Indicate whether the following processes are spontaneous or nonspontaneous. (a) Liquid water freezing at a temperature below its freezing point (b) Liquid water freezing at a temperature above its freezing point (c) The combustion of gasoline (d) A ball thrown into the air (e) A raindrop falling to the ground (f) Iron rusting in a moist atmosphere
Answers
Answer:
The correct answer is -
(a) spontaneous;
(b) nonspontaneous;
(c) spontaneous;
(d) nonspontaneous;
(e) spontaneous;
(f) spontaneous
Explanation:
(a) Liquid water freezing at a temperature below its freezing point.
in this reaction, there is no requirement of external energy or force to make it possible due to the fact that the temperature of the environment is less than the freezing point of water. So, the water will spontaneously freeze.
(b) Liquid water freezing at a temperature above its freezing point. the melting of ice takes place with the increase or absorbing the heat which is a nonspontaneous,
(c) The combustion of gasoline produces gas molecules, and release energy which means it is an exothermic reaction, the combustion of gasoline is a spontaneous reaction.
(d) A ball is thrown into the air is not a natural process and it requires force or energy to be complete and works against gravity, Therefore, it is a nonspontaneous reaction.
(e) A raindrop falling to the ground is a naturally occurring reaction without any external force and in the direction of gravity, thus it is a spontaneous reaction.
(f) Iron rusting in a moist atmosphere is a spontaneous reaction as it does not require energy to occur and it naturally takes place. It is a redox reaction.
Iron + chlorine gas yields iron (III) chloride
Answers
Answer:
\(2Fe +3Cl_{2} -> 2FeCl_{3}\)
Explanation:
In order to have a balanced chemical reaction, you need coefficients of 2, 3 and 2 before each substance in this equation. Chlorine is diatomic so it occurs as a molecular pair when a gas.
name two physical properties that could be used to distinguish between the material in each pair 1,cork and lead 2,copper and silver 3,water and benzene 4,sulphur and iron?
Answers
The physical properties of each material can be used to tell each apart. One example of these properties is their respective densities and polarities, among others.
In the case of Cork and Lead, we can name differences such as:
Densities, since lead has a very high density when compared to the low density of corkColors, given that lead is a blueish silver color and cork is not. Melting points are another way to tell them apart since lead melts at 330 degrees while cork begins to melt at 200.As for copper and silver, though not identical in numbers, both elements share a wide range of properties. One physical property to be used when telling them apart is color, as silver has its own aptly named color whilst copper shines in a bright brown-red coloration.
As opposed to the previous group, benzene and water can often be physically identical. However, this does not mean that they share all physical properties. One example of this and a property that can tell them apart is their polarity, given that water is highly polar and benzene is not polar at all.
Of the groups listed, the grouping of sulfur and iron is the easiest to differentiate given that they possess distinct:
ColorMelting pointsBoiling pointsOdorsConductivitiesand so on.
These are some properties that are different between each grouped elements and can be used by scientists or students to differentiate the substances from one another.
To learn more visit:
https://brainly.com/question/18327661?referrer=searchResults
1. How many moles of carbon dioxide will be produced if 4 moles of ethane are burned in the presence of oxygen? Show work
below.
2 C₂H6+5 02 --> 4 CO2 + 6 H₂O
a. 4 moles
b. 6 moles
c. 8 moles
d. 12 moles
Answers
Answer:
It should be c. 8 moles
how much is the velocity of a body when it travals 600 m in 5 minutes
Answers
Answer:
7.2km/h.
Explanation:
Therefore, we get the speed of the man crossing a 600m long street in 5 minutes as 7.2km/h.
How many moles of iron are in (2.0x10^-36) atoms of iron (Fe)?
Answers
Answer:
Approx. 20 moles of iron
Explanation:
There are approx. 6.022 ×1023 iron atoms per mole of ion. This quantity, this number of iron atoms has a mass of 55.85 ⋅ g.
How is severe weather different from regular weather?
Answers
Answer:
Normal weather is like rn with no hurricanes or anything dangerous
Severe weather, is a weather that is that can cause a lot of harm.
With enough ____________,
_______________, and
__________, the original
sedimentary or igneous rock is
changed to ______________ rock.
Answers
Answer: with enough heat, pressure, and time, the original sedimentary or igneous rock is changed to metamorphic rock.
Explanation:
Hydrogen chloride gas and oxygen react to form water vapor and chlorine gas. What volume of chlorine would be produced by this reaction if of oxygen were consumed
Answers
Reaction of O₂ with HCl is as follow,
4 HCl + O₂ → 2 H₂O + 2 Cl₂
Let suppose we are given with 1 mole of HCl gas, Then,
According to eq,
89.6 L (4 moles) of HCl when reacted produced = 44.8 L (2 moles) Cl₂ gas
So,
22.4 L (1 mole) of HCl will produce = X L of Cl₂ gas
Solving for X,
X = (22.4 L × 44.8 L) ÷ 89.6 L
X = 11.2 L of Cl₂ Gas
lphins... Acid. (b) Chlorine reacts with red hot iron powder to give Iron(III) Chloride but not Iron (II) Chloride. Explain. (1Mark)
Answers
(a) Because acid is caustic, dolphins can perish from exposure to it. Acids are compounds that give other things protons (H+). Acid can react with the proteins and lipids in dolphins' skin when they come into touch with it, leading to chemical burns and damage to the underlying tissue. Systemic consequences from this include death.
(b) Because chlorine is a potent oxidizer, it interacts with red-hot iron powder to produce Iron(III) chloride (FeCl3) rather than Iron(II) chloride (FeCl2). FeCl3 is created when chlorine at high temperatures rapidly accepts electrons from iron atoms. Contrarily, iron interacts with HCl, a less potent oxidizer than chlorine, to produce FeCl2.
Learn more about chlorine at :
https://brainly.com/question/31560014
#SPJ1
Calcium nitrate reacts with ammonium fluoride to make calcium fluoride and ammonium nitrate. When (4.479x10^1) mL of (4.61x10^-1) M calcium nitrate was added to (7.332x10^1) mL of (1.5835x10^0) M ammonium fluoride, 0.731 grams of calcium fluoride were isolated. How many moles of ammonium fluoride were initially added in this experiment (not necessarily reacted)?
Answers
The moles of ammonium fluoride initially added in this experiment was 0.0216 moles.
What is mole?Mole is a unit of measurement that is used in chemistry to measure the amount of a substance. It is a very important unit of measurement because it allows chemists to accurately measure the amount of a substance that is being used in a reaction. The mole is defined as the amount of a substance that contains the same number of particles as there are atoms in 12 grams of carbon-12..
First, we need to calculate the moles of calcium nitrate in the solution. We can do this by using the molarity and volume of the solution:
(4.61x10⁻¹ M)*(4.479x10¹ mL) = 0.0216 moles of calcium nitrate
(0.731 g)*(1 mol/55.847 g) = 0.0131 moles of calcium fluoride
(0.0216 moles)*(1 mol/1 mol)
= 0.0216 moles of ammonium fluoride
Therefore, the moles of ammonium fluoride initially added in this experiment was 0.0216 moles.
To learn more about mole
https://brainly.com/question/29367909
#SPJ1
Aluminum sulfate reacts with barium chloride to form the insoluble compound, barium sulfate. The reaction proceeds according to the balanced equation below:
1Al2(SO4)3 + 3BaCl2 → 2AlCl3 + 3BaSO4
Marie reacts 150 g of aluminum sulfate with 200 g of barium chloride in order to produce insoluble barium sulfate for her crystallography studies. Determine the limiting and excess reactants for this reaction.
Molar mass aluminum sulfate: 342.15 g/mol
Molar mass barium chloride: 208.23 g/mol
Molar mass barium sulfate: 233.38 g/mol
Answers
Answer: \(BaCl_2\) is the limiting reagent and \(Al_2(SO_4)_3\) is the excess reagent.
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}\)
\(\text{Moles of aluminium sulphate}=\frac{150g}{342.15g/mol}=0.438moles\)
\(\text{Moles of barium chloride}=\frac{200g}{208.23g/mol}=0.960moles\)
The balanced chemical reaction is:
\(Al_2(SO_4)_3+3BaCl_2\rightarrow 2AlCl_3+3BaSO_4\)
According to stoichiometry :
3 moles of \(BaCl_2\) require = 1 mole of \(Al_2(SO_4)_3\)
Thus 0.960 moles of \(BaCl_2\) will require=\(\frac{1}{3}\times 0.960=0.320moles\) of \(Al_2(SO_4)_3\)
Thus \(BaCl_2\) is the limiting reagent as it limits the formation of product and \(Al_2(SO_4)_3\) is the excess reagent as it is left.
4 C3H6(g) + 6 NO(g) → 4 C3H3N(g) + 6 H2O(g) + N2(g) How many grams of C3H3N can be obtained from 651 kg of C3H6
Answers
Answer
820866.45 grams C₃H₃N can be obta
Explanation
Given balanced equation:
\(4C_3H_6\mleft(g\mright)+6NO\mleft(g\mright)\to4C_3H_3N\mleft(g\mright)+6H_2O\mleft(g\mright)+N_2\mleft(g\mright)\)Given reacting mass of C₃H₆ = 651 kg
What to find:
The mass of C₃H₃N obtained from 651 kg of C₃H₆
Step-by-step soltion:
From the Periodic Table:
Molar mass of C₃H₆ = 42.08 g/mol
Molar mass of C₃H₃N = 53.06 g/mol
You need to convert the given reacting mass of C₃H₆ from kilograms, (kg) to grams, (g).
Conversion factor:
1 kg = 1000 g
So 651 kg = 651 x 1000 = 651,000 grams
From the balanced equation above;
(4 mol x 42.08 g/mol) = 168.32 g C₃H₆ produced (4 mol x 53.06 g/mol) = 212.24 g C₃H₃N
Therefore 651,000 g C₃H₆ will produce
\(\frac{651000\text{ g }C_3H_6\times212.24\text{ }C_3H_3N}{168.32\text{ g }C_3H_6}=820866.45\text{ grams }C_3H_3N\)Melting point for strontium ( Sr )
Answers
Answer:
the melting point is 1431 degrees f or 777.2 c
Answer:
1,431° Fahrenheit or 777° Celsius
Determine the theoretical yield, limiting reactant when 0.50 g of Cr and 0.75 g of H3PO4 react according to the following chemical equation?
2Cr + 2 H3PO4 --> 2CrPO4 + 3H2
Answers
The theoretical yield of the reaction is 1.13 g. The acid is the limiting reactant.
What is the theoretical yield?In a given chemical reaction, the theoretical yield can only be obtained from the balanced reaction equation. We have been given the balanced reaction equation in the question so we can work from there.
Number of moles of Cr = 0.50 g /52 g/mol = 9.6 * 10^-3 moles
Number of moles of acid = 0.75 g/98 g/mol = 7.7 * 10^-3 moles
Given that the reaction is 1:1, the limiting reactant would be the acid.
The theoretical yield is obtained from;
Number of moles of product * molar mass of product
We substitute to obtain;
7.7 * 10^-3 moles * 147 g/mole (since the reaction is 1:1)
= 1.13 g
Learn more about theoretical yield:https://brainly.com/question/14966377
#SPJ1
An element has 2 stable isotopes. One has 13 amu and 1.07% abundant . The second has 12 amu and 98.93% abundant. What is the average atomic mass of the element
Answers
The average atomic mass of the element is 12.0107 amu.
To calculate the average atomic mass of the element in question, we can use the following formula:
average atomic mass = (mass of isotope 1 x abundance of isotope 1) + (mass of isotope 2 x abundance of isotope 2)
where "mass of isotope 1" is the mass of the first stable isotope (13 amu in this case), "abundance of isotope 1" is the percentage of that isotope in the element (1.07% in this case), "mass of isotope 2" is the mass of the second stable isotope (12 amu in this case), and "abundance of isotope 2" is the percentage of that isotope in the element (98.93% in this case).
Substituting the given values in the formula, we get:
average atomic mass = (13 amu x 1.07%) + (12 amu x 98.93%)
average atomic mass = (0.1391 amu) + (11.8716 amu)
average atomic mass = 12.0107 amu
Therefore, the average atomic mass of the element is 12.0107 amu.
This means that on average, one atom of this element weighs 12.0107 atomic mass units (amu), which is slightly heavier than the most abundant isotope (12 amu) due to the presence of the less abundant isotope (13 amu). This concept is important in chemistry because the mass of atoms plays a crucial role in determining their chemical and physical properties. The knowledge of the average atomic mass of an element is important in a wide range of applications, including analytical chemistry, geochemistry, and nuclear physics.
Know more about atomic mass here:
https://brainly.com/question/3187640
#SPJ11
Rank the following molecules in order of decreasing acid strength (1= most acidic, 3= least acidic)

Answers
Among the given carboxylic acids, the acidic strength varies in the order A>B>C. This is due to the inductive effect.
What is inductive effect?
An inductive effect is an effect in which a permanent dipole arises because of the unequal distribution of electrons in a molecule. This is of 2 types, positive I effect and negative I effect.
In carboxylic acids, due to inductive effect, the presence of any halogen groups on the adjacent carbon increases the acidity of the carboxylic acid. This is due to the stabilization of carboxylate conjugate base by halogen atoms. The higher the number of halogens, more is the acidic strength of the carboxylic acids.
Therefore, higher the number of fluorine atoms on the adjacent carbon of the carboxylic acid group, higher is the acidic strength because of the inductive effect. The order of the acidic strength varies in the order A>B>C.
To learn more about inductive effect click on the given link https://brainly.com/question/20596181
#SPJ1
60 points!! Look at picture please don’t troll
Answers
Platinum is a transition metal and forms two different ions, Pt2+ and Pt4+. Write the formulas for the compounds for each platinum ion with bromide ions.
Answers
The formulas for the compounds formed between platinum ions and bromide ions are Platinum (II) bromide (PtBr2) and Platinum (IV) bromide (PtBr4)
In both of these compounds, the bonding between the platinum ion and the bromide ions is primarily ionic in nature.
In platinum (II) bromide (PtBr2), each platinum ion is surrounded by two bromide ions, and each bromide ion is bonded to one platinum ion. The platinum ion has a +2 charge, and the two bromide ions have a -1 charge each, so the overall charge of the compound is neutral.
In platinum (IV) bromide (PtBr4), each platinum ion is surrounded by four bromide ions, and each bromide ion is bonded to one platinum ion. The platinum ion has a +4 charge, and the four bromide ions have a -1 charge each, so the overall charge of the compound is neutral.
The bond between the platinum ion and the bromide ions is a result of the attraction between their opposite charges.
Read more about Ionic Compounds:
https://brainly.com/question/20348031
#SPJ4
How many kilojoules of heat are needed to raise the temperature of 10g of aluminum from 22 degrees C to 55 degrees C, if the specific heat of aluminum is .901 j/gc?
Answers
Answer:
name four agricultural inputs are subsidized by the government
0.297 kJ of heat is needed to raise the temperature of 10g of aluminum from 22 degrees Celsius to 55 degrees Celsius.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Given,
mass = 10g
c = 0.901J/g⁰C
Initial temperature (T₁) = 22⁰C
Final Temperature (T₂) = 55⁰C
Q = mcΔT
= 10 × 0.901 × (55 -22)
= 297.33 J = 0.297 kJ
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
]All organic compounds contain the element carbon but, not all compounds containing the element “carbon”are organic .Justify this statement.
Answers
The statement "All organic compounds contain the element carbon, but not all compounds containing the element 'carbon' are organic" can be justified based on the definition and characteristics of organic compounds.
Organic compounds are compounds primarily composed of carbon and hydrogen atoms, often with other elements like oxygen, nitrogen, sulfur, and phosphorus. These compounds are typically associated with living organisms and are known for their unique properties and behavior, including the ability to form complex structures, exhibit covalent bonding, and undergo organic reactions.
On the other hand, there are compounds that contain carbon but are not classified as organic. One notable example is carbon dioxide (\(CO_{2}\)), which is a simple inorganic compound composed of carbon and oxygen. Carbon dioxide does not possess the characteristic properties of organic compounds, such as the ability to form long chains or undergo organic reactions.
Additionally, there are inorganic compounds like carbonates (such as calcium carbonate) and carbides (such as calcium carbide) that contain carbon but are not considered organic. These compounds have distinct chemical and physical properties different from those of organic compounds.
In summary, while all organic compounds contain carbon, not all compounds containing carbon are organic. The classification of a compound as organic or inorganic depends on its overall molecular structure, bonding, and characteristic properties.
Know more about molecular structure here:
https://brainly.com/question/27789666
#SPJ8
Which statement describes how phase changes
can be diagrammed as a substance is heated?
A. The phase is on the y-axis and thels
temperature is on the x-axis.
B. The temperature is on the y-axis and the
phase is on the x-axis.
C. The time is on the y-axis and the temperature
is on the x-axis.
D. The temperature is on the y-axis and the time
is on the x-axis.
Answers
Answer: d
Explanation:
On edg
6.25 x 10^22 Molecules of BR2 = ?moles
Answers
0.104 mol Br₂
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Avogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Stoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
6.25 × 10²² molecules Br₂
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
Set up: \(\displaystyle 6.25 \cdot 10^{22} \ molecules \ Br_2(\frac{1 \ mol \ Br_2}{6.022 \cdot 10^{23} \ molecules \ Br_2})\)Multiply/Divide: \(\displaystyle 0.103786 \ mol \ Br_2\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
0.103786 mol Br₂ ≈ 0.104 mol Br₂
What temperature are the objects in space that x-rays come from?
A. thousands of degrees Celsius
B. millions of degrees Celsius
C. billions of degrees Celsius
D. hundreds of degrees Celsius
Answers
Answer:
B. millions of degrees Celsius
Balance the following equations:
I
Na +
Br2 →
NaBr
C4H6 +
02 →
CO2 +
H2O
3)
LiOH +
H3PO4 →
Li3PO4 +
H2O
4)
CaSO4 +
KNO3 →
Ca(NO3)2 +
K2SO4
5)
H2O2 →
H2O +
02
Answers
ANSWERS :-
1)
\(\red{2}Na + Br2 → \red{2}NaBr\)
2)
\(\blue{2}C4H6 + \blue{11}O2 → \blue{8}CO2 + \blue{6}H2O\)
3)
\(H3PO4 + \green{3}LiOH → \green{3}H2O + Li3PO4\)
4)
\(Ca(NO3)2 + K2SO4 → CaSO4 + \pink{2}KNO3\)
5)
\(\orange{2}H2O2→\orange{2}H2O+O2\)
(mark me brainliest please I need 3 more :))
\(\large\sf\red{thank \: you}\)
If 38.6 grams of iron react with an excess of bromine gas, what mass of FeBr2 can form?
Answers
Answer:
›› FeBr2 molecular weight. Molar mass of FeBr2 = 215.653 g/mol. This compound is also known as Iron(II) Bromide. Convert grams FeBr2 to moles or moles FeBr2 to grams. Molecular weight calculation: 55.845 + 79.904*2 ›› Percent composition by element
Explanation:
If 38.6 grams of iron react with an excess of bromine gas, the mass of FeBr2 can form is 149 grams.
What is mass?Mass is defined as a way to gauge how much matter there is in a substance or thing. The kilogram (kg) is the fundamental SI unit of mass, while lower masses can also be measured in grams (g). Atoms make up everyday matter. A majority of an atom's mass is contained in its nucleus.
Given Fe = 38.6 g.
Fe has a molar mass = 55.845 g/mol.
Given mass/molar mass equals 38.6g/55.845gmol-1, or 0.6912 moles of iron.
The reaction is described as Fe + Br2 FeBr2.
One mole Fe yields 1 mole of FeBr2.
FeBr2 would be produced from 0.6912 moles of Fe.
FeBr2 has a molar mass of 215.65 g/mol.
Moles of FeBr2 x Molar mass of FeBr2
= 215.65 g/mole x 0.6912 mole
= 149.06 g FeBr2 produced is the formula.
Thus, if 38.6 grams of iron react with an excess of bromine gas, the mass of FeBr2 can form is 149 grams.
To learn more about mass, refer to the link below:
https://brainly.com/question/19694949
#SPJ2
When the equation
_Pb2+ + Au²+ →__pb4+ + _Au
is correctly balanced using the smallest whole-number coefficients, the coefficient of Pb2+ will be
1.1
2.2
3.3
4.4
Submit Answer
Hide Toolbar
Answers
The answer is option 2 , the smallest whole-number coefficients, the coefficient of Pb²⁺ will be 2.
What is a Balanced Chemical Equation ?
Law of conservation of mass states that mass can neither be created nor be destroyed but it can only be transformed from one form to another form.
This also means that total mass on the reactant side must be equal to the total mass on the product side.
There are two types of numbers that appear in chemical equations.
There are subscripts, which are part of the chemical formulas of the reactants and products;
and there are coefficients that are placed in front of the formulas to indicate how many molecules of that substance is used or produced.
Balance The Equation:
Pb²⁺ + Au²⁺ = Pb⁴⁺ + Au
Label Each Compound With a Variable
Label each compound with a variable to represent the unknown coefficients.
aPb²⁺ + bAu²⁺ = c Pb⁴⁺ + d Au
Create a System of Equations
Create an equation for each element where each term represents the number of atoms of the element in each reactant or product.
Pb: 2a + 0b = 4c + 0d
Au: 0a + 2b = 0c + 1d
e: 0a + -1b = -1c + 0d
2a - 4c = 0
2b - 1d = 0
-1b + 1c = 0
Solve For All Variables
Use substitution,
Substitute Coefficients and Verify Result
2Pb²⁺ + Au²⁺ = Pb⁴⁺ + 2 Au
So the answer is 2 , option 2
To know more about Balanced Chemical Equation
https://brainly.com/question/15052184
#SPJ1
If a gas is cooled from 343.0 K to 283.15 K and the volume is kept constant what final pressure would result if the original pressure was 760.0 mm HG?
Answers
Answer:
627.4 mmHg
Explanation:
From Gay-Lussac law:
\( \frac{p1}{t1} = \frac{p2}{t2} \)
p1 = 760 mmHg
t1 = 343 K
t2=283.15 K
\(p2 = p1 \times \frac{t2}{t1} \)
\(p2 = 760 \times \frac{283.15}{343.0} \)
\(p2 = 627.4 \: mmhg\)
If a gas is cooled from 343.0 K to 283.14 K and the volume is kept constant the final pressure would be 627.4 mm Hg if the original pressure was 760.0 mm Hg.
Gay-Lussac's LawGay-Lussac's law states that the pressure of a given mass of gas at a constant volume varies directly with the temperature of the gas. Gay-Lussac's law defined the relationship between pressure and temperature of a gas when kept at a constant volume.
According to the Gay-Lussac's LawP ∝ T (When V = constant)
or, P = kT
or, \(\frac{P_1}{T_1} = \frac{P_2}{T_2}\)
Here,
\(P_{1}\) = 760.0 mm Hg,
\(T_{1}\)= 343.0 K
\(T_{2}\) = 283.15 K
Now put the values in above formula, we get
\(\frac{P_1}{T_1} = \frac{P_2}{T_2}\)
\(\frac{760.0\ \text{mm Hg}}{343.0K} = \frac{P_2}{283.15\ K}\)
\(P_{2} = \frac{760.0\ \text{mm Hg} \times 283.15\ K}{343.0\ K}\)
\(P_{2} = \frac{215194\ \text{mm Hg}}{343}\)
\(P_{2}\) = 627.4 mm Hg
Thus, we can say that if a gas is cooled from 343.0 K to 283.14 K and the volume is kept constant the final pressure would be 627.4 mm Hg if the original pressure was 760.0 mm Hg.
Learn more about Gay-Lussac's Law here: https://brainly.com/question/24691513
#SPJ2