Answers
Answer:
0.67 moles of NH3 are required to react with 32.2 grams of O2.
Explanation:
To find the number of moles of NH3 required to react with 32.2 grams of O2, you will need to balance the chemical equation for the reaction and use the balanced equation to determine the mole ratio between the reactants.
The balanced equation for the reaction of NH3 with O2 is:
2 NH3 + 3 O2 -> 2 N2 + 6 H2O
From this equation, we can see that for every 2 moles of NH3 that react, 3 moles of O2 are also consumed. Therefore, the mole ratio between NH3 and O2 is 2:3.
Since we are given the mass of O2 in grams and we want to find the number of moles of NH3, we can use the molar mass of O2 to convert the mass to moles. The molar mass of O2 is 32.0 g/mol. Therefore, 32.2 grams of O2 is equivalent to 32.2 / 32.0 = 1.00625 moles of O2.
To find the number of moles of NH3 required, we can use the mole ratio to convert from moles of O2 to moles of NH3. Since the ratio is 2:3, we can multiply the number of moles of O2 by (2/3) to find the number of moles of NH3:
1.00625 moles O2 * (2 moles NH3 / 3 moles O2) = 0.67 moles NH3
This means that 0.67 moles of NH3 are required to react with 32.2 grams of O2.
Related Questions
2
Select the correct answer
in a redex reaction, what folle does the reducing agent play?
OA. it gives up electrons
OB. it keeps electrons
OC. it takes electrons
OD. it takes onygen atoms
Answers
Answer:
A. it gives up electrons
Explanation:
In a redox reaction, the reducing agent is the element or compound that undergoes oxidation and gives up electrons. The oxidizing agent is the element or compound that undergoes reduction and gains electrons.
Hope that helps.
Part D What evidence can be used to support the fact that oxidation, reduction, or both took place in test tube 5?
Answers
Copper atoms (Cu) were created by reducing copper ions (Cu2+). An illustration of a reduction reaction is this. (Mg2+).To create Mg2+ ions, Mg atoms lost their electrons. An example of an oxidation reaction is this.
Oxidation and reduction occurred in test tube 5. Here is the equation for the reaction that took place:MgSO4 (aq) + Cu (s) CuSO4 (aq) + MgCuSO4's blue hue was neutralised to a solid copper hue. Copper atoms (Cu) were created by reducing copper ions (Cu2+). An illustration of a reduction reaction is this. Cu atoms are created when Cu2+ gains electrons. Due to the presence of magnesium ions (Mg2+), the solution was greenish-yellow.To create Mg2+ ions, Mg atoms lost their electrons. An example of an oxidation reaction is this. Two electrons were lost by the magnesium atom, leaving two electrons behind.
As a result, test tube 5 experienced both oxidation and reduction.It is possible to prove that oxidation, reduction, or both took place through the reaction's electron transfer and colour changes. A reduction reaction was seen when copper ions transformed into copper atoms. An oxidation reaction was evident when magnesium atoms transformed into magnesium ions.
For more question on reaction
https://brainly.com/question/11231920
#SPJ8
Calculate the percent mass of hydrogen in ethane (C2H6).
Answers
Answer:
80.% carbon and 20.% hydrogen
Explanation:
Ethane has molecular formula C2H6. So What is the composition of carbon and hydrogen in ethane. its alot to explain but yea thats wht i got
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
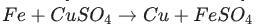
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
Which of the following occurs in an ionic bond? *
O Oppositely charged ions attract.
OTwo atoms share two electrons.
Two atoms share more than two electrons.
Like-charged ions attract.
Answers
Answer:
Oppositely charged ions attract.
Explanation:
In an ionic bond oppositely charged ions attracts one another by electrostatic attraction.
To form ionic bonds, chemical species must lose or gain electrons. Most metals will lose their electrons due to electropositive nature. Non-metals gains the electrons. When a substance lose electrons, it becomes positively charged. A non-metal gaining electrons becomes negatively charged. The attraction between these two species forms the ionic bond.What circumstances during the French Revolution permitted the metric system to gain a foothold?
Answers
Answer:
The trade was difficult with the old system.
Explanation:
The existing system for the measurement of goods did not work well in the trade because there are different system used by the people of different cities so metric system was used in the whole country in place of the old system of measurement during the French Revolution in order to make the trade easier and quick across the country. This metric system is extensively used in the trade as compared to the old system of measurement due to its easiness and fast.
HELP PLEASE !
Stuart Wilkinson, the engineer of "Chew-Chew" said, "we stole the idea of eating food
from the biological world, but we are marrying that idea to useful robotic capabilities."
Which of the following would not be an application of Stuart Wilkinson's theory?
O
A. a garden cultivator that feeds on soil
B. a leaf mulcher that feeds on foliage
C. a lawn mower that feeds on grass clippings
D. a recycling truck that feeds on petroleum
E. a trash compactor that feeds on garbage
Answers
The following which would not be an application of Stuart Wilkinson's theory as regards marrying the idea of useful robotic capabilities in terms of eating food is a recycling truck that feeds on petroleum and is denoted as option D.
What is a Robot?This is referred to as a machine which is able to replicate certain human movements and functions automatically through the use of an external control device.
Since Stuart Wilkinson's theory intends to marry the idea of useful robotic capabilities in terms of eating food then a recycling truck that feeds on petroleum isn't accurate because petroleum isn't a source of food.
Read more about Robot here https://brainly.com/question/27788887
#SPJ1
the number of mole of iron produced from 0.216 mole of aluminum is:
3 FeO (l) + 2 Al (l) → 3 Fe (l) + 1 Al2O3
Answers
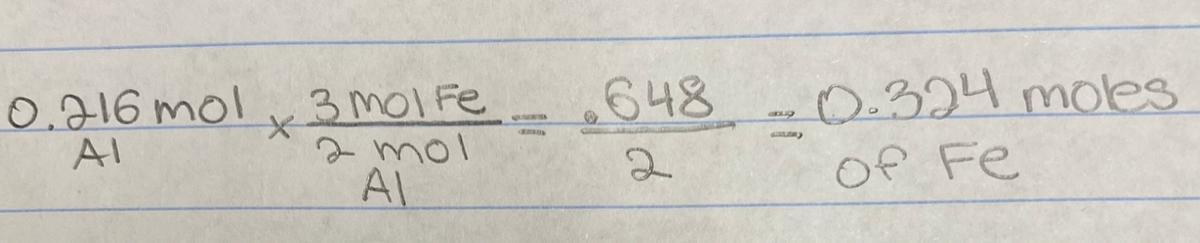
Which term related to electromagnetism applies only to magnetic force
Answers
What is the half-life in minutes of a compound if 75.0 percent of a given sample decomposes in 40.0 minutes? Assume first-order kinetics.
Answers
Answer:
See below
Explanation:
.75 = 1/2^(40/h)
log .75 / ( log 1/2) = 40 / h
h = half life = 96.37683 min
To solve this type of question we must know the concept of rate law. The half-life of a compound is 23 years for 75% of a given sample of the compound decomposes in 40 minutes.
What is the expression for rate law for first order kinetics?There are two kinds of rate law in chemical kinetics one is differential rate law and other is integrated rate law
The rate law for first order kinetics is
\(k=\frac{2.303}{t}log\frac{a}{a-x}\)
Where
k - rate constant
t - time passed by the sample
a - initial amount of the reactant
a-x - amount left after the decay process
The expression for the half-life of the compound is
\(t_{1/2} = \frac{0.693}{K}\)
Where k is the rate constant
t = 40 minutes
a = 100 g
a-x = 100 g - 75g = 25 g
(Since it is given that 75% of the given compound decomposes)
Then,
\(k=\frac{2.303}{40}log\frac{100}{25}\)
=0.03years⁻¹
\(t_{1/2} = \frac{0.693}{0.03}\)
=23 years
Therefore, the half-life of the given compound is 23years.
Learn more about rate law for first order kinetics here:
https://brainly.com/question/12593974
#SPJ5
which option correctly describes a covalent species that has four electron groups around the central atom? select all that apply.
Answers
The option that correctly describes a covalent species that has four electron groups around the central atom are:
The ideal bond angle for a four-electron system is 109.5°.If all four electron pairs are bonding pairs, the shape of the system is tetrahedral.A bent shape is observed if there are two bonding pairs and two lone pairs.What is the covalent species that has four electron groups around the central atom?It should be noted that when a molecule have four electron groups and it is been found around the central atom and this will orients the four groups in the direction of a tetrahedron.
However when there are four atoms attached to these electron groups, in Tetrahedral Geometry, molecular shape will also be also tetrahedral.
Instance of this is Methane (CH 4), in conclusion, in the case whereby all four electron pairs are bonding pairs, then it can be concluded that the shape of the system is tetrahedral.
Learn more about atom at:
https://brainly.com/question/6258301
#SPJ1
missing options:
The ideal bond angle for a four-electron system is 109.5°.
If all four electron pairs are bonding pairs, the shape of the system is tetrahedral.
The eletron are not bonding in the system.
A bent shape is observed if there are two bonding pairs and two lone pairs.
Do you have to have both number and unit to be considered a measurement
Answers
Answer: Yes!
To be considered a measurement, you need the number AND the unit. Without the unit, the measurement is just a number, and without the number, the measurement is just a word :)
How many kilojoules of heat are needed to raise the temperature of 10g of aluminum from 22 degrees C to 55 degrees C, if the specific heat of aluminum is .901 j/gc?
Answers
Answer:
name four agricultural inputs are subsidized by the government
0.297 kJ of heat is needed to raise the temperature of 10g of aluminum from 22 degrees Celsius to 55 degrees Celsius.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Given,
mass = 10g
c = 0.901J/g⁰C
Initial temperature (T₁) = 22⁰C
Final Temperature (T₂) = 55⁰C
Q = mcΔT
= 10 × 0.901 × (55 -22)
= 297.33 J = 0.297 kJ
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
The reaction of nitrogen and hydrogen to make ammonia is important because it provides fertilizer for growing food, and is because ammonia is needed to make other nitrogen-containing compounds. At room temperature, ∆G° and ∆H° for the reaction are both negative.
N2(g) + 3 H2(g) --> 2 NH3(g)
Which two of the following statements about this reaction are true?
- Adding an appropriate catalyst makes the reaction more spontaneous
- Increasing the temperature lowers the activation energy of the reaction
- Increasing the temperature makes the reaction less spontaneous
- The entropy change for the reaction is positive
- Increasing the pressure makes the reaction more spontaneous
Answers
The two statements that are true about the reaction are:
Adding an appropriate catalyst makes the reaction more spontaneous Increasing the temperature lowers the activation energy of the reactionWhat is a catalyst?A catalyst is described as a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction.
If we happen to increase the temperature, it provides more kinetic energy to the reactant molecules and this makes it more likely to overcome the activation energy barrier and engage in the reaction.
Learn more about catalyst at:
https://brainly.com/question/666246
#SPJ1
Two examples of potential energy are ________________________ and ______________________. Examples can include _________________________ and __________________________________.
Answers
Two examples of potential energy are gravitational potential energy and elastic potential energy.
What is Gravitational Potential Energy ?Gravitational potential energy is the energy stored in an object due to its position in a gravitational field. For example, a book placed on a shelf has gravitational potential energy because it has the potential to fall to the ground due to the force of gravity.
What is Elastic Potential Energy ?Elastic potential energy is the energy stored in an object when it is deformed, such as when a spring is compressed or stretched. For example, a compressed spring has elastic potential energy because it has the potential to return to its original shape and release the stored energy when it is released.
Other examples of potential energy include chemical potential energy, which is the energy stored in the bonds between atoms in a molecule, and nuclear potential energy, which is the energy stored in the nucleus of an atom.
To know more about Chemical potential energy , visit :
https://brainly.com/question/11440456
#SPJ1
What is the formula for manganese (IV) oxide?
A. MnO4
B. Mn40
C. MNO2
D. Mn20
Answers
The formula of manganese (IV) oxide is MNO2
The formula for manganese (IV) oxide is \(MnO_4\). Therefore, the correct option is A.
A manganese atom is bonded to four oxygen atoms to produce an inorganic compound known as manganese(IV) oxide with the chemical formula \(MnO_4\). It is often referred to as manganese dioxide. The +4 oxidation state of a manganese compound is indicated by the Roman numeral "IV" in the name.
Pyrolusite, a naturally occurring mineral, is a dark brown or black solid consisting of manganese(IV) oxide. Due to its favorable properties, it is often employed in a wide variety of applications. It acts as a catalyst, especially when breaking down hydrogen peroxide. Additionally, it is used to make batteries, ceramics, glass and pigments.
Therefore, the correct option is A.
Learn more about chemical formula, here:
https://brainly.com/question/32018188
#SPJ6
2) What is the pH when the concentration of [H*] is 4.22 x 108
3) What is the pH when the pOH is 7
4) What is the concentration of [OH¹¹] when the pH is 4.3
5) What does it mean to be diprotic?
6) What does amphoteric mean?
7) WHat can you use to measure pH?
8) WHat does a buffer solution do?
9) What does titration do?
10) What is the difference between a strong acid and a weak acid?
Answers
2. The pH is 4.22 × 10⁸. 3. pH is 7, 4. pOH is 9.7, 5. diprotic is explained below, 6. Amphoteric is explained below, 7. pH meter, 8. A buffer solution is explained below, 9. Titration is explained below, 10. Difference between strong and weak acids is explained below.
2. pH = -log[H⁺]
pH = -log(4.22 x 10⁻⁸)
pH ≈ 7.375
3. pH + pOH = 14
pH + 7 = 14
pH = 14 - 7
pH = 7
4. pH + pOH = 14
pOH = 14 - pH
pOH = 14 - 4.3
pOH ≈ 9.7
Now,
[H⁺] × [OH⁻] = 1.0 x 10⁻¹⁴
[OH⁻] = 1.0 x 10⁻¹⁴ / [H⁺]
[OH⁻] = 1.0 x 10¹⁴ / 10^(-pOH)
[OH⁻] = 1.0 x 10⁻¹⁴ / 10^(-9.7)
[OH⁻] ≈ 1.99 x 10⁻⁶ M
5. Being diprotic means that a molecule or ion can donate or release two protons (H⁺ ions) in an acid-base reaction.
6. Amphoteric refers to a substance that can act as both an acid and a base.
7. The pH can be measured using a pH meter or a pH indicator.
8. A buffer solution is a solution that can resist changes in pH when small amounts of acid or base are added to it.
9. Titration is a laboratory technique used to determine the concentration of a solution by reacting it with a solution of known concentration (titrant) of another substance.
10. A strong acid is an acid that completely ionizes in water, releasing all of its hydrogen ions. A weak acid is an acid that does not completely dissociate into ions when dissolved in water.
Learn more about pH, here:
https://brainly.com/question/2288405
#SPJ1
If you have 3.5 L of He to blow up balloons... at STP...
a) How many moles of He do you have?
b) How many grams of He do you have?
Answers
Answer:
B
Explanation:
What is the correct formula that would result from the combination of the two ionic species? Cu2+ and SO42-
Answers
CuSO4.
brainliest???
help me please please

Answers
Answer:
this shows that the plates are very active in that region. The plate collide a lot.
Explanation:
What is the volume (in liters) of 1.51 x10^24 molecules of Argon gas at STP?
Answers
Answer:
Explanation:
The molar volume of a gas at STP (Standard Temperature and Pressure), which is equal to 22.4 liters.
There are 6.02x10^23 molecules in 1 mole of Argon.
So for 1.51x10^24 molecules of Argon, the volume at STP should be
(1.51x10^24 / (6.02x10^23)) * 22.4
= 56.19 liters
Moles of Argon:-
No of molecules/Avagadro's constant1.51×10^24/6.022×10^23=2.5molNow
1mol of gas contains 22.4L2.5mol contains:-\(\\ \rm\Rrightarrow 2.5(22.4)=56L\)
Name the monosaccharides that make up each of the
disaccharides and give a common food source of each disaccharide.
Answers
Answer:
Glucose. Fructose. Galactose.
Explanation:
Glucose +fructose=succrose(normal sugar)
Glucose+Glucose= Maltose(wheat sugar)
Glucose+Galactose= Lactose(milk sugar)
C + H2O → CO + H2
What is the starting substance?
Answers
Answer:
Explanation:
Carbon and water = carbon dioxide and hydrogen
Choose the best answer for this question.
Describe way(s) that your speed could change as you jog along a park's path.
O speed up
all of these, except none of these
O none of these
O slow down
Answers
Your speed could change in several ways as you jog along a park's path. Option 2.
Speed of joggingOne possible way is that you could speed up if you increase your pace or start running instead of jogging. This could happen if you feel more energized, motivated, or if you need to catch up with someone.
Conversely, your speed could slow down if you get tired, experience muscle fatigue, or encounter an uphill section of the path that requires more effort. Other factors such as weather conditions, terrain, or distractions can also affect your speed.
Therefore, the correct answer would be "all of these, except none of these," as there are various factors that can cause changes in your jogging speed along a park's path.
More on speed can be found here: https://brainly.com/question/14969657
#SPJ1
Pls help me asap I don’t know how to do this

Answers
Answer:
\(3\)Explanation:
Here, we want to know the number of moles of water molecules present in the hydrate
Firstly, we need to get the percentage by mass of water
To get this, we have to divide the percentage lost to heating by the total mass and convert to a percentage
Mathematically, we have that as:
\(\frac{450-376.76}{450}\times\text{ 100 \% = 16.276 \%}\)What this means is that the percentage by the molar mass of water is still 16.276%
To get the number of moles of water, let us call it n
The molar mass of water divided by the total mass of the hydrate as a percentage is equal to 16.276%
Thus, we have it that;
The molar mass of n moles of water is (18 * n = 18n g/mol : 1 mole of water has a mass of 18g)
For the anhydrous part, the molar mass is 278 g/mol
Thus, we have the percentage as:
\(\begin{gathered} \frac{18n}{278\text{ + 18n}}\times100\text{ \% = 16.276} \\ \end{gathered}\)From the above, we can get the value of n
Thus, we have that as:
\(\begin{gathered} \frac{100(18n)}{278\text{ + 18n}}\text{ = 16.276} \\ \\ 16.276(278\text{ + 18n\rparen = 1800n} \\ 4524.60\text{ +}292.96n\text{ = 1800n} \\ 1800n-292.96n\text{ = 4524.60} \\ 1507.04n\text{ = 4524.60} \\ n\text{ = }\frac{4524.60}{1507.04} \\ n\text{ = 3} \end{gathered}\)The number of hydrate molecule is 3
If a chemist wishes to produce 500 mL of 2.0 M HCl, how much concentrated 12 M HCl should he measure out? (dilution problem)
Answers
To solve the dilution problem, we can use the formula:
C₁V₁ = C₂V₂
Where:
C₁ is the initial concentration,
V₁ is the initial volume,
C₂ is the final concentration, and
V₂ is the final volume.
Given:
C₁ = 12 M (concentration of the concentrated HCl),
V₂ = 500 mL (final desired volume),
C₂ = 2.0 M (final desired concentration).
Let's solve for V₁:
C₁V₁ = C₂V₂
(12 M)(V₁) = (2.0 M)(500 mL)
Cross multiplying:
12V₁ = 2.0 × 500
12V₁ = 1000
V₁ = 1000 / 12
V₁ ≈ 83.33 mL
Therefore, the chemist should measure out approximately 83.33 mL of concentrated 12 M HCl to produce 500 mL of 2.0 M HCl.\(\)
Please help me. I can’t give too much points bc I don’t have too much.
QUANTUM=
The relationship between energy and frequency=
E = hy
EN
h=
V=
Albert Einstein: PHOTONS
Answers
During a day that is 39°F outside, it is also mid-fall. There is a nearby pond, what assumptions can you make about the temperature within that pond on that day?

Answers
The temperature should be between 30 °F and 60 °F because the temperature outside is 39 °F, so the water in the pond should have a temperature range that includes this temperature.
Therefore, the answer is B.
Why does the solubility of alkaline earth metal hydroxides in water increase down the group?
Answers
Answer:
The solubility of alkaline earth metal hydroxides in water increases down the group because the size of the metal cation increases as you move down the group. This increase in size results in a decrease in the cation's charge density, which makes it less able to attract and hold onto hydroxide ions. As a result, the hydroxides become more soluble in water as you move down the group. Additionally, the lattice energies of the hydroxides decrease down the group, making it easier to break apart the crystal lattice structure and dissolve the hydroxides in water.
PLEASE HELP!!!
How many calories are in 4,180 joules?
Answers
Answer:
To convert joules to calories, you can use the conversion factor:
1 calorie = 4.184 joules
To find out how many calories are in 4,180 joules, divide the given value by the conversion factor:
4,180 joules / 4.184 joules per calorie = 0.9 calories (approximately)
Therefore, there are approximately 0.9 calories in 4,180 joules.