Answers
The number of particles of copper produced when 3.85 grams of Copper (II) Chloride is consumed is approximately \(1.728 * 10^2^2\) particles.
To determine the number of particles of copper produced when 3.85 grams of Copper (II) Chloride is consumed with excess aluminum, we need to use stoichiometry and the balanced chemical equation for the reaction.
The balanced chemical equation for the reaction between Copper (II) Chloride (CuCl2) and aluminum (Al) is:
\(3CuCl_2 + 2Al\) -> \(2AlCl_3 + 3Cu\)
From the balanced equation, we can see that for every 3 moles of\(CuCl_2\)consumed, 3 moles of Cu are produced.
First, we need to calculate the number of moles of \(CuCl_2\) in 3.85 grams. To do this, we divide the mass of\(CuCl_2\) by its molar mass. The molar mass of \(CuCl_2\) can be calculated by summing the atomic masses of its constituent elements: Cu (63.55 g/mol) and Cl (35.45 g/mol).
Molar mass of\(CuCl_2\) = 63.55 g/mol (Cu) + (2 * 35.45 g/mol) (Cl) = 134.45 g/mol
Number of moles of CuCl2 = 3.85 g / 134.45 g/mol ≈ 0.0287 mol
Since the stoichiometry of the reaction states that 3 moles of CuCl2 produce 3 moles of Cu, we can conclude that 0.0287 mol of CuCl2 will produce 0.0287 mol of Cu.
Finally, to calculate the number of particles (atoms or molecules) of copper produced, we multiply the number of moles of Cu by Avogadro's number, which is approximately \(6.022 * 10^2^3\)particles/mol.
Number of particles of Cu = 0.0287 mol * \(6.022 * 10^2^3\) particles/mol
Therefore, the number of particles of copper produced when 3.85 grams of Copper (II) Chloride is consumed is approximately \(1.728 * 10^2^2\)particles.
Know more about copper here:
https://brainly.com/question/24856041
#SPJ8
Related Questions
what is 1.01 km to mm in scientific notation
Answers
Answer:
= 3.456 × 1011
Explanation:
Please help!!!!!!!!!!!!!!!!!!

Answers
Answer:
5
Explanation:
Arsenic is in row 4 which means it has 4 electron energy levels. The bottom number in the box on the PT is the energy level with the MOST energy.
What mass of glucose must be metabolized in order to produce 223 g of water? C6H12O6 + 6O2 → 6CO2 + 6H2O
Answers
371.4 g of glucose must be metabolized to produce 223 g of water.
What is the chemical equation for the complete combustion of glucose?The balanced chemical equation for the complete combustion of glucose is:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O
According to the equation, for every 1 mole of glucose consumed, 6 moles of water are produced.
The molar mass of glucose is:
6(12.01 g/mol) + 12(1.01 g/mol) + 6(16.00 g/mol) = 180.18 g/mol
To calculate the mass of glucose required to produce 223 g of water, we need to first convert the mass of water to moles:
223 g / 18.015 g/mol = 12.38 mol H₂O
Now we can use the mole ratio from the balanced equation to calculate the moles of glucose required:
1 mol glucose / 6 mol H₂O = x mol glucose / 12.38 mol H₂O
x = 2.06 mol glucose
Finally, we can calculate the mass of glucose:
mass = moles × molar mass
mass = 2.06 mol × 180.18 g/mol = 371.4 g
Therefore, 371.4 g of glucose must be metabolized to produce 223 g of water.
Learn more about glucose here:
https://brainly.com/question/30548064
#SPJ1
b) Draw a diagram that would represent a greater overall energy change for an exothermic reaction
Giving brainliest!
Answers
The diagram the represents the overall energy change for an exothermic reaction is shown in the image attached to this answer.
What is an exothermic reaction?We know that the exothermic reaction has to do with the kind of reaction in which there is the giving off or the heat of the reaction. This implies that the energy of the reacxtsnts would be greater than the energy of nteh products. As such the excess energy would be given off in the reaction.
One of the ways by which we can quickly know that a given reaction is exothermic is when we try to feel the reaction vessel. If indeed the reaction as we know it is an exothermic reaction then it follows that the vessel that is used for the reaction would be hot and then there would be an evolution of heat by the reaction system.
Learn more about exothermic reaction:https://brainly.com/question/10373907
#SPJ1

2 NaClO3 → 2 NaCl + 3 O2
Calculate the mass of O2 produced as the result of the decomposition of 843 g of NaClO3.
Answers
Taking into account the reaction stoichiometry, 380.12 grams of O₂ are produced as the result of the decomposition of 843 g of NaClO₃.
Reaction stoichiometryIn first place, the balanced reaction iS:
2 NaClO₃ → 2 NaCl + 3 O₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
NaClO₃: 2 molesNaCl: 2 molesO₂: 3 molesThe molar mass of the compounds is:
NaClO₃: 106.45 g/moleNaCl: 58.45 g/moleO₂: 32 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
NaClO₃: 2 moles ×106.45 g/mole= 212.9 gramsNaCl: 2 moles ×58.45 g/mole= 116.9 gramsO₂: 3 moles ×32 g/mole= 96 gramsMass of O₂ formedThe following rule of three can be applied: if by reaction stoichiometry 212.9 grams of NaClO₃ form 96 grams of O₂, 843 grams of NaClO₃ form how much mass of O₂?
mass of O₂= (843 grams of NaClO₃× 96 grams of O₂) ÷212.9 grams of NaClO₃
mass of O₂= 380.12 grams
Finally, 380.12 grams of O₂ are formed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
Please help I’ll make brainlist

Answers
Explanation:
c realsed from the galaxie
Answer:
C
Explanation:
A P E X
how to convert anisole to salicylic acid
Answers
Answer:
ipfiyf8y97guog7
Explanation:
oyviyvhlvoyvhlvouuoupvpu
what is the boiling pressure in a mixture? how is this boiling pressure generated for the mixture? explain with the figure.
Answers
When the combined vapour pressure of a miscible liquid mixture reaches atmospheric pressure, the solution boils. This vapour pressure denotes the boiling pressure in a mixture.
What causes boiling under pressure?The air pressure directly impacts a liquid's boiling point. The weight of the air molecules above the liquid is the factor putting more pressure on the liquid. This is considered to as atmospheric pressure in an open system. The higher the boiling point and the more energy needed to bring liquids to a boil, the higher the pressure.
What is the combination distillation boiling point?Because volatile compounds regularly have different boiling points, when a mixture is distilled, the components commonly separate from the mixture. The temperature at which the external pressure operating on the surface of a liquid equals the vapour pressure of the liquid phase of a compound is termed as the boiling point.
Learn more about pressure here:
brainly.com/question/17645330
#SPJ4
Heart, 5 stars, Brainiest, and 40 coins if right! Answer needed ASAP
Which statement describes what happens when Bret runs up a flight of stairs?
A. His kinetic energy is destroyed.
B. His potential energy decreases.
C. His kinetic energy is converted into potential energy.
D. His potential energy is converted into kinetic energy.
Answers
Answer:
His kinetic energy is converted into potential energy.
Explanation:
If 1 gram of radium decays to 1/2 gram in 1622 Years so, 6 grams decay to 3 grams in
a)9732 years
b) 3244 years
c) 4866 years
d) 1622years
Answers
Answer:
Explanation:
Since 1 gram of radium decays to 1/2 gram in 1622 years that means the half-life of radium is 1622 years.
So, 6 grams also decay to 3 grams in 1622years
heating curve iron
at what temperature does the substance begins to boil
at what temperature does a substance begin to melt
at what temperature is a substance for a liquid and a gas
at what temperature is the substance both a solid and a liquid

Answers
The substance begins to boil at 2750⁰C, the substance begins to melt at 1500⁰C, the temperature at which the substance is both a liquid and a gas at 2750⁰C, temperature is the substance both a solid and a liquid at 1500⁰C.
Heating curves are the graphical correlations between heat added to a substance. When viewed from a cooling perspective, ie. loss of heat, it is the cooling curve.
The gradient of the cooling curve is related to the heat capacity, the thermal conductivity of the substance, and the external temperature. The more heat is required to change the temperature of the substance, the slower it cools, so the smaller the gradient of the curve. The higher the thermal conductivity, the faster heat is transferred, so the faster the substance cools.
Learn more about Heating curve, here:
https://brainly.com/question/29592874
#SPJ1
In an experiment Teresa's measures 15.5 mL of water she must have used a
Answers
Answer:
transfer pipet that had markings every 0.1 mL.
Explanation:
The nucleus of an atom contains which subatomic partcles?
Answers
Answer:
The nucleus contains two types of subatomic particles, protons and neutrons. The protons have a positive electrical charge and the neutrons have no electrical charge. A third type of subatomic particle, electrons, move around the nucleus. The electrons have a negative electrical charge.
Solid Snake is being chased up the communications tower. Solid Snake is 75 kg and is carrying 18 kg of equipment. If Solid Snake climbs 27 flights of stairs, each 5.5 m in height, how much work was done against gravity?
a.
4.04 kJ
b.
109.15 kJ
c.
135.34 kJ
d.
5.01 kJ
Answers
Solid Snake (75 kg) carries 18 kg of equipment. If Solid Snake climbs 27 flights of stairs, each 5.5 m in height, the work done against gravity is 135.34 kJ (c).
What is work?In physics, work is the energy transferred to or from an object via the application of force along a displacement.
In this problem, the force that does the work is the one that opposes the weight.
Step 1: Calculate the total weight of the system.Solid Snake is 75 kg and is carrying 18 kg of equipment. The total mass is:
m = 75 kg + 18 kg = 93 kg
We can calculate the weight using Newton's second law of motion.
F = m . g = 93 kg . 9.8 m/s² = 911.4 N
Step 2: Calculate the total displacement.Solid Snake climbs 27 flights of stairs, each 5.5 m in height.
27 . 5.5 m = 148.5 m
Step 3: Calculate the work done.We will use the definition of work.
w = F . s = (911.4 N) . (148.5 m) . (1 kJ/1000 J) = 135.34 kJ
Solid Snake (75 kg) carries 18 kg of equipment. If Solid Snake climbs 27 flights of stairs, each 5.5 m in height, the work done against gravity is 135.34 kJ (c).
Learn more about work here: https://brainly.com/question/62183
d) Would the following molecules be attracted to a magnet? Briefly explain i. B ii. C iii. HCI Tutorial Roo to be handed in Monday 9th May 2022 b 16.00

Answers
The field of magnetic materials research and development is frequently reinvented.
Thus, Work on permalloys, ferrites, transport processes, and magnetic resonance in bulk and thin film samples dominated the magnetic material.
Magnetic "bubble films"—perpendicularly magnetized domains that could store and alter information—and the quick advancements in amorphous magnetic alloys drew a lot of attention in the 1970s.
The introduction of Fe-Nd-B permanent magnets and the dramatic acceleration of activity in magnetic thin films and surfaces, as well as continuous advancements in amorphous magnetic alloys, were all seen in the 1980s.
Thus, The field of magnetic materials research and development is frequently reinvented.
Learn more about Magnetic material, refer to the link:
https://brainly.com/question/31728739?
#SPJ1
4. Identify the conjugate acid – base pairs in the following equations;
H2PO4- + OH- HPO42- + H2O
Answers
Answer:The correct options is, H2O and H3O+
Explanation:
According to the Bronsted Lowry conjugate acid-base pair concept, an acid is a substance which donates protons and a base is a substance which accepts protons.
From the given options, and represents a base-conjugate acid pair.
The balanced reaction will be :H2O +H+ = H3O+
In this, H2O is a base which accepts a proton to form conjugate acid .
Hence, the correct options is,H2O and H3O+
Taking into account the Brønsted-Lowry acid-base theory, the conjugate acid – base pairs are H₂PO₄²⁻/HPO₄⁻ and H₂O/OH⁻.
Brønsted-Lowry acid-base theoryThe Brønsted-Lowry acid-base theory (or the Brønsted-Lowry theory) identifies acids and bases based on whether the species accepts or donates protons or H⁺.
According to this theory, acids are proton donors while bases are proton acceptors. That is, an acid is a species that donates an H⁺ proton while a base is a chemical species that accepts an H⁺ proton from the acid.
So, reactions between acids and bases are H⁺ proton transfer reactions, causing the acid to form its conjugate base and the base to form its conjugated acid by exchanging a proton.
In other words, a conjugate base is an ion or molecule resulting from the acid that loses the proton, while a conjugate acid is an ion or molecule resulting from the base that gains the proton:
acid + base ⇄ conjugate base + conjugate acid
Conjugate acid – base pairs in this caseIn this case, you know
HPO₄⁻ + OH⁻ → H₂PO₄²⁻ + H₂O
HPO₄⁻ behaves like acid because donates an H⁺ proton while OH⁻ behaves like base because accepts an H⁺ proton from the acid.
So, H₂PO₄²⁻ is the conjugate base of the HPO₄⁻ and H₂O is conjugate acid of OH⁻.
In summary, the conjugate acid – base pairs are H₂PO₄²⁻/HPO₄⁻ and H₂O/OH⁻.
Learn more about the Brønsted-Lowry acid-base theory:
brainly.com/question/12916250
brainly.com/question/1191429
brainly.com/question/4000152
brainly.com/question/12808135
#SPJ2
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
n a combination redox reaction, two or more ____________ , at least one of which is a(n) ____________ , form a(n) ____________ . General Reaction: ____________ In a decomposition redox reaction, a(n) ____________ forms two or more ____________ , at least one of which is a(n) ____________ . General Reaction: ____________ In double-displacement (metathesis) reactions, such as precipitation and acid-base reactions, ____________ of two ____________ exchange places; these reactions ____________ redox processes.General Reaction: ____________ In solution, single-displacement reactions occur when a(n) ____________ of one ____________ displaces the ____________ of another. Since one of the ____________ is a(n) ____________ , a
Answers
Answer:
In a combination redox reaction, two or more reactants, at least one of which is a(n) element, form a(n) compound. General Reaction: X + Y > Z
In a decomposition redox reaction, a(n) compound forms two or more products, at least one of which is a(n) element. General Reaction: Z>X+Y
In double-displacement (metathesis) reactions, such as precipitation and acid-base reactions, atoms (or ions) of two compounds exchange places; these reactions are not redox processes. General Reaction: AB+CD>AD+CB
In solution, single-displacement reactions occur when a(n) atom of one element displaces the atom of another. Since one of the reactants is a(n) element, all single-displacement reactions are redox processes. General Reaction: X+YZ>XY+Z
Explanation:
In a combination redox reaction, two or more reactants, at least one of which is a(n) element, form a(n) compound.
General Reaction: X + Y > Z
In the reaction scheme above, X combines with Y to give Z as a product.
In a decomposition redox reaction, a(n) compound forms two or more products, at least one of which is a(n) element.
General Reaction: Z>X+Y
In the reaction scheme above, Z decomposes to X and Y
In double-displacement (metathesis) reactions, such as precipitation and acid-base reactions, atoms (or ions) of two compounds exchange places; these reactions are not redox processes since there are no changes occurring in the oxidation number of the atoms (or ions) involved.
General Reaction: AB+CD>AD+CB
In the reaction scheme above, B and D exchange places in their respective compounds
In solution, single-displacement reactions occur when a(n) atom of one element displaces the atom of another. This type of reaction is due to the difference in the reactivities of the elements. The more reactive atom of one element displaces the least reactive atom of another element from its solution.
Since one of the reactants is a(n) element, all single-displacement reactions are redox processes.
General Reaction: X+YZ>XY+Z
In the reaction scheme above, X displaces Z from the compound YZ.
which nucleus experiences a stronger net force holding its protons and neutrons togeter unanium or or barium
Answers
Answer:
Uranium experiences a stronger net force holding its protons and neutrons together than barium.
Explanation:
Which part of the cell is the cathode?
X
Y
W
Z

Answers
Y
Answer:
I think negative cell Y is cathode
Okghhjhyuknji
Hdhdnsbdhjsms dnddms dnjdke
Answers
Answer:
thanks for the points
take care bye
What is Kb for the conjugate base of HF (Ka = 6.8 × 10⁻⁴)?
Answers
The Kb for the conjugate base of HF, Fluoride ion is 1.470588 * 10^-11
The conjugate base of HF (hydrofluoric acid) is fluoride ion, F-
Ka of HF or hydrofluoric acid is given as 6.8 × 10⁻⁴
Kw = Ka * Kb
Where,
Kb = Base dissociation constant
Kw = Water dissociation constant or equilibrium constant
Ka = Acid dissociation constant
Kw = [H+] [OH-] = 1.0 X 10-14 at room temperature
The formula can be rewritten as,
Kb = (Kw / Ka)
= (10^-14) / (6.8 * 10^-4)
= 1.470588 * 10^-11
Therefore, the kb for the conjugate base of HF, Fluoride ion is 1.470588 * 10^-11
To know more about Conjugate base
https://brainly.com/question/13872450
#SPJ1
Which shows an isomer of the molecule below?

Answers
Answer:
The answer is B, in this case.
Explanation:
An isomer is a molecule with the same number of atoms as another compound, but they differ in arrangement of the atoms.
1.75 L of a 12.0 M HCI stock solution is diluted to a new volume 25.0 L. What is the concentration of
this new solution?
Answers
The concentration of the new solution, given that the stock solution has a concentration of 12.0 M is 0.84 M
How do i determine the concentration of the new solution?First, we shall list out the given parameters from the question. This is shown below:
Volume of stock solution (V₁) = 1.75 LConcentration of stock solution (M₁) = 12.0 MVolume of new solution (V₂) = 25.0 L Concentration of new solution (M₂) =?Using the dilution formula, we can easily obtain the concentration of the new solution as follow:
M₁V₁ = M₂V₂
12 × 1.75 = M₂ × 25
21 = M₂ × 25
Divide both side by 25
M₂ = 21 / 25
= 0.84 M
Thus, we can conclude that the concentration of the new solution is 0.84 M
Learn more about dilution:
https://brainly.com/question/15022582
#SPJ1
What is the gravitational potential energy of a 1500-kg truck resting on top of a 550-m hill on earth?( earth’s gravitational pull is 9.8m/s2).
Answers
Answer:
E = 8085 kJ
Explanation:
Given that,
The mass of a truck, m = 1500 kg
Height, h = 550 m
We need to find the gravitational potential energy of the truck. It can be calculated as follows :
\(E=mgh\)
Put all the values,
\(E=1500\times 9.8\times 550\\\\E=8085000\ J\\\\or\\\\E=8085\ kJ\)
So, the gravitational potential energy is 8085 kJ.
Predict and explain the structure of the major and minor products when hydrogen bromide is added to 2-methylbut-2- ene, (Ch3)2CCHCH3
Pls help with homework!!!!
Answers
When hydrogen bromide is added to 2-methylbut-2-ene, two products are expected to be produced: 2-bromo-2-methylbutane (major product) and 3-bromo-2-methylbutane (minor product).
The addition of HBr to 2-methylbut-2-ene follows the Markovnikov addition rule. This means that the hydrogen atom and the bromine atom will add to the carbon atoms in the double bond, such that the hydrogen atom adds to the carbon with the greater number of hydrogen atoms, and the bromine atom adds to the carbon with the lesser number of hydrogen atoms.
In this case, the hydrogen atom will attach to the second carbon atom, which has three hydrogen atoms, while the bromine atom will attach to the third carbon atom, which has only one hydrogen atom. This produces the major product, 2-bromo-2-methylbutane.
The formation of the minor product, 3-bromo-2-methylbutane, occurs due to the rearrangement of the carbocation intermediate formed during the addition reaction. The carbocation can rearrange either by shifting a methyl group from the second to the third carbon, or by shifting a hydrogen atom from the third to the second carbon. This rearrangement produces the minor product, 3-bromo-2-methylbutane.
In conclusion, the addition of HBr to 2-methylbut-2-ene produces two products: 2-bromo-2-methylbutane (major product) and 3-bromo-2-methylbutane (minor product). The major product forms due to Markovnikov addition rule, while the minor product forms due to carbocation rearrangement.
for more such questions on products
https://brainly.com/question/16859279
#SPJ8
When 6.13 g of a certain molecular compound X are dissolved in 90. g of formamide (NH2COH), the freezing point of the solution is measured to be 2.1 °C. Calculate the molar mass of X. If you need any additional information on formamide, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits.
Answers
Answer:
321.6 g/Mol
Explanation:
mass of solvent in kilograms = 90g/1000 = 0.09 Kg
Given that;
ΔTf = Kf . m . i
Where;
Kf = freezing point constant = 4.25 °C/Kg mol
m = molality of the solution
i = Van't Hoff factor = 1 (since the substance is molecular)
ΔTf = freezing point of pure solvent - freezing point of solution
freezing point of pure solvent = 3 °C
ΔTf = 3 °C - 2.1 °C
ΔTf = 0.9 °C
0.9= 4.25 * 6.13/M/0.09 * 1
0.9= 26.0525/M * 1/0.09
0.9 = 26.0525/0.09 M
0.9 * 0.09M = 26.0525
M = 26.0525/0.9 * 0.09
M= 321.6 g/Mol
a chemical reaction experiment was carried out with the objective of comparing if a new catalyst b would give higher yields than the old catalyst a. the experiment was run on five different batches of raw material which were known to be quite different from one another. each batch was divided into two portions to which a or b was applied at random. the data collected are given in the following table: Catalyst 10 30 28 18 23 22 21 12 a). Explain the experimental design (b)_ Carry out the appropriate t-test.
Answers
According to the problem, we need to determine whether new catalyst B would give higher yields than old catalyst A. The given data is a paired sample, because both the catalysts were experimented on 6 different batches of raw materials.
(a) Explain the experimental design.
Here the data collected is from 6 different raw materials, which were divided into two portions. Therefore, two data were obtained for each of the 6 raw materials, which implies in scientific terms that repeated data was obtained for each of the 6 raw materials. Thus, this type of design is known as Repeated-Measures Design.
(b) Carry out the appropriate t test.
For testing whether the new catalyst B will give higher yields than old catalyst A, a Paired-Sample t-test needs to be performed. The test will be performed using R Studio. The R codes and output are as below.
R CODE
# Load the Data A <- c(9,19,28,22,18,8) B <- c(10,22,30,21,23,12) # Paired Sample t-test t.test(A,B,paired = TRUE,alternative = "less")
R OUTPUT
# Paired Sample t-test > t.test(A, B, paired = TRUE, alternative = less) Paired t-test = data: A and B t = -2.6458, df = ">
The decision rule for this test is: "If the p-value < 0.05, then reject the null hypothesis, under 0.05 level of significance; otherwise accept the null hypothesis".
Decision: As p-value (= 0.02283) < 0.05, so we decide to REJECT the null hypothesis, under 0.05 level of significance.
Conclusion: As the null hypothesis is to be rejected, so we can conclude that "there is sufficient evidence conclude that the new catalyst B gives a higher yield than old catalyst A".
(c) Construct a 95% confidence interval for the difference between catalysts A and B.
From part (b),
we have obtained the R output about the 95% confidence interval for the difference between catalysts A and B as:
Therefore, the 95% confidence interval for the difference between catalysts A and B is (-∞, -0.56).
In frequency statistics, the confidence interval (CI) is the range of estimated values for an unknown parameter. Confidence intervals are computed at the specified confidence level. A 95% confidence level is the most common, but other levels such as 90% and 99% are sometimes used . The confidence level represents the proportion of corresponding CIs over time that contain the true value of the parameter. For example, 95% of all intervals computed at the 95% level must contain the true value of the parameter.
Factors that affect the width of the CI include confidence level, sample size, and within-sample variability. All other things being equal, the larger the sample, the narrower the confidence interval. Similarly, the more varied the sample, the wider the confidence interval, and the higher the confidence level, the wider the confidence interval.
Learn more about confidence interval here : https://brainly.com/question/16742675
#SPJ4
Which describes the correct procedure when converting a number from scientific notation to standard notation? A If the power of 10 is positive, move the decimal point to the left. BIf the power of 10 is positive, move the decimal point to the right. CIf the number being converted is greater than 10, move the decimal point to the left. DIf the number being converted is greater than 10, move the decimal point to the right.
Answers
Answer:
I think it's B
Explanation:
I think it is b because if the number is positive the zeroes will be on the left so you move the decimal to the right to get rid of the zeroes.
Need help with this please thanks
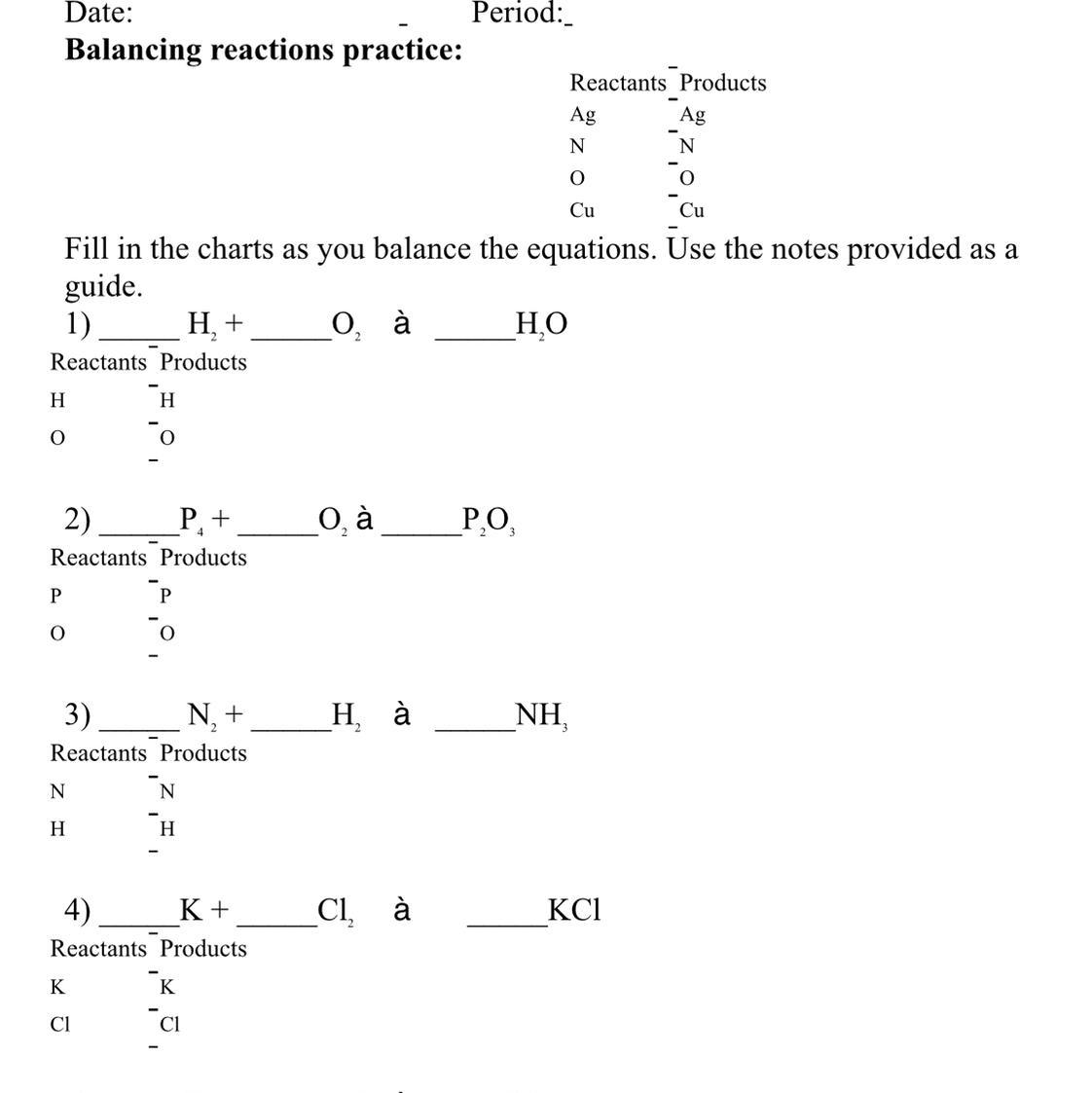
Answers
Answer: 1. \(2H_2+O_2\rightarrow 2H_2O\)
2. \(P_4+3O_2\rightarrow 2P_2O_3\)
3. \(N_2+3H_2\rightarrow 2NH_3\)
4. \(2K+Cl_2\rightarrow 2KCl\)
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
The given equations are balanced as:
1. \(2H_2+O_2\rightarrow 2H_2O\)
2. \(P_4+3O_2\rightarrow 2P_2O_3\)
3. \(N_2+3H_2\rightarrow 2NH_3\)
4. \(2K+Cl_2\rightarrow 2KCl\)