How many significant figures are in 0.00102?
Answers
Answer:
3
Explanation:
102 are significant figures
Answer:
3 digits
Explanation:
The first nonzero digit is counted as a significant digit and the zero inbetween 2 nonzero digits count too.
Therefore, the significant digits are 0.00102.


Related Questions
Silver nitrate and iron (III) chloride are reacted. 27.0 g silver nitrate and 43.5 g iron (III) chloride are used in the reaction.
3 AgNO3 + FeCl3 --> 3 AgCl + Fe(NO3)3
1. Using the limiting reactant, calculate how many grams of silver chloride are produced.
Answers
Using limiting reactant, 22.8 grams of silver chloride are produced.
What is limiting reactant?The limiting reactant is the reactant that is completely consumed in a chemical reaction and limits the amount of product that can be formed. It is the reactant that is present in the smallest stoichiometric amount compared to the other reactants involved in the reaction
Equation:To determine the limiting reactant, we need to calculate the amount of product that can be produced by each reactant and compare the results.
First, we need to convert the given masses of silver nitrate and iron (III) chloride into moles:
27.0 g AgNO₃ * (1 mol AgNO₃/169.87 g AgNO₃) = 0.159 mol AgNO₃
43.5 g FeCl₃ * (1 mol FeCl₃/162.2 g FeCl₃) = 0.268 mol FeCl₃
Next, we need to use the balanced chemical equation to determine the amount of product that can be produced by each reactant:
From the balanced chemical equation, we know that 3 moles of AgCl are produced for every 1 mole of FeCl₃ reacted.
Amount of AgCl produced by AgNO₃:
0.159 mol AgNO₃ * (3 mol AgCl/3 mol AgNO₃) = 0.159 mol AgCl
Amount of AgCl produced by FeCl₃:
0.268 mol FeCl₃ * (3 mol AgCl/1 mol FeCl₃) = 0.804 mol AgCl
Since AgNO₃ produces less AgCl (0.159 mol) than FeCl₃ (0.804 mol), AgNO₃ is the limiting reactant.
Finally, we can calculate the mass of AgCl produced using the amount of AgNO₃ reacted:
0.159 mol AgNO₃* (3 mol AgCl/3 mol AgNO₃) * (143.32 g AgCl/1 mol AgCl) = 22.8 g AgCl
To know more about limiting reactant, click here
https://brainly.com/question/14225536
#SPJ1
type the correct answer in each box balance the equation

Answers
Answer:
5SiO2 + 2CaC2 ➡ 5Si + 2CaO + 4CO2
Explanation:
This question involves balancing the above equation. An equation is said to be BALANCED when all the atoms of each element in the reactant side equates that in the product side.
According to this question, a chemical reaction is given as follows: SiO2 + CaC2 = Si + CaO + CO2. Based on observation, the atoms of elements are Silicon, oxygen, calcium and carbon are not the same on the reactants and products side. Based on this, the balanced equation is:
5SiO2 + 2CaC2 ➡ 5Si + 2CaO + 4CO2
HELP! IM GIVING BRAINLIST.What is salinity?
Question 1 options:
The concentration of dissolved salt in a liquid
to become incorporated with a liquid in forming a solution
a liquid mixture in which the smaller component (solute) is distributed evenly through the major component (solvent)
Answers
Answer:eighter a b or c
nah i am playing it is a
Explanation:
Answer:
A is correct i took the quiz
Explanation:
Answer the question on the picture please.

Answers
The right answer is :
Ca(NO3)2 salt
HCN weak acid
Ca(OH)2 strong base
Li2SO4 salt
H2SO4 strong acid
H2SO3 weak acid
HF weak acid
CeH12O6 molecule
glucose molecule
NH3 weak base
A salt is a substance created when an acid and a base are neutralised. Ca(NO3)2 and Li2SO4 are categorised as salts in this list.
A base is a chemical that receives hydrogen ions (H+) whereas an acid is a compound that contributes hydrogen ions (H+) in a solution. HCN, H2SO4, H2SO3, and HF are acids on this list, whereas Ca(OH)2 and NH3 are bases.
Strong acids and bases fully dissociate in solution, which means that all of the molecules of the acid or base separate into their individual ions. A weak acid or base, on the other hand, only partially dissociates in solution. HCN, H2SO3, HF, and NH3 are weak acids, whereas Ca(OH)2 and H2SO4 are strong acids and bases, respectively.
Learn more about strong acid at:
https://brainly.com/question/31143763
#SPJ1
what element have 5 electron shells and 6 valance electrons
Answers
Answer:
Tellurium
Explanation:
It is stated that element have six valence electron thus the given element must be belongs to oxygen family.
There are five members present in oxygen family.
Oxygen, sulfur, selenium, tellurium, polonium
Electronic configuration of oxygen:
O₈ = [He] 2s² 2p⁴
It means given element is not oxygen because it has two electronic shell.
Electronic configuration of sulfur:
S₁₆ = [Ne] 3s² 3p⁴
It means given element is not sulfur because it has three electronic shell.
Electronic configuration of selenium:
Se₃₄ = [Ar] 3d¹⁰ 4s² 4p⁴
It means given element is not selenium because it has four electronic shell.
Electronic configuration of tellurium:
Te₅₂ = [kr] 4d¹⁰ 5s² 5p⁴
The given element is tellurium because it has six valence electrons and five electronic shell.
Fusion reactions power the _____ in space. Humans currently use fusion in _____ , but researchers are seeking more applications.

Answers
Answer:
Sun and the stars, nothing humans don't use fusion at all
Explanation:
Nuclear fusion reactions power the sun in space. Humans currently use fusion in nuclear weapons by means of nuclear reactions, but researchers are seeking more applications.
What are nuclear reactions?There are two types of nuclear reactions which are nuclear fusion and nuclear fission .They involve the combination and disintegration of the element's nucleus respectively.
In nuclear fission, the nucleus of the atom is bombarded with electrons of low energy which splits the nucleus in to two parts .Large amount of energy is released in the process.It is used in nuclear power reactors as it produces large amount of energy.
In nuclear fusion,on the other hand, is a reaction which occurs when two or more atoms combine to form a heavy nucleus.Large amount of energy is released in the process which is greater than that of the energy which is released in nuclear fission process.
Learn more about nuclear reactions,here:
https://brainly.com/question/12649087
#SPJ7
suppose the average person in your sample does reduce liquor consumption by 1.5 ounces (y¯ = −1.5). what is the range of potential values that you are 70onfident contains µ?
Answers
The range of potential values for the population mean (µ) with a 70% confidence interval, given that the sample mean (y¯) shows a reduction in liquor consumption by 1.5 ounces.
To calculate this range, we need to know the sample size (n), standard deviation (σ), and the appropriate critical value (z-score) for a 70% confidence interval. Unfortunately, you haven't provided all the necessary information to perform the calculation.However, I can guide you on how to find the range once you have the missing information. Here are the steps:1. Find the critical z-score for a 70% confidence interval using a z-score table or calculator.
2. Calculate the standard error (SE) using the formula: SE = σ / √n, where σ is the standard deviation and n is the sample size.
3. Multiply the critical z-score by the standard error: z * SE.
4. Determine the range by adding and subtracting the result from step 3 to/from the sample mean (y¯ = -1.5).Once you follow these steps, you'll obtain the range of potential values for µ with a 70% confidence level.
For more such question on reduction
https://brainly.com/question/29631179
#SPJ11
Anyone know this pleaase help

Answers
Answer:
Explanation:
I'll answer question 3 for you. But just ask one question at a time.
it's really not fair to ask a bunch of questions.
so for 3 we know it's hydroCarbons. or \(H_{2}\) b/c of how Hydrogen is diatomic, C , for Carbon. Next, we know 8 Carbon atoms have attached themselves. so do you happen to know the Lewis diagram for Hydrogen and Carbon? look it up if not. so we know Carbon is 6 on the periodic chart, so it's normally got 6 electrons. I mentioned Lewis structures b/c now you have to picture how the electrons are going to "stick" the atoms together. Recall they like to be in groups of 8 :P so the diatomic Hydrogen is going to stick to one Carbon atom well. Also, Hydrogen is never the central atom, it just "hangs around" :DD , so I added a picture of the Lewis structure for 8 carbon atoms, but, you can see there are double bond connections and the question you asked doesn't say if those are allowed. I feel like the professor has misguided you in this question. b/c HydroCarbons come in a big variety of complex connections, so almost any answer would be correct. but probably 16 is good. but that's not even an option. Maybe they just want you to recognize that Hydrogen is diatomic and comes in twos. so maybe 10 is the best answer. or A)

(a) Briefly describe the phenomena of superheating and supercooling.(b) Why do these phenomena occur?
Answers
(a) Superheating is a phenomenon where a liquid is heated above its boiling point without actually boiling.
(b) Superheating and supercooling occur because they represent a state of thermodynamic instability
(a) This occurs when the liquid is free of impurities or nucleation sites that can trigger boiling. Supercooling is the opposite phenomenon, where a liquid is cooled below its freezing point without actually freezing. This occurs when the liquid is pure and there are no nucleation sites for the formation of ice crystals.
(b). In the case of superheating, the liquid is at a temperature above its boiling point but is prevented from boiling due to the absence of nucleation sites. In the case of supercooling, the liquid is at a temperature below its freezing point but is prevented from freezing due to the absence of nucleation sites. These phenomena can be observed in nature and can have practical applications in various fields, such as materials science and engineering.
learn more about superheating Refer: https://brainly.com/question/24249319
#SPJ11
Superheating and supercooling are two phenomena that occur when a substance is heated or cooled beyond its boiling or freezing point, respectively.
Superheating is when a liquid is heated above its boiling point without boiling. This occurs because the liquid is in a stable state with no nucleation sites for bubbles to form. When a nucleation site is introduced, such as when the liquid is disturbed or when a foreign object is added, the liquid will rapidly boil and can potentially cause a dangerous explosion. Supercooling, on the other hand, is when a liquid is cooled below its freezing point without solidifying. This occurs because the liquid is also stable with no nucleation sites for ice crystals to form. When a nucleation site is introduced, such as when the liquid is agitated or when a foreign object is added, the liquid will rapidly freeze.These phenomena occur because a substance's boiling or freezing point is dependent on pressure, and when the pressure is decreased or increased, the boiling or freezing point will also change. Additionally, the lack of nucleation sites in a superheated or supercooled substance means that the substance is not able to transition to a new state until a nucleation site is introduced.
Learn more about Superheating here:
https://brainly.com/question/31496362
#SPJ11
In 2006, scientists decided that Pluto
had more in common with
than the original 8 planets of the solar
system.
A: the other, newly discovered, dwarf planets
b: the moon
c: the asteroids
Answers
Scientists decided that Pluto are more in common with than the original 8 planets of the solar system will be the other, newly discovered, dwarf planets. Option A is correct.
In 2006, scientists redefined the definition of a planet, leading to the reclassification of Pluto as a dwarf planet. This decision was influenced by the discovery of other celestial bodies in the outer regions of the solar system that shared similar characteristics with Pluto.
The other, newly discovered, dwarf planets" is correct. Scientists recognized that there were other objects in the solar system, such as Eris, Haumea, Makemake, and Ceres, that shared similar characteristics with Pluto and were classified as dwarf planets alongside Pluto.
This reclassification highlighted the presence of a population of objects in the outer regions of the solar system known as the Kuiper Belt, which consists of numerous icy bodies similar to Pluto. By considering the properties and characteristics of these newly discovered dwarf planets, scientists concluded that Pluto had more in common with these objects than with the original eight planets of the solar system.
The moon and the asteroids are not accurate. While the moon and asteroids are significant celestial objects in the solar system, they do not represent the specific group of objects that are considered dwarf planets and have characteristics similar to Pluto.
Hence, A. is the correct option.
To know more about planets here
https://brainly.com/question/26756957
#SPJ2
g How many of the atoms in p-toluidine are expected to have approximate bond angles consistent with sp2 hybridization
Answers
There are 11 atoms in p-toluidine that are expected to have approximate bond angles consistent with sp² hybridization.
Hybridization is a concept that describes how atomic orbitals fuse together to form new hybrid orbitals in order to produce the molecules. The sp² hybridization in organic compounds is one of the most common hybridization types found. It has a tetrahedral arrangement of four electron domains, which is the most common shape for sp² hybridization. The approximate bond angles of sp² hybridization are approximately 120 degrees.
P-toluidine has a total of eleven atoms, with the carbon atoms in the amine group being sp² hybridized. The carbon atom in the carboxyl group is sp³ hybridized, and the other five carbon atoms are sp³ hybridized. Therefore, there are eleven atoms in p-toluidine that are expected to have approximate bond angles consistent with sp² hybridization.
Learn more about hybridization here:
https://brainly.com/question/29020053
#SPJ11
A thermos contains 150 cm3 of coffee at 85 8C. To cool the coffee, you drop two 11-g ice cubes into the thermos. The ice cubes are initially at 0 8C and melt completely. What is the final temperature of the coffee
Answers
The final temperature of the coffee in the thermos will be a result of the heat transfer between the coffee and the melting ice cubes.
When the ice cubes are dropped into the thermos containing coffee, heat transfer occurs between the two substances until they reach thermal equilibrium. The heat transfer process involves the transfer of heat from the coffee to the ice cubes, causing them to melt.
To calculate the final temperature, we can use the principle of conservation of energy. The heat gained by the ice cubes equals the heat lost by the coffee.
First, let's calculate the heat gained by the ice cubes. The specific heat capacity of ice is approximately 2.09 J/g°C. Since each ice cube weighs 11 g and starts at 0°C, the heat gained by the ice cubes is:
Q_ice = (mass_ice) x (specific heat capacity_ice) x (change in temperature)
= (11 g) x (2.09 J/g°C) x (final temperature - 0°C)
Next, let's calculate the heat lost by the coffee. The specific heat capacity of coffee is assumed to be the same as water, which is approximately 4.18 J/g°C. The initial temperature of the coffee is 85°C, and its volume is 150 cm³. Using the density of water (1 g/cm³), we can find the mass of the coffee
mass_coffee = (density_water) x (volume_coffee)
= (1 g/cm³) x (150 cm³)
Q_coffee = (mass_coffee) x (specific heat capacity_coffee) x (final temperature - 85°C)
Since the heat gained by the ice cubes is equal to the heat lost by the coffee, we can set up the equation:
Q_ice = Q_coffee
Substituting the respective values and solving the equation will give us the final temperature of the coffee.
Learn more about:Thermos
brainly.com/question/29137878?
#SPJ11
nitrogen (n) normally forms three covalent bonds with a valence of five. however, ammonium has four covalent bonds, each to a different hydrogen (h) atom (h has a valence of one). what do you predict to be the charge on ammonium?
Answers
The charge on ammonium is +1, indicating that it has one less electron than protons. In ammonium, nitrogen forms four covalent bonds with four hydrogen atoms, using four of its valence electrons. Each hydrogen atom contributes one valence electron to the bonding process.
As a result, the total number of valence electrons in ammonium is 5 (from nitrogen) + 4 (from four hydrogen atoms) = 9. Since nitrogen's valence electron count is less than its usual five, it has an electron deficiency. This deficiency is balanced by a positive charge on the ammonium ion.
In summary, the charge on ammonium is +1 because nitrogen forms four covalent bonds with hydrogen atoms, resulting in an electron deficiency in the nitrogen atom.
To know more about valence electrons visit:-
https://brainly.com/question/31264554
#SPJ11
Can someone help me thx

Answers
Answer:
salt i know the answer and even sugar is
Marco was looking at this picture of two boats sitting differently in the water. He decided to compare the way the two boats sit in the water to the way land is behaving in Greenland.
Answers
I don't know
Explanation:
because it does not give an question
What human activity constitutes to air pollution?
A) construction
B) reforestation
C)habitats restoration
D)dam development
Answers
Answer:
The answer is A. Construction. The production of raw materials for construction in factories is harmful for the environment due to CO2 emissions. The chemicals used in construction and the diesel used by construction vehicles are also harmful to the environment.
Explanation:
In an ecosystem , human activity which constitutes to air pollution is construction.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the ecosystem through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.There are different types of ecosystems.
Learn more about ecosystem,here:
https://brainly.com/question/19267717
#SPJ6
what phase change is represented by the transition from b to c? a) fusion b) sublimation c) vaporization d) condensation e) solidification (deposition)
Answers
sublimation/deposition A-solid, B- liquid, C-gas is represented by the transition from b to c
Sublimation is the direct transition of a material from a solid form to a gas state in scientific words. It only happens at certain temperatures and pressures and does not go through the typical liquid stage. Actually, sublimation printing employs heat to fuse ink and cloth together. On special paper, a design is first printed. When heated, the inks that are employed transform into gas, mix with the cloth, and leave a permanent imprint on the fabric.
what phase change is represented by the transition from b to c? a) fusion b) sublimation c) vaporization d) condensation e) solidification (deposition)
Learn more about sublimation here:
https://brainly.com/question/28626755
#SPJ4

can you help me with the chemistry presentation? the topic is the impact of fossil fuels on the environment, can you tell me what you should write? I have to present up to 5 minutes
Answers
Fossil fuels, when burnt, can lead to the release of greenhouse gases to the environment (atmosphere) ultimately causing global warming.
What are fossil fuels?Fossil fuels are any fuel derived from hydrocarbon deposits such as coal, petroleum, natural gas and, to some extent, peat.
These fuels are non-renewable, and their burning generates the greenhouse gas called carbon dioxide.
Carbondioxide is a notorious greenhouse gas that results from the burning of fossil fuels such as petroleum and coal. Greenhouse gases have the ability to trap and release heat into the atmosphere causing global warming in the process.
Learn more about fossil fuels at: https://brainly.com/question/2029072
#SPJ1
microwave ovens use radiation with 11.2 cm to heat food. the imcrowaves are asborebd by h2o molecules, and the energy is comepletely transofrmed to heat. what is the minimum number of moles of microwave photons necessay to convert 100g of ice at 0
Answers
We have given that :
Wavelength (λ) = 11.2 cm
= 0.112 m
Temperature(T) = 0°C = 273 K
Specific Heat capacity of water(c) = 4.184 J/g•K
So,Formula for Quantity of heat(Q) required
To raise the temperature is Q=mc∆T
where,{m is mass of ice}
{C is heat capacity}
{∆t is change in temperature}
Thus ,
Q=(100g)(4.184J/g•K)(273K)
Q=114223.2 J
Now,Energy of photon E =hc/(λ)
where,{h is plant constant = 6.6×10^(-34)}
{c is speed of light = 3×10^(-8)}
{λ is wavelength}
Thus ,
E = [6.6 × 10^(-34)]×[3×10⁸] J
E =1.77 ×10 ^(-24) J
To get number of of photon n = Q/E
n = 114223.2/1.77 ×10 ^(-24)
n = 6.45×10¹⁸
Therefore number of mole required= number of photon × avogadro number
= 6.45×10¹⁸ × 6.02 × 10²³
Number of mole of photon =38.82×10⁴¹
Learn more about photons here https://brainly.com/question/20912241?referrer=searchResults
#SPJ4
which gas has the fastest gas particles assuming they are at the same temperature nitrogen, oxygen or chlorine
Answers
NH3 has the least molar mass, it'll diffuse faster than the others.
What is Molar Mass?
We all wish to know how many molecules are there in a given material. Atoms and molecules are very small in both size and mass. One sample mole's weight is the molar mass. To get the molar mass, connect the atomic masses (atomic weights) of each atom in the molecule. Using the mass listed in the Periodic Table or Atomic Weight Table, determine the atomic mass for each element.
Typically, molar mass is stated in either grammes (g) or kilogrammes (kg) (kg).
The diffusion rate of a particular gas is inversely proportional to the square root of the molar mass of that gas.
As for molar masses go,
NH3= 17gm/mol
O2= 32 gm/mol
N2= 28gm/mol
CO2= 44gm/mol
NH3 has the least molar mass, it'll diffuse faster than the others.
Learn more about Molar Mass from given link
https://brainly.com/question/837939
#SPJ4
quizlet 30.0g consider the reaction a 2b → 3c. if the molar mass of c is twice the molar mass of a, what mass of c is produced by the complete reaction of 10.0 g of a?
Answers
The mass of C produced by the complete reaction of 10.0 g of A is 15.0 grams.
To determine the mass of C produced by the complete reaction of 10.0 g of A, we need to use the molar masses and the stoichiometry of the reaction.
Molar mass of C is twice the molar mass of A.
The reaction is 2A → 3C.
Let's start by finding the molar masses of A and C. Let's assume the molar mass of A is "M" g/mol. Therefore, the molar mass of C would be "2M" g/mol.
Using the molar masses, we can calculate the number of moles of A in 10.0 g of A:
Number of moles of A = Mass of A / Molar mass of A
= 10.0 g / M g/mol
= 10.0 / M mol
According to the stoichiometry of the reaction, 2 moles of A react to produce 3 moles of C. So, the number of moles of C produced can be calculated as follows:
Number of moles of C = (3/2) * Number of moles of A
= (3/2) * (10.0 / M) mol
To find the mass of C produced, we multiply the number of moles of C by its molar mass:
Mass of C = Number of moles of C * Molar mass of C
= [(3/2) * (10.0 / M) mol] * (2M g/mol)
= (3/2) * 10.0 g
= 15.0 g
Therefore, the mass of C produced by the complete reaction of 10.0 g of A is 15.0 grams.
For more such questions on mass , Visit:
https://brainly.com/question/24191825
#SPJ11
We determine the mass of C produced by the complete reaction of 10.0 g of A is 15.0 grams.
How do we calculate?We know that molar mass of C is twice the molar mass of A.
The reaction is given as 2A → 3C.
we go ahead to calculate the number of moles of A in 10.0 g of A:
Number of moles of A = Mass of A / Molar mass of A
Number of moles of A= 10.0 g / M g/mol
Number of moles of A= 10.0 / M mol
we do same for C according to the stoichiometry of the reaction:
Number of moles of C = (3/2) * Number of moles of A
Number of moles of C = (3/2) * (10.0 / M) mol
Mass of C = Number of moles of C * Molar mass of C
Mass of C = [(3/2) * (10.0 / M) mol] * (2M g/mol)
Mass of C = (3/2) * 10.0 g
Mass of C = 15.0 g
In conclusion, the mass of C produced by the complete reaction of 10.0 g of A is 15.0 grams.
Learn more about number of moles at:
https://brainly.com/question/15356425
#SPJ4
classify the following energy sources as renewable and non-renewable energy sources (natural gas cool biomass and geothermal energy)
Answers
Answer:
bio mass is renewable
natural gas non renewable geothermal energy renewable
Explanation:
biomass is by palnts and animals geothermals is unlimeted because its made out of the earths core
why do organisms need to respond to their environment
Answers
I Hope this will Help You:-
Organisms need to detect and respond to changes in their internal and external environment. This is because the conditions inside our body must be carefully controlled for it to function effectively and survive. The control systems that allow organisms to respond to changes are incredibly important.
Which action is an example of chemical weathering?
Answers
Chemical weathering is an example of Airborne oxygen reacts with the iron content of rocks, causing them to change color.
Chemical weathering is a process in which things disintegrate due to chemical reactions, mostly with water and compounds dissolved in it, rather than mechanical processes.
A) Freezing water widens fissures in rocks is a physical phenomenon in which water stuck inside crevices in rocks expands when frozen at lower temperatures. In this method, no chemical changes are seen.
B) Plant roots force their way into cracks in rocks and finally shatter them. This is a physical process in which plant roots search for water and nutrients in fissures in rocks and eventually break them. There are no chemical changes visible here.
C) Oxygen in the air reacts with iron content in rocks, causing them to change color; as we can see, the color of rocks is changing as a result of a chemical reaction between oxygen in the air and iron content in the rocks. This process is also characterized by rock disintegration. As a result, this is referred to as chemical weathering.
D) Strong winds physically peel soft rock away, leaving harder rock behind There is no visible chemical change.
As a result, the correct solution is Option C), which provides completely adequate modifications to be classified as chemical weathering.
To learn more about Chemical weathering, Here :
https://brainly.com/question/14686073?referrer=searchResults
#SPJ4
In the early 1900's many scientists thought that an atom consisted of a positive substance with negative charges scattered throughout the substance. Then Ernest Rutherford completed an experiment that changed the concept of an atom. His discovery led to the understanding that an atom. His discovery led to the understanding that an atom consists of mostly empty space with
Answers
The question is incomplete; the complete question is;
In the early 1900s many scientists thought that an atom consisted of a positive substance with negative charges scattered throughout the substance. Then Ernest Rutherford completed an experiment that changed the concept of an atom. His discovery led to the understanding that an atom consists mostly of empty with space with-
Protons orbiting a dense nucleus made of electrons and neutrons
Electrons orbiting a dense nucleus made of protons and neutrons
Neutrons and protons orbiting a cloud of electrons
Electrons and protons orbiting a cloud of neutrons
Answer:
Electrons orbiting a dense nucleus made of protons and neutrons
Explanation:
Rutherford established the nuclear theory of the atom by his famous experiment. In his experiment, alpha particles were used to bombard a thin gold foil and the movement of the particles was observed on a moveable zinc sulphide screen.
It was discovered from the experiment that the atom was mostly made up of empty space. The electrons orbit a dense nucleus comprising of protons and neutrons.
need help with this.

Answers
HELP ASAP!!! Which two energy transformations are shown in the photo? A. Chemical energy → electromagnetic energy B. Chemical energy → thermal energy C. Thermal energy → electrical energy D. Thermal energy → chemical energy

Answers
Answer:
Chemical energy _ thermal energy
What group could X be in if it forms ions with ammonium in the ratio of (NH4)3X?
X could be in Group
Answers
If it forms ions with ammonium in the ratio of (NH4)3X, X could be in Group IV A. Group IVA (14) metals form cations with +4 charge, although tin (Sn) and lead (Pb) can form cations having +2 charge.
What does the term "coordinate bond" mean?A covalent link (a shared pair of electrons) in which both electrons originate from the same atom is known as a coordinate bond (also known as a dative covalent bond). Two atoms sharing a pair of electrons make a covalent connection. Because the electron pair is drawn to both nuclei, the atoms are kept together.
How is a coordinate bond recognized?An arrow pointing from the donor to the acceptor, with a positive charge on the donor and a negative charge on the acceptor, is used to symbolize a coordinate bond.
To learn more about coordinate bond visit:
brainly.com/question/12857081
#SPJ1
Ayúdenme por favor
Please help me
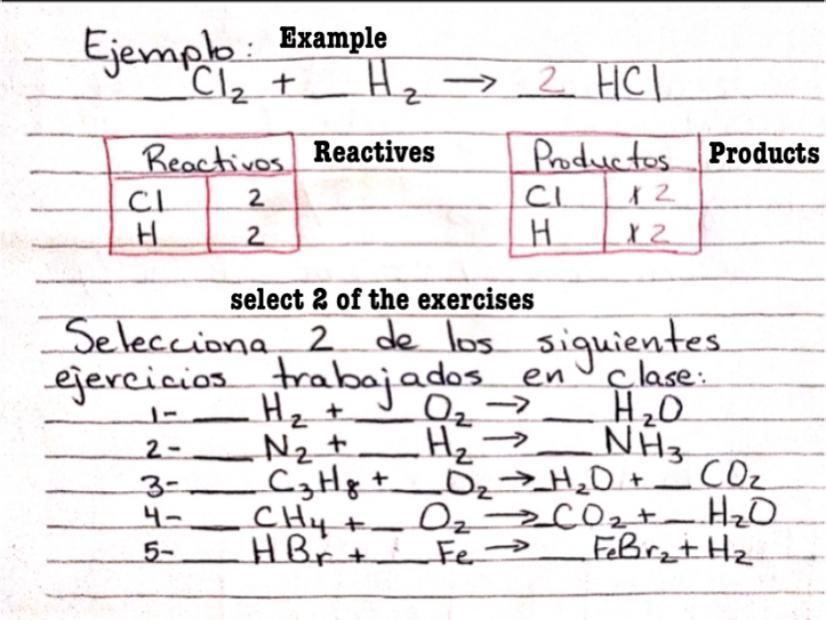
Answers
2H2+O2===>2H2O
N2+3H2==>2NH3
C3H8+5O2==>4H20+3CO2
CH4+2O2===>CO2+2H2O
2HBr +Fe==>FeBr2 +H2
I hope it helped
7. True or false. Where air masses collide, weather changes
Answers
Answer:true
Explanation: