How many moles of LINO3 are
equivalent to 525 g LINO3?
(LINO3 = 68.95 g/mol)
[?] mol LINO3
Answers
Answer:
Explanation:
number of moles = mass / molar mass
= 525 / 7+14+(3*14)
= 525 / 63
= 8.33 mol
Related Questions
Common alkaline batteries produce electricity through an electrochemical reaction between zinc metal and manganese(V). Use the form below to complete both the oxidation and reduction half reactions as well as the balanced overall reaction. Zn° + 2 4+
Answers
The oxidation reduction reaction are given below.
Oxidation half reaction:
Zn° →Zn² + 2e-
Reduction half reaction:
2Mn^5 +4e^- → 2Mn^2+
Oxidation and reduction reaction explained.
Belo w are the oxidation and reduction reaction of the common alkaline batteries to produce electricity.
Oxidation half reaction:
Zn° →Zn² + 2e-
Reduction half reaction:
2Mn^5 +4e^- → 2Mn^2+
To balance the overall reaction, we need to multiply each half reaction by appropriate coefficients to ensure that the electrons cancel out.
Here is the balance overall reaction.
2Zn° + 2Mn^5 → 2Zn² + 2Mn²+
The balanced equation shows that in alkaline batteries, zinc metal is oxidized to form zinc ion, while manganese ions are reduced to manganese(II) ions. The oxidation reduction reaction generate an electric current as a result of the flow of electrons.
Learn more about oxidation and reduction below.
https://brainly.com/question/13892498
#SPJ1
what does Le châteliers principle state?
Answers
Hope this helps!
what compound has a molar mass of 37.11 g/mol
Answers
Answer:
Explanation:
The closest I can come to this is
Li C2H5 which would really be weird, but it comes to nearly 37.11. Everything depends on the periodic table you are using.
C2 = 2 * 12.011 = 24.022
H5 = 5 * 1.008 = 5.040
Li = 1 * 6.94 6.94
Total 36.002
3. A polyatomic ion is formed from more than one atom.
True or false
Answers
Answer:
true
Explanation:
when cu(oh)2(s)cu(oh)2(s) is heated, copper(ii) oxide and water are formed. write a balanced equation for the reaction.
Answers
The balanced equation for the thermal decomposition of copper(II) hydroxide (Cu(OH)₂) into copper(II) oxide (CuO) and water (H₂O) is Cu(OH)₂(s) → CuO(s) + H₂O(g). In this reaction, when Cu(OH)2(s) is heated, it decomposes into copper(II) oxide (CuO) and water (H2O). The balanced equation shows that one mole of Cu(OH)₂ produces one mole of CuO and one mole of H₂O.
In this reaction, copper(II) hydroxide is decomposed into copper(II) oxide and water when it is heated. The solid copper(II) hydroxide is converted into solid copper(II) oxide, and water is produced in the gaseous state. It is important to note that the equation is balanced, meaning that the number of atoms of each element is the same on both sides of the equation. In this case, there is one copper atom, two oxygen atoms, and two hydrogen atoms on each side of the equation.
Learn more about the balanced equation: https://brainly.com/question/11904811
#SPJ11
pls help help help!
i give 20 points
pls

Answers
Answer:
His name is Harold
Explanation:
The radioisotope phosphorus-32 is used in tracers for measuring phosphorus uptake by plants. The half-life of phosphorus-32 is 14.3 days. How much time is required for the activity of a sample of phosphorus-32 to fall to 7.34 percent of its original value
Answers
Answer:
54 days
Explanation:
We have to use the formula;
0.693/t1/2 =2.303/t log Ao/A
Where;
t1/2= half-life of phosphorus-32= 14.3 days
t= time taken for the activity to fall to 7.34% of its original value
Ao=initial activity of phosphorus-32
A= activity of phosphorus-32 after a time t
Note that;
A=0.0734Ao (the activity of the sample decreased to 7.34% of the activity of the original sample)
Substituting values;
0.693/14.3 = 2.303/t log Ao/0.0734Ao
0.693/14.3 = 2.303/t log 1/0.0734
0.693/14.3 = 2.6/t
0.048=2.6/t
t= 2.6/0.048
t= 54 days
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
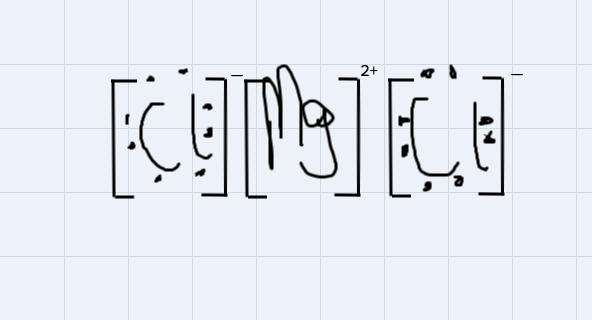
1.
At constant pressure. 50 milliliters (mL) of a gas
at 20°C is heated to 30° C. The new volume of
the gas in milliliters (ml) is equal
Answers
Answer:
\(\boxed {\boxed {\sf V_2=75 \ mL}}\)
Explanation:
Since the pressure is constant, the only variables we need to work with are temperature and volume. We will use Charles's Law, which states the volume of a gas is directly proportional to the temperature. The formula is:
\(\frac{V_1}{T_1}=\frac{V_2}{T_2}\)
Originally, the gas was 50 milliliters at 20 degrees celsius. Substitute these values into the left side of the equation.
\(\frac{50 \ mL}{20 \textdegree C}=\frac{ V_2}{T_2}\)
We don't know the volume of the new gas, but we know the temperature was changed to 30 degrees celsius.
\(\frac{50 \ mL}{20 \textdegree C}=\frac{ V_2}{30 \textdegree C}\)
Since we are solving for the new volume, we must isolate the variable. It is being divided by 30 °Cand the inverse of division is muliplication. Multiply both sides by 30 °C.
\(30 \textdegree C*\frac{50 \ mL}{20 \textdegree C}=\frac{ V_2}{30 \textdegree C}* 30 \textdegree C\)
\(30 \textdegree C*\frac{50 \ mL}{20 \textdegree C}= V_2\)
The units of degrees celsius cancel, so we are left with milliliters as the units.
\(30*\frac{50 \ mL}{20}= V_2\)
\(\frac{1500 \ mL}{20}= V_2\)
\(75 \ mL=V_2\)
The new volume of the gas is 75 milliliters.
Which one please help

Answers
Answer:
C
Explanation:
Adding another Li will balance the equation
2LiNO3 + CaBr2 → Ca(NO3)2 + 2LiBr
According to Maslow, humans devote all their efforts to satisfying ________ needs until they are met. Only when these needs are met can people focus their attention on satisfying the next level of needs.
Answers
According to Maslow, humans devote all their efforts to satisfying lower-level needs until they are met. Only when these needs are met can people focus their attention on satisfying the next level of needs.
Maslow refers to Abraham Maslow, an influential psychologist known for his theory of human motivation and the hierarchy of needs. Maslow's hierarchy of needs is a psychological theory that explains human motivation and the progression of needs from basic survival to higher-level psychological fulfillment.
According to Maslow, human needs can be organized into a hierarchical structure, with lower-level needs needing to be satisfied before higher-level needs become motivating factors. The hierarchy consists of five levels:
Physiological Needs: These are the most basic needs necessary for survival, such as food, water, shelter, and sleep. These needs must be fulfilled before higher-level needs become relevant.
According to Maslow, humans devote all their efforts to satisfying lower-level needs until they are met. Only when these needs are met can people focus their attention on satisfying the next level of needs. These lower-level needs include physiological needs such as food, water, and shelter, as well as safety needs such as feeling secure and free from harm. Once these needs are satisfied, individuals can then focus on satisfying higher-level needs such as social belonging, self-esteem, and ultimately self-actualization.
To know more about devote visit:
https://brainly.com/question/11212628
#SPJ11
suppose 7.00 mol of NaOH reacted with 2.5 mol CaBr2 how many Moles of NaBr would be produced
Answers
When 7.00 mol of NaOH reacts with 2.5 mol of CaBr2, 5.0 mol of NaBr will be produced.
To determine the number of moles of NaBr produced, we need to look at the balanced chemical equation for the reaction between NaOH and CaBr2.
The balanced equation is:
2NaOH + CaBr2 -> 2NaBr + Ca(OH)2
According to the balanced equation, 2 moles of NaOH react with 1 mole of CaBr2 to produce 2 moles of NaBr.
Given that 7.00 mol of NaOH and 2.5 mol of CaBr2 are available, we can determine the limiting reactant. The limiting reactant is the one that is completely consumed first and determines the maximum amount of product that can be formed.
To find the limiting reactant, we compare the moles of each reactant to their stoichiometric coefficients in the balanced equation:
For NaOH: 7.00 mol NaOH * (1 mol CaBr2 / 2 mol NaOH) = 3.50 mol CaBr2
For CaBr2: 2.5 mol CaBr2
The limiting reactant is CaBr2 since it has the smaller amount. Therefore, 2.5 mol of CaBr2 will react completely.
From the balanced equation, we know that 2 moles of NaBr are produced for every 1 mole of CaBr2. Therefore, the number of moles of NaBr produced will be:
2.5 mol CaBr2 * (2 mol NaBr / 1 mol CaBr2) = 5.0 mol NaBr
for more such questions on mol
https://brainly.com/question/29367909
#SPJ11
nucleic acid and carbohydrates are both types of what
Answers
Answer:
macromolecules.
Explanation:
Nucleic acids and carbohydrates are both types of organic molecules known as macromolecules.
Ionic bonding is the result of electron transfer. _______________ electrons and become positive ions. While, _______________ gain electrons an become negative ions.
Answers
Answer:
metal lose electronsnon metal gain electronsWhen the View from Earth is a new moon phase, the View from the Moon would be a
Please help
Answers
Answer:
When the view from Earth is a new moon phase, the view from the Moon would be a full Earth phase.
Explanation:
This is because the new moon phase occurs when the Moon is located between the Earth and the Sun, with the side of the Moon facing the Earth being unlit by the Sun. From the Moon's perspective, the Earth would be fully illuminated by the Sun, and the side of the Earth facing the Moon would be in the full Earth phase.
Why is glass useful for making eyeglasses
Answers
Answer:
because it's the glass that goes on eyeglasses
Answer:
Because it is transparent
Which of these does not describe an ethical dilemma associated with the
field of prosthetics?
A. People may get prostheses to gain an advantage over others.
B. Prostheses allow people to regain bodily functions that have been
lost due to injury or disease.
C. If a prosthesis is rejected by a patient's body, the result could be
death.
D. Athletes might use prostheses to enhance the function of body
parts.
Answers
Prostheses allow people to regain bodily functions that have been lost due to injury or disease.

Prostheses allow people to regain bodily functions that have been lost due to injury or disease does not describe an ethical dilemma associated with the field of prosthetics. Hence, option B is correct.
What is ethical dilemma?An ethical dilemma is a situation where a person is faced with two or more moral principles, values or duties that are in conflict with one another, and the person must choose between them, but there is no clear or obvious right or wrong decision.
In other words, an ethical dilemma is a complex situation that requires a person to weigh the competing moral principles involved and make a difficult decision that may have significant consequences.
Ethical dilemmas can arise in various contexts, such as in personal relationships, in the workplace, or in professional fields like healthcare, law, and business. Here, option B does not describe an ethical dilemma.
Find more on ethical dilemmas:
https://brainly.com/question/28221102
#SPJ7
What is the trend in ionization energy as you go down a group?
answer choices
a. Down the group Ionization energy increases because atomic radius decreases.
b. Down the group Ionization energy decreases because atomic radius decreases.
c. Down the group Ionization energy decreases because atomic radius increases.
d. Down the group Ionization energy increases because atomic radius increases.
Answers
The trend in ionization energies as you go down a group is C. Going down the group Ionization energies decrease as the atomic radius increase.
Definition of Ionization EnergyIonization energy is the energy required to remove an electron from a neutral atom in gaseous form. Also as with the atomic radius, the periodicity of this one element also has the following tendencies:
In a group from top to bottom, the ionization energy of an element decreases because the atomic radius increases, so that the attraction of the nucleus to the outermost electrons becomes weaker and the ionization energy decreases.
In one period, the ionization energy of the elements increases from left to right because the atomic radius is getting smaller, so that the attraction of the nucleus to the outermost atom is getting stronger and the ionization energy is increasing.
Learn more about Ionization Energy at: https://brainly.com/question/11723795
#SPJ4
Why does the sun appear very large compared to the other stars
The other stars are much smaller than our sun.
O The sun is actually the largest star in the universe.
The sun is so bright that it appears larger than the other stars.
The other stars are much farther away from Earth than our sun.
Answers
Answer:
the other stars are much farther away from Earth than our sun
g what is the name of the potassium salt of m-ethylbenzoic acid? a. m-ethyl potassium benzoate b. potassium m-ethylbenzoate c. potassium m-ethylbenzoic d. potassium m-ethylbenzoic acid
Answers
The name of the potassium salt of m- ethyl benzoic acid is potassium m- ethyl benzoate. The correct option is (b).
Potassium m- ethyl benzoate is formed when m- ethyl benzoic acid reacts with potassium hydroxide in an aqueous solution. This reaction is an example of an acid-base reaction, where the acid (m- ethyl benzoic acid) donates a proton (H+) to the base (potassium hydroxide) to form an ionic salt. The general equation for this reaction is:
m- ethyl benzoic acid + potassium hydroxide → potassium m- ethyl benzoate + water
The m- ethyl group of m- ethyl benzoic acid replaces the hydrogen atom in the carboxyl group of the acid. This produces an anion of m- ethyl benzoate, which is then balanced out by the positively charged potassium ion (K+).
Potassium m- ethyl benzoate is an odorless white crystalline solid. It is soluble in water, alcohol, and other organic solvents, and it is used in the synthesis of various drugs and chemicals.
Therefore, the correct answer is (b).
To know more about potassium m- ethyl benzoate, refer here:
https://brainly.com/question/12370141#
#SPJ11
correct name for Ti(SO4)2?
Answers
Answer:
Titanium(IV) Sulfide
Explanation:
How much more average Kinetic Energy do molecules have at 50°C compared to 25°C?
Answers
The kinetic energy is 0.5177 × 10⁻²¹ J more at 50°C compared to 25°C.
The average kinetic energy of a molecule is directly proportional to the absolute temperature of a gas.
KE = ( 3/2 ) ( R / Nₐ ) T
Where T is the temperature of the molecule, R is the gas constant, and Nₐ is Avogadro's number.
Now, R = 8.314 J/mol.K
Avogadro's number, Nₐ = 6.022 × 10²³ atoms/ mol
The average kinetic energy at 50° C is:
T = 50° C = 323 K
KE₁ = ( 3/2 ) × ( R / Nₐ ) × T₁
KE₁ = ( 3 × 8.314 × 323 ) / ( 2 × 6.022 × 10²³ )
KE₁ = 668.90 × 10⁻²³ J
KE₁ = 6.6890 × 10⁻²¹ J
The average kinetic energy at 25°C is:
KE₂ = ( 3/2 ) × ( R / Nₐ ) × T₂
KE₂ = ( 3 × 8.314 × 298 ) / ( 2 × 6.022 × 10²³ )
KE₂ = 617.13 × 10⁻²³ J
KE₂ = 6.1713 × 10⁻²¹ J
Now,
The average kinetic energy of the molecules at 50° C compared to 25° C is:
KE = KE₁ - KE₂
KE = 6.6890 × 10⁻²¹ - 6.1713 × 10⁻²¹
KE = 0.5177 × 10⁻²¹ J
Hence, the average kinetic energy is 0.5177 × 10⁻²¹ J more at 50° C compared to 25° C.
Learn more about kinetic energy here:
https://brainly.com/question/8101588
#SPJ9
An atom has 11 protons, 12 neutrons, and 11 electrons. What is the identity of the element?
Answers
Answer:
It's a Sodium Atom
9. What four guidelines are useful in balancing an
equation?
Answers
Answer:
Explanation:
To show the reactants and products, write the imbalanced equation.
Calculate the number of atoms of each element on each side of the reaction arrow.
To make the number of atoms of each element the same on both sides of the equation, multiply coefficients (the numbers in front of the formulas). ...
Check your work by indicating the state of matter of the reactants and products.
Which cofactor would most likely carry the e⁻ necessary for a reaction which converts acetaldehyde to ethanol?1.coenzyme A2.NADPH3.NADH4.FADH₂
Answers
The cofactor that would most likely carry the electrons necessary for a reaction converting acetaldehyde to ethanol is 3. NADH. This is because NADH is involved in various redox reactions and serves as an electron carrier, providing the necessary electrons for the reduction of acetaldehyde to ethanol.
A cofactor is a non-protein chemical compound that is required for the activity of certain enzymes. Enzymes are proteins that catalyze chemical reactions, and some of them require the presence of a cofactor to function properly.
Cofactors can be divided into two main types: inorganic cofactors and organic cofactors, also known as coenzymes. Inorganic cofactors include metal ions such as iron, copper, and zinc, which are involved in redox reactions and electron transfer processes. Organic cofactors are usually derived from vitamins and are often involved in reactions that transfer chemical groups between molecules, such as acetyl, methyl, and phosphate groups.
To learn more about Cofactor Here:
https://brainly.com/question/13004767
#SPJ11
What period has the highest ionization energy?
Answers
Answer:helium or the noble gases
Explanation: The ionization energy decreases from top to bottom in groups, and increases from left to right across a period. Thus, helium has the largest ionizing energy
Use your own words to define Resultant:
Use your own words to define Resultant
Answers
Answer:
the outcome of something
What will occur when the following chemical reaction reaches dynamic equilibrium? 3H2 + N2 3 2NH3 O A. No further chemical reactions will occur in either direction. B. The concentrations of all reactants and products will be the same C. The product will form at the same rate at which it decomposes. D. The concentrations will continue to change gradually over time.
Answers
Answer:
The product will form at the same rate at which it decomposes.
Explanation:
hope it helps
Answer: The product will form at the same rate at which it decomposes.
Explanation: I took the test
The element copper with a relative atomic mass of 63.55 contains atoms of mass numbers 63 and 65. Calculate the fraction of each isotope.
Answers
Explanation:
Difference between 63 and 65 = 2.
(63.55 - 63) / 2 * 100% = 27.5%.
(65 - 63.55) / 2 * 100% = 72.5%.
Hence there is 72.5% of the isotope with atomic mass 63 and 27.5% of the isotope with atomic mass 65 in copper.
question.
Which of the following events must occur at point 6 in order to transform sedimentary rock into igneous rock?
A
Shifting tectonic plates push the sedimentary rock toward the poles, where it freezes and hardens into igneous rock.
B
Extreme winds and storms weather the sedimentary rock into particles, which collect in rivers and are compacted into igneous rock.
C
Rising oceans erode mountains of sedimentary rock into particles, which sink and get compacted into layers that form igneous rock.
D
Tectonic plates push against each other, driving sedimentary rock under Earth's surface, where it melts into magma and turns into igneous rock as it cools

Answers
Answer:
I belive the answer is D