How much iron can be recovered from 25. 0g of fe2o3.
Answers
15 grams of iron can be recovered from 25.0g of Fe2O3.
To solve this question, the first step is to determine the molar mass of Fe2O3
Fe: 2 x 55.85 g/mol = 111.7 g/molO: 3 x 16.00 g/mol = 48.00 g/mol
Total molar mass of Fe2O3 = 159.7 g/mol
The next step is to find the moles of Fe2O3 that are present in 25.0g
moles = mass / molar mass
moles = 25.0 g / 159.7 g/mol
moles = 0.1564 mol
Finally, using the balanced chemical equation for the reaction of Fe2O3 with carbon monoxide (CO), we can determine the theoretical yield of iron
Fe2O3 + 3CO → 2Fe + 3CO21 mol
Fe2O3 produces 2 mol FeSo,
0.1564 mol Fe2O3 produces (2/1 x 0.1564 mol) = 0.3128 mol Fe
The molar mass of Fe is 55.85 g/mol, so the mass of iron that can be recovered is
mass = moles x molar mass
mass = 0.3128 mol x 55.85 g/mol
mass = 17.46 g
The actual yield may be less than the theoretical yield due to various factors, such as the efficiency of the reaction. Assuming a 85% yield, the amount of iron that can be recovered is
recovered mass = theoretical yield x % yield
recovered mass = 17.46 g x 85%
recovered mass = 14.8 g
Therefore, the main answer to this question is that 15 grams of iron can be recovered from 25.0g of Fe2O3.
To know more about molar mass, click here
https://brainly.com/question/31545539
#SPJ11
Related Questions
How many moles are in 25.5g of helium?
Answers
which of the following phrases describes how the position of an electron relates to its energy?
A. the farther an electron is from the nucleus, the lower its energy.
B. the farther an electron is from the nucleus, the greater its energy.
C. the electrons within the core of the atom possess the highest energy.
D. the closer an electron is to the nucleus, the higher the energy level it is in.
correct answer is B.
Answers
An electron with a negative energy is attached to the nucleus by the attraction between its opposing charges. The closer it is to the nucleus, the more closely connected it is. The farther an electron is from the nucleus, the greater its energy. The correct option is B.
The electron's energy grows as it goes away from the nucleus and gets less and less negative. When the electron is not subject to the nucleus's attracting Coulomb attraction, it has zero potential energy and only kinetic energy, which is always positive. The electron has greater energy the further it travels.
On the other hand, the nucleus's weak forces of attraction hold electrons that are moving away from it.
Thus the correct option is B.
To know more about energy of electrons, visit;
https://brainly.com/question/13445068
#SPJ1
which empirical gas law describes the relationship between the volume and temperature of a gas when the number of moles and pressure are constant? please choose the correct answer from the following choices, and then select the submit answer button. answer choices
Answers
Charles's Law helps us understand the relationship between temperature and volume of a gas when other factors, such as pressure and number of moles, remain constant. The empirical gas law that describes the relationship between the volume and temperature of a gas when the number of moles and pressure are constant is Charles's Law.
This can be expressed mathematically as: V₁/T₁ = V₂/T₂
Where V₁ and V₂ represent the initial and final volumes of the gas, and T₁ and T₂ represent the initial and final temperatures of the gas, respectively. The relationship can also be stated as: V/T = constant
To illustrate this law, let's consider an example. Imagine a balloon filled with a fixed number of moles of gas at a constant pressure. If we were to heat the balloon by placing it in a warm environment, the temperature of the gas inside the balloon would increase.
To know more about empirical gas law visit:
brainly.com/question/31191366
#SPJ11
Given the balanced chemical equation below, how many grams of oxygen are needed to produce 221.3 grams H2O? Report your answer to the hundredths place.C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Answers
Answer: 392.71 g of O2 would be necessary to produce 221.3g of H2O
Explanation:
The question requires us to calculate the mass of oxygen gas (O2) necessary to produce 221.3g of H2O, given the following balanced equation:
\(C_6H_{12}O_6+6O_2\rightarrow6CO_2+6H_2O\)The problem presented can be classified as a mass to mass stoichiometry problem. To solve it, we'll need to follow the steps:
mass of H2O → moles of H2O → moles of O2 → mass of O2
First, let's determine the number of moles of H2O contained in 221.3g of it, knowing that the molar mass of H2O is 18.02g/mol:
\(n=\frac{m}{MM}\rightarrow n_{H_2O}=\frac{221.3g}{18.02g/mol}=12.28mol\)(where n is the number of moles, m is the mass of the sample and MM corresponds to the molar mass of the compound)
Therefore, 12.28 moles of H2O need to be produced in the reaction.
Next, let's determine the number of moles of O2 necessary to produce 12.28 moles of H2O. According to the balanced chemical equation, 6 moles of O2 are necessary to produce 6 moles of H2O:
6 mol H2O ------------------- 6 mol O2
12.28 mol H2O ------------- x
Solving for x, we have that 12.28 moles of O2 would be necessary to produce 12.28 moles of H2O.
At last, we need to convert the calculated number of moles of O2 to its correspondent mass, in grams. Knowing that the molar mass of O2 is 31.98g/mol, we can calculate the mass of O2 as:
\(\begin{gathered} n=\frac{m}{MM}\rightarrow m=n\times MM \\ \\ m_{O_2}=12.28mol\times31.98g/mol=392.71g \end{gathered}\)Therefore, 392.71 g of O2 would be necessary to produce 221.3g of H2O.
Natasha adds sugar to the water, a little at a time. She stops adding sugar when no more will
dissolve in the water.
Suggest two things she can do to make the test
Answers
Natasha is adding sugar to the water, a little at a time, and also she stopped adding sugar when no more sugar can be dissolved in the water. The two things she can do to involve the test are the same amount of water and the same water temperature.
When sugar is added to water, after a time it will stop dissolving since it reaches saturation point, so the amount, as well as temperature of the water, can be used to make the test. Sugar serves as a solute, which means that when it is added to water, the amount of vapor produced will decrease. Therefore, in order to achieve the boiling point after adding sugar (solute) to water, additional heat will be needed. As a result, the water's boiling point rises.
The amount of water stays the same because the sugar particles get trapped in the gaps between the water molecules. As the temperature of the water increases, it takes less time for the sugar to dissolve. The sugar can dissolve more readily into the gaps between the water molecules when the temperature is higher, hastening the formation of the solution.
Learn to know more about sugar dissolving on
https://brainly.com/question/26239531
#SPJ9
What are the main organs of the muscular system?
Answers
The muscular system is an organ system consisting of skeletal, smooth and cardiac muscles. It permits movement of the body, maintains posture and circulates blood throughout the body.
Answer:
Skeletal, cardiac muscles.
Explanation:
It helps move the body around. Your welcome :)
33. A student finds that the mass of 25ml of an unknown liquid is 20.5g. What is the density of this substance?
Answers
Answer:
Density, \(d=0.82\ g/cm^3\)
Explanation:
It is given that,
Mass of an unknown liquid, m = 20.5 g
The volume of unknown liquid is 25 mL
We need to find the volume of the brass object. Mass per unit volume of an object is called its density.
We know that, 1 mL = 1 cm³
So, 25 mL = 25 cm³
\(d=\dfrac{m}{V}\)
Putting the values, we get :
\(d=\dfrac{20.5\ g}{25\ cm^3}\\\\d=0.82\ g/cm^3\)
So, the density of this substance is \(0.82\ g/cm^3\)
why is the temperature at which density is measured usually specified
Answers
Density is a crucial physical quantity for a variety of materials, including liquids, gases, and solids. The temperature at which density is measured is usually specified because density is temperature-dependent and changes with temperature.
This implies that a small change in temperature will have a substantial effect on density.Let's have a look at the connection between temperature and density:T and ρ are the symbols used to represent temperature and density, respectively. It is common knowledge that substances expand when heated and contract when cooled. In the same vein, as temperature increases, the space between the molecules increases, resulting in a decrease in density. Conversely, if the temperature decreases, the molecules move closer together, and the density increases. When we consider the relationship between temperature and density, we can infer that density is directly proportional to temperature. As a result, when the temperature changes, so does the density, and this fluctuation must be specified. Therefore, specifying the temperature at which density is measured ensures accuracy and consistency in measurements. It also allows us to make appropriate comparisons between density values obtained from various sources. In conclusion, specifying temperature when measuring density ensures consistency and accuracy in results.
To know more about Density visit :
brainly.com/question/29775886
#SPJ11
Which action gives the best method for neutralizing spilled acid?
a. add sodium bicarbonate to the spill
b. neutralize the spill with a strong base
c. pour water over the spill
d. mop up the spill with paper towels
Answers
The best method for neutralizing a spilled acid depends on the type of acid and the severity of the spill. However, in general, the recommended method is to add a neutralizing agent, such as sodium bicarbonate, to the spill. This will help to neutralize the acid and prevent it from spreading or causing damage to the surrounding area.
Using a strong base to neutralize the spill can also be effective but requires more caution as it can be dangerous if not handled properly. Pouring water over the spill can be helpful to dilute the acid and prevent it from spreading, but it may not fully neutralize the acid. Mopping up the spill with paper towels is not recommended as it can spread the acid and increase the risk of injury. It is important to wear protective gear, such as gloves and goggles, when handling spilled acid and to follow proper procedures for clean-up and disposal. Neutralizing spilled acid is a critical process that requires a careful approach to prevent accidents and injuries. In case of acid spills, it is essential to act quickly to prevent the acid from causing further damage. Neutralizing the spill with a suitable neutralizing agent such as sodium bicarbonate is the best method as it ensures that the acid is completely neutralized and does not cause further harm. Pouring water over the spill can be helpful, but it does not fully neutralize the acid and may not prevent it from spreading. It is important to handle spilled acid with caution and to wear protective gear to minimize the risk of injury. Proper procedures for clean-up and disposal should be followed to ensure that the acid is properly contained and disposed of.
To know more about Water visit:
https://brainly.com/question/31641293
#SPJ11
It takes 547 kJ to remove one mole of electrons from the atoms at the surface of a solid metal.
What is the maximum wavelength of light capable of doing this?
Answers
According to the relation of variables in the electromagnetic spectrum the maximum wavelength of light is 36.3 ×10\(^-\)³¹ m.
What is electromagnetic spectrum ?The electromagnetic spectrum consists of electromagnetic radiation consists of waves made up of electromagnetic field which are capable of propogating through space and carry the radiant electromagnetic energy.
The radiation are composed of electromagnetic waves which are synchronized oscillations of electric and magnetic fields . They are created due to change which is periodic in electric as well as magnetic fields.
In the given problem,energy is related to wavelength by the formula, λ=hc/E,λ=6.626×10\(^-34\)×3×10⁸/547×1000=36.3×10\(^-31\) m.
Thus, the maximum wavelength of light is 36.3×10\(^-31\) m.
Learn more about electromagnetic spectrum,here:
https://brainly.com/question/23727978
#SPJ1
which chemical waste situation should always be supervised or performed by an instructor? note that you still may need to alert your instructor about the described incident, even if you clean it up yourself.
Answers
It is always best practice to have a qualified instructor supervise any chemical waste situation, even if you are capable of cleaning it up yourself.
This is because the instructor will be able to ensure the safety of all individuals involved and ensure that the waste is disposed of properly.
Additionally, you should always alert the instructor about any described incident, even if you are able to clean it up yourself, as this will ensure that appropriate action is taken.
When disposing of chemical waste, it is important to ensure that the waste is disposed of according to local regulations. Depending on the type of waste, there may be specific requirements for how to dispose of it safely and legally. Additionally, it is important to ensure that all containers used for storing or transporting the chemical waste are labeled properly and securely closed.
Learn more about chemical waste:
https://brainly.com/question/30322250
#SPJ4
What is another name for the sugars organisms use for energy?1. Proteins2. Nucleic acids3. Carbohydrates4. Lipids
Answers
Answer:
carbohydrates
Explanation:
i took the test
Calculate the [H3O+] of a solution that is 0. 20 M in HF and 0. 10 M in NaF. Ka = 7. 2 × 10-4 for HF
Answers
Answer: 1.44 × 10^-3 M.
Explanation: To calculate the [H3O+] of the solution, we need to consider the dissociation of HF (hydrofluoric acid) and the subsequent formation of H3O+ ions. Since HF is a weak acid, it partially dissociates in water.
The dissociation reaction of HF can be represented as follows:
HF + H2O ⇌ H3O+ + F-
The equilibrium constant for this reaction, Ka, is given as 7.2 × 10^-4.
Given:
[Molar concentration of HF] = 0.20 M
[Molar concentration of NaF] = 0.10 M
Assuming that the dissociation of HF is limited by the available F- ions from NaF, we can use the concept of the common ion effect to calculate the [H3O+] in the solution.
Since NaF is a salt that dissociates completely into Na+ and F- ions, the [F-] in the solution is equal to the concentration of NaF, which is 0.10 M.
Let's denote the concentration of [H3O+] as x M.
Using the equilibrium expression for the dissociation of HF:
Ka = [H3O+][F-] / [HF]
Substituting the known values:
7.2 × 10^-4 = x * 0.10 / 0.20
Simplifying the equation:
x = (7.2 × 10^-4) * (0.20 / 0.10)
x = 1.44 × 10^-3
Therefore, the [H3O+] of the solution is approximately 1.44 × 10^-3 M.
someone help me with this rly quick
105006hm/ds to. m/s
convert and show the works
Answers
Answer:
10500600m/s
1ds=100m/s
Please help
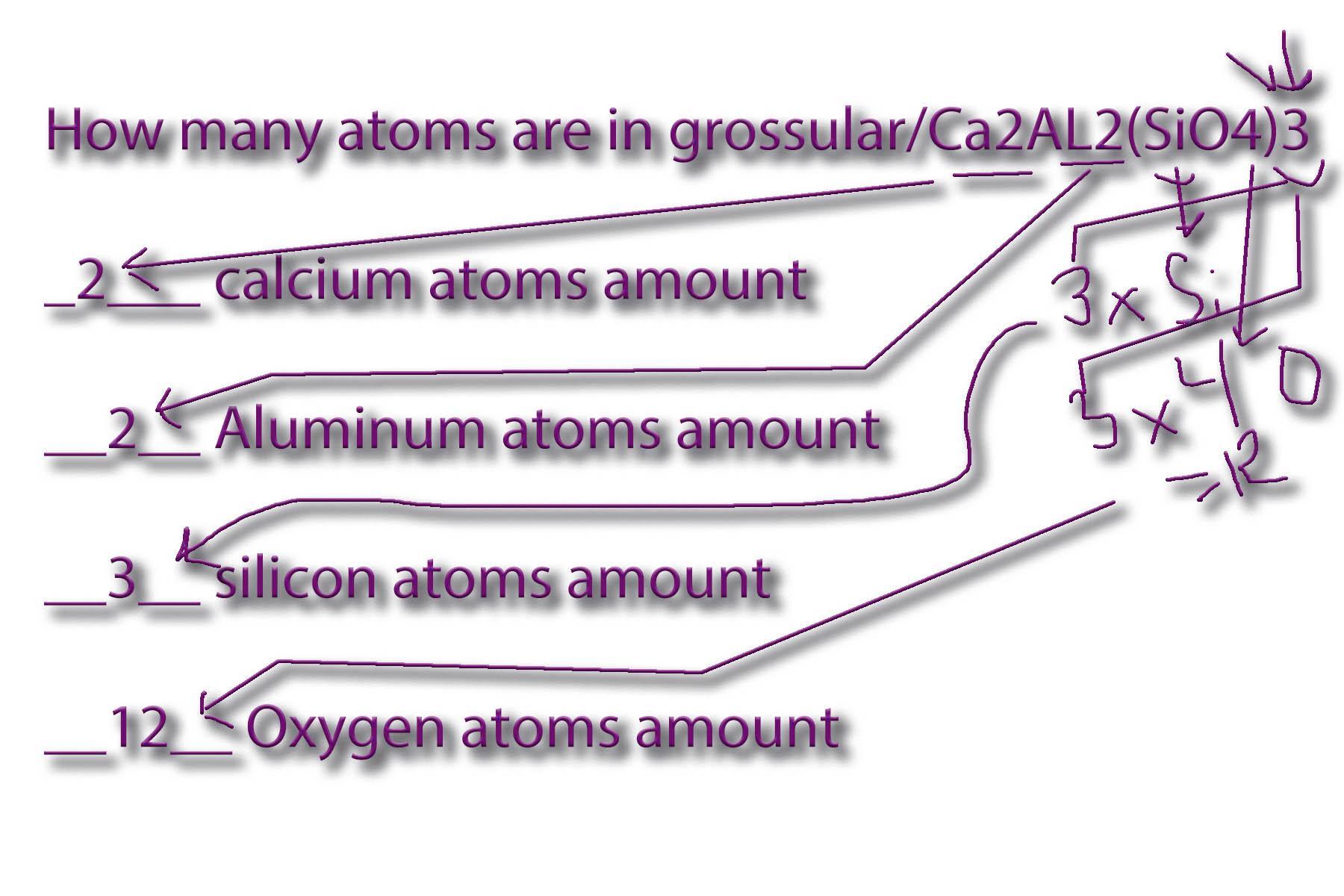
How many atoms are in grossular/Ca2AL2(SiO4)3
____ calcium atoms amount
____ Aluminum atoms amount
____ silicon atoms amount
____ Oxygen atoms amount
Answers
Answer:
See below
Explanation:
How many atoms are in grossular/Ca2AL2(SiO4)3
_2___ calcium atoms amount
__2__ Aluminum atoms amount
__3__ silicon atoms amount
__12__ Oxygen atoms amount
See attached worksheet
what will you suggest to a person who is suffering from the problem of acidity after overeating?
Answers
Answer:
If someone is suffering from the problem of acidity after overeating Baking soda solution would be suggested as a remedy as it is basic in nature, it neutralises excess acid in the stomach.
The balanced equation below represents the reaction that occurs inside the airbag:
2NaN 3 (s) à 2Na(s) + 3N 2 (g)
How many grams of NaN 3 are needed to produce the number of moles you calculated in problem 1 above?
Answers
Approximately 402.27 grams of sodium azide (NaN₃) must decompose to fill a 55.0-gallon airbag.
What is ideal gas law?The ideal gas law is a fundamental equation that describes the behavior of ideal gases under a wide range of conditions. It relates the pressure (P), volume (V), temperature (T), and number of moles of gas (n) of an ideal gas through the equation:
PV = nRT
where R is the gas constant, which has a value of 8.314 J/(mol·K) or 0.0821 L·atm/(mol·K) in SI units.
We know that the volume of the airbag is 55.0 gallons, so we need to convert this to liters using a conversion factor.
1 gallon = 3.78541 liters
Therefore, the volume of the airbag in liters is:
55.0 gallons x 3.78541 liters/gallon = 208.20 liters
Next, we need to calculate the number of moles of N₂ gas that would be produced from the decomposition of NaN₃ required to fill the airbag.
From the balanced equation:
2 NaN₃ (s) -> 2 Na (s) + 3 N₂ (g)
We can observe that two moles of NaN₃ result in three moles of N₂. Therefore, the number of moles of N2 produced is:
moles of N2 = (2/3) x moles of NaN₃
To fill the airbag, we need enough N₂ gas to occupy a volume of 208.20 liters. One mole of any gas has a volume of 22.4 litres at standard temperature and pressure (STP).
Therefore, the number of moles of N₂ required is:
moles of N₂ = (208.20/22.4) = 9.29 moles
Now we can use the balanced equation to calculate the number of moles of NaN₃ required:
2 NaN₃ (s) -> 2 Na (s) + 3 N2 (g)
For every 3 moles of N₂ produced, we need 2 moles of NaN3. Therefore, the number of moles of NaN₃ required is:
moles of NaN₃ = (2/3) x moles of N₂ = (2/3) x 9.29 = 6.19 moles
Finally, we can use the molar mass of NaN₃ to calculate the mass required:
mass of NaN₃ = moles of NaN₃ x molar mass of NaN₃
The molar mass of NaN₃ is:
Molar mass of NaN₃ = (1 x 22.99) + (3 x 14.01) = 65.01 g/mol
Therefore, the mass of NaN₃ required to fill the airbag is:
mass of NaN₃ = 6.19 moles x 65.01 g/mol = 402.27 grams
To know more about Ideal gas law, visit:
https://brainly.com/question/28206895
#SPJ1
Evaluate the following reactions:
REACTION 1. The hydrolysis of phosphoenolpyruvate (PEP) to pyruvate and inorganic phosphate (Pi) is represented by the reaction: PEP + H2O ---> Pyruvate + Pi + H+ and has a ΔG’of: -61.9 kJ mol -1.
REACTION 2. The hydrolysis of ATP is represented by the reaction: H2O + ATP --> ADP + Pi + H+ and has a ΔG’ of: -30.5 kJ mol-1.
a. What is the ratio of Pyruvate versus PEP under the equilibrium conditions in REACTION 1?
b. What is the ratio of ATP versus ADP under standard conditions for equilibrium in REACTION 2?
c. Under cellular conditions, these reactions are thermodynamically coupled. PEP in REACTION 1 can drive the synthesis of ATP in REACTION 2. Write the net coupled chemical equation for the synthesis of ATP from ADP and inorganic phosphate using the hydrolysis of PEP into Pyruvate. Show REACTION 1 and REACTION 2 in either forward or reverse direction AND the final overall coupled reaction equation.
d. Calculate the ΔG’ for the overall net coupled reaction. Using complete sentences, indicate whether the overall net coupled reaction would be spontaneous or non-spontaneous and why. Remember, show your work indicating the common intermediates and using appropriate units to receive full credit.
e. Calculate the ratio of products and reactants for the overall net coupled reaction.
Answers
a. Under equilibrium conditions, the ratio of Pyruvate to PEP is \(3.78 * 10^6.\)
b. Under standard conditions for equilibrium, the ratio of ATP to ADP is 441.
c. This equation shows that PEP can drive the synthesis of ATP from ADP and Pi. REACTION 1 is in the forward direction, while REACTION 2 is in the reverse direction.
d. The overall net coupled reaction is exergonic or spontaneous because the ΔG' is negative (-92.4 kJ/mol). This means that the reaction releases energy and can proceed spontaneously without the addition of energy.
e. At equilibrium, the concentrations of PEP, ADP, Pi, H+, Pyruvate, and ATP will be equal.
a. The equilibrium constant (Keq) for REACTION 1 can be calculated using the equation:
ΔG° = -RT ln(Keq)
where R is the gas constant (8.314 J mol^-1 K^-1), T is the temperature in Kelvin (assumed to be 298 K), and ΔG° is the standard free energy change. Rearranging the equation to solve for Keq gives:
Keq = e^(-ΔG°/RT)
Substituting the given values, we get:
Keq =\(e^{(-(-61.9 kJ mol^{-1})/(8.314 J mol^{-1} K^{-1} * 298 K))}\) = \(2.10 * 10^8\)
The equilibrium constant expression for the hydrolysis of PEP can be written as:
Keq = [Pyruvate][Pi][\(H^+\)] / [PEP][\(H_2O\)]
At equilibrium, the ratio of products to reactants is equal to Keq. Therefore, the ratio of Pyruvate to PEP is:
[Pyruvate] / [PEP] = Keq / ([Pi][\(H^+\)]/[\(H_2O\)])
Substituting the given values, we get:
[Pyruvate] / [PEP] = \((2.10 * 10^8) / ((1 M)(10^{-7} M) / (55.5 M))\)
[Pyruvate] / [PEP] = \(3.78 * 10^6\)
b. The equilibrium constant (Keq) for REACTION 2 can be calculated in the same way as in part (a):
Keq = e^(-ΔG°/RT) = \(e^{(-(-30.5 kJ mol^{-1})/(8.314 J mol^{-1} K^{-1} * 298 K))} = 1.26 * 10^5\)
The equilibrium constant expression for the hydrolysis of ATP can be written as:
Keq = [ADP][Pi][\(H^+\)] / [ATP][\(H_2O\)]
At equilibrium, the ratio of products to reactants is equal to Keq. Therefore, the ratio of ATP to ADP is:
[ATP] / [ADP] = [\(H_2O\)] / ([Pi][\(H^+\)]/([ATP]Keq))
Substituting the given values, we get:
[ATP] / [ADP] = \((55.5 M) / ((1 M)(10^{-7} M)/(1 M)(1.26 * 10^5))\)
[ATP] / [ADP] = 441
c. The net coupled chemical equation for the synthesis of ATP from ADP and inorganic phosphate using the hydrolysis of PEP into Pyruvate can be written as:
PEP + ADP + Pi --> Pyruvate + ATP
This equation shows that PEP can drive the synthesis of ATP from ADP and Pi. REACTION 1 is in the forward direction, while REACTION 2 is in the reverse direction.
d. To calculate the ΔG' for the overall net coupled reaction, we need to sum up the ΔG' of the individual reactions.
ΔG'net = ΔG'1 + ΔG'2
ΔG'1 = -61.9 kJ/mol
ΔG'2 = -30.5 kJ/mol
ΔG'net = -61.9 kJ/mol + (-30.5 kJ/mol)
ΔG'net = -92.4 kJ/mol
e. The overall net coupled reaction can be written as follows:
PEP + ADP + Pi + \(H^+\) → Pyruvate + ATP
The ratio of products and reactants for the overall net coupled reaction can be calculated using the equilibrium constant (Keq). The equilibrium constant is defined as the ratio of the concentrations of products to the concentrations of reactants at equilibrium.
Keq = [Pyruvate][ATP]/[PEP][ADP][Pi][\(H^+\)]
At equilibrium, Keq = 10^(ΔG'net/(-RT))
where R is the gas constant (8.314 J/molK), T is the temperature (in Kelvin), and ΔG'net is the standard free energy change of the reaction.
Assuming standard conditions of 25°C (298 K), we get:
Keq = \(10^{(-92400/(8.314*298))\)
Keq = \(2.1 * 10^{27\)
The ratio of products to reactants is given by the coefficients in the balanced equation:
PEP : ADP : Pi : H+ : Pyruvate : ATP = 1 : 1 : 1 : 1 : 1 : 1
For more question on equilibrium click on
https://brainly.com/question/19340344
#SPJ11
What is the specific heat of a 75.01 g piece of an unknown metal that exhibits a 45.2°C temperature change upon absorbing 1870 J of heat?
Answers
Answer:
\(Cp=0.552\frac{J}{g\°C}\)
Explanation:
Hello,
In this case, the formula to compute the required heat based on the mass, specific heat and change in temperature is widely known as:
\(Q=mCp\Delta T\)
In such a way, since we are asked to compute the specific heat, we solve for it as shown below:
\(Cp=\frac{Q}{m\Delta T}=\frac{1870J}{75.01g*45.2\°C} \\ \\Cp=0.552\frac{J}{g\°C}\)
Best regards.
The specific heat of the metal exhibiting 45.2 \(\rm ^\circ C\) temperature change has been 0.55 J/g.\(\rm ^\circ C\).
The specific heat can be defined as the amount of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius.
The expression can be given as:
Q = mc\(\Delta\)T
where, Q = heat required = 1870 J
m = mass = 75.01 g
c = specific heat capacity
\(\Delta\)T = change in temperature = 45.2 \(\rm ^\circ C\)
1870 J = 75.01 g \(\times\) c \(\times\) 45.2 \(\rm ^\circ C\)
c = \(\rm \dfrac{1870}{75.01\;\times\;45.2}\)
c = 0.55 J/g.\(\rm ^\circ C\)
The specific heat of the metal exhibiting 45.2 \(\rm ^\circ C\) temperature change has been 0.55 J/g.\(\rm ^\circ C\).
For more information about the specific heat capacity, refer to the link:
https://brainly.com/question/13369050
Question 40 (1 point) A single cell of inferior temporal cortex: a) Responds best to a complex visual pattern such as a face or hand. Ob) Responds best to a sinewave pattern of light and dark of a particular orientation, location, and frequency. Responds best to a simple visual pattern such as a stationary edge, bar, or c) line. d) Responds best to a simple visual pattern such as an edge, bar, or line, that moves at a particular direction and speed.
Answers
A single cell of inferior temporal cortex responds best to a complex visual pattern such as a face or hand. Option A is the correct answer.
Inferior temporal cortex: It is a part of the brain that is responsible for recognizing objects and faces, as well as other visual processing. It is located in the lower part of the temporal lobe, behind the ears. Research has demonstrated that individual neurons in the inferior temporal cortex react selectively to specific object characteristics. When we view a particular object, a large number of neurons in the inferior temporal cortex are activated, and the pattern of activation across these neurons represents the object's identity.
Based on research conducted, a single cell of inferior temporal cortex responds best to a complex visual pattern such as a face or hand. This can be concluded from the given options as option A reads: Responds best to a complex visual pattern such as a face or hand.
More on inferior temporal cortex: https://brainly.com/question/32875654
#SPJ11
given that the standard free energy change of atp hydrolysis is –30.5 kj/mol, what is the change in free energy in a cell at 37 °c where the atp and pi are both 10 mm and adp is 10 um?
Answers
The standard free energy change of the ATP hydrolysis is –30.5 kJ/mol, the change in the free energy in the cell at the 37 °C where the ATP and Pi are both 10 mm and ADP is 10 um is - 42.4 kJ/mol.
The equation is as :
ATP ----> ADP + Pi + H⁺
The temperature = 37 °C = 37 + 273 = 310 K
The ADP = 0.01 mM
The free energy change is as :
ΔG = ΔG° + RT lnQ
ΔG = - 30.5 kJ/mol + 8.314 J/mol × 310 ln ( 0.01 × 10 / 10 )
ΔG = -30.5 kJ/mol - 11.869 kJ /mol
ΔG = - 42.4 kJ/mol
To learn more about free energy here
https://brainly.com/question/30449907
#SPJ4
suppose naoh is added to the following system when it is at equilibrium. nh3(aq) h2o(l) nh4 (aq) oh- (aq) a. in which direction will the reaction shift after the naoh is added? b. will this stress increase or decrease the value of the reaction quotient, q? justify your answer. c. will the rate of the forward reaction exceed the rate of the reverse reaction before equilibrium is re-established? justify your answer. d. when equilibrium is re-established will the rate of the forward reaction exceed the rate of the reverse reaction? justify your answer.
Answers
It will raise the concentration of OH- (aq), which will cause equilibrium to move in the other direction, causing the concentration of OH- (aq) to fall in order to restore balance.
Describe equilibrium and provide a sample :The Latin term libra, which signifies weight or balance, is where the word equilibrium first appeared. Equilibrium can be visualized as an interesting story that is currently in rest. a vehicle that is going steadily. a chemical process where both the forward and backward rates of reaction are equal.
\(NH3(aq) + H2O (l) < === > NH4+ (aq) + OH- (aq)\)
NaOH is added to system \(: NaOH (aq) === > Na+ (aq) + OH- (aq)\)
(i) It will raise the concentration of OH- (aq), causing equilibrium to shift in a way that lowers the conc of OH- (aq) to attain equilibrium again: thus equilibrium will shift towards reactants (Left) .
(ii) Q\(= [OH-] [NH4+]/ [NH3] [H2O]\)
with increase in concentration of OH- (aq) (stress) ,value of Q will increase, since OH- appear in numerator .
(iii) Before equilibrium is re-established, rate of reverse reaction will exceed forward reaction, not vice-versa. As conc. of OH-(aq)decreases in towards reactants.
(iv) When equilibrium is re-established, rate of reverse reaction will be equal to rate of forward reaction, to maintain equilibrium.
To know more about Equilibrium visit :
https://brainly.com/question/29901689
#SPJ4
(14)
Consider the following elementary reaction equation.
NO3 (g) + CO (g) yields NO2 (g) + CO2 (g)
What is the order with respect of NO3?
What is the overall order of the reaction?
Classify the reaction as unimolecular, bimolecular, or termolecular
Answers
The order with respect to NO₃ is 1. The overall order of the reaction is 2. It is classified as a bimolecular reaction.
The elementary reaction equation is given as: NO₃ (g) + CO (g) → NO₂ (g) + CO₂ (g). To determine the order with respect to NO₃, we need to know the reaction rate law. Since it is an elementary reaction, the rate law can be directly written from the stoichiometry. The rate law for this reaction is: Rate = k[NO₃][CO], where k is the rate constant.
The order with respect to NO₃ is 1, as its concentration is raised to the power of 1 in the rate law. To find the overall order of the reaction, we sum the exponents of the concentration terms in the rate law: overall order = 1 (from NO₃) + 1 (from CO) = 2. Therefore, the overall order of the reaction is 2.
Since the reaction involves two reacting species (NO₃ and CO) colliding to form products, it is classified as a bimolecular reaction. Bimolecular reactions involve two reacting molecules coming together to form the products, in contrast to unimolecular reactions (involving a single reactant molecule) or termolecular reactions (involving three reactant molecules).
Learn more bout rate law here: https://brainly.com/question/16981791
#SPJ11
what is non polar with the absorbent?
Answers
A non-polar absorbent is a substance that can absorb or soak up other substances, but it only absorbs substances that are also non-polar.
Non-polar substances are those that do not have a separation of electric charge, meaning they do not have positive and negative ends.
Examples of non-polar absorbents include oils and fats. These substances can absorb other non-polar substances, such as other oils, but they cannot absorb polar substances, such as water. This is because polar and non-polar substances have different chemical properties and do not mix with each other.
Learn more about non-polar at:
https://brainly.com/question/30763634
#SPJ11
How many formula units are in 3.52 moles of NaF
Answers
The number of formula units in 3.52 moles of NaF is 2.119 × 10²⁴ formula units.
How to calculate formula units?Formula unit refers to the empirical formula of an ionic compound (that does not possess individual molecules) for use in stoichiometric calculations.
The formula unit of a substance can be calculated by multiplying the number of moles in the substance by Avogadro's number.
According to this question, there are 3.52 moles of NaF in a substance. The formula unit can be calculated as follows:
formula unit of NaF = 3.52 × 6.02 × 10²³
Formula unit = 2.119 × 10²⁴ formula units.
Learn more about formula units at: https://brainly.com/question/21494857
#SPJ1
which is stronger H2SO4 (sulfuric acid) or NaOH (sodium hydroxide)? It takes 8 drops of sulfuric acid added to sodium hydroxide for it to turn yellow and 14 drops of sodium hydroxide for sulfuric acid to turn purple so which one is stronger?
Answers
Answer: H2SO4 (sulfuric acid)
Explanation: Oops mb i just double cheked
what assumptions are you making about the temperature of gas space in the graduated cylinder? why do you think this is acceptable?
Answers
The assumptions being made about the temperature of the gas space in the graduated cylinder are that it remains constant and is equal to the ambient temperature.
This assumption is made because it simplifies calculations and measurements involving gas properties. Since most experiments are conducted under controlled conditions, it is reasonable to assume that the temperature of the gas space remains constant. Additionally, the graduated cylinder is typically exposed to the surrounding environment, allowing the gas inside to reach equilibrium with the ambient temperature.
Assuming that the temperature of the gas space in the graduated cylinder remains constant and equal to the ambient temperature is acceptable because it simplifies the analysis and is generally valid for most experimental setups.
To know more about gas properties, visit:
https://brainly.com/question/857678
#SPJ11
what does new substances often have that are different from the reactants
Answers
Answer
The new substances often have different combinations of atoms different from the reactants.
Explanation
The reactants and the new substances in a chemical reaction contain the same atoms, but they are rearranged during the reaction. As a result, the atoms end up in different combinations in the new substances. This makes the products new substances that are chemically different from the reactants.
When a liquid is heated, the average (blank) energy of its
particles will increase.
Answers
Answer:
trueee is the answer heh need points
When a liquid is heated, the average kinetic energy of its particles will increase.
What is Energy?The ability to do work is called as energy. Work done is product of force and displacement. Energy is of different types as sound energy, kinetic energy, potential energy, electrical energy, light energy etc.
Law of conservation of energy states that energy can neither be created nor destroyed, but it can be transferred from one form to another.
Molecules can move so they show kinetic energy, molecules can be there at many positions so they can show potential energy, molecules can vibrate so they can show sound energy but molecules can only absorb light hence they can't have light energy.
Average kinetic energy = 3KT/2
If temperature increases, kinetic energy also increases.
Therefore, When a liquid is heated, the average kinetic energy of its particles will increase.
Learn more about Kinetic energy, here:
https://brainly.com/question/26472013
#SPJ2
Question 1 of 10
What does a low number on the pH scale say about a solution?
O A. The solution is an acid,
O B. The solution is changing.
O C. The solution is a base.
O D. The solution sg neutral.