Answers
Answer:
A 12-liter balloon contains 0.52 moles of helium gas. answer is 0.52
Explanation:
hope this helps
Related Questions
College Chemistry Help Please 2

Answers
The net ionic equation is a simplified form of the balanced chemical equation, where spectator ions are removed.
What is equation?An equation is a mathematical statement that expresses the equality or inequality of two expressions. It is composed of two expressions, separated by an equal sign (=). The expressions can be numbers, variables, or a combination of both. Equations are used to describe relationships between different values, such as the slope of a line, the area of a rectangle, or the speed of a car. Equations are also used to solve problems and make predictions.
The potassium and bromide ions remain unchanged on both sides of the equation and hence can be removed. The net ionic equation thus becomes Sn²⁺(aq) + S²⁻(aq) → SnS(s).
To learn more about equation
https://brainly.com/question/28818351
#SPJ1
The density of air at ordinary atmospheric pressure and 25 ∘C is 1.19 g/L. What is the mass of the air in a room that measures 14.5×16.5×6.0 ft?
Answers
Answer:
40,827.34 g
Explanation:
First find the volume of the room and then multiply it by the density of air at ordinary atmospheric pressure and 25 ∘C.
In this case, the room has a length of 14.5 ft, a width of 16.5 ft, and a height of 6.0 ft.
volume = 14.5 ft x 16.5 ft x 6.0 ft = 1209.25 ft^3
To convert ft^3 to L, we can use the conversion factor 1ft^3 = 28.316846592 L
1209.25 ft^3 = 1209.25 * 28.316846592 = 34,416.99 L
Calculate the mass of the air in the room by multiplying the volume of the room by the density of air:
mass = 34,416.99 L x 1.19 g/L = 40,827.34 g
So, the mass of the air in the room is 40,827.34 g
Lead (ll) iodide (PbI2) has a solubility of 1.52×10 to the -3 mol/L.
1. write the dissolution reaction to PbI2 including all states.
2. Write the expression for Ksp for Pbl2.
3. What is the concentration of Pb2+ in the equilibrium solution?
4. What is the concentration of I- in the equilibrium solution?
5. Calculate the solubility product of Pbl2.

Answers
Answer:
A. PbI2(s) ===> Pb2+(aq) + 2I-(aq)
B. Ksp = [Pb2+][I-]^2
C. 1.52 x 10^-3 M. It is equal to the moles/L of PbI2 that go into solution.
D. 2 x 1.52 x 10^-3 = 3.04 x 10^-3 M
E. Ksp = (1.52x10^-3)(2.31x10^-6) = 3.51 x 10^-9
Explanation:
In the given question, \(\rm PbI_2(s) \rightleftharpoons Pb^{2+}(aq) + 2I^-(aq)\) is the dissolution reaction of \(\rm PbI_2\) in water, \(\rm Ksp = [Pb^{2+}][I^-]^2\) is expression for Ksp for \(\rm PbI_2\), \(1.52\times 10^{-3 }\) mol/L is the concentration of \(\rm Pb^{2+ }\) in the equilibrium solution, \(3.04\times 10^{-3}\) mol/L is the concentration of I- in the equilibrium solution and
\(1.40\times 10^{-8}\) is the solubility product of \(\rm PbI_2\), respectively.
A reaction is a process that involves the transformation of one or more substances into one or more different substances.
1. The dissolution reaction of \(\rm PbI_2\) in water is:
\(\rm PbI_2(s) \rightleftharpoons Pb^{2+}(aq) + 2I^-(aq)\)
2. The expression for Ksp for \(\rm PbI_2\) is:
\(\rm Ksp = [Pb^{2+}][I^-]^2\)
Where, \(\rm [Pb^{2+}]\) is the concentration of \(\rm Pb^{2+ }\) ions in solution and \(\rm [I^-]\) is the concentration of \(\rm I^-\) ions in solution.
3. The solubility of \(\rm PbI_2\) is \(1.52\times 10^{-3 }\) mol/L. Since \(\rm PbI_2\) dissociates into one \(\rm Pb^{2+ }\) ion and two \(\rm I^-\) ions, the concentration of \(\rm Pb^{2+ }\) in solution is equal to the solubility of \(\rm PbI_2\), which is \(1.52\times 10^{-3 }\) mol/L.
\(\rm [Pb^{2+}] = 1.52\times 10^{-3}\ mol/L\)
4. Since \(\rm PbI_2\) dissociates into one \(\rm Pb^{2+ }\) ion and two \(\rm I^-\) ions, the concentration of \(\rm I^-\) ions in solution is twice the solubility of \(\rm PbI_2\) .
\(\rm [I^-] = 2 \times 1.52\times 10^{-3 }\ mol/L\)
= \(3.04\times 10^{-3}\) mol/L
5. The solubility product of \(\rm PbI_2\) can be calculated using the expression for Ksp and the concentrations of \(\rm Pb^{2+ }\) and \(\rm I^-\) ions in solution.
\(\rm Ksp = [Pb^{2+}][I^-]^2\)
= \(1.52\times 10^{-3 }\) \(\times\) \(\rm (3.04\times 10^{-3} mol/L)^2\)
= \(1.40\times 10^{-8}\)
Therefore, the dissolution reaction of \(\rm PbI_2\) in water, expression for Ksp for \(\rm PbI_2\), the concentration of Pb2+ in the equilibrium solution, the concentration of I- in the equilibrium solution and solubility product of \(\rm PbI_2\) is mentioned above.
Learn more about reaction here:
https://brainly.com/question/16737295
#SPJ4
In the following reaction how many moles of NaCI are needed to produce 5.4 miles of Na2O
NaCI+MgO= Na2O+MgCI2
Answers
To produce 5.4 moles of \(Na_{2} O\) in the given reaction, 10.8 moles of NaCl are required.
The given chemical equation is a balanced equation, which means it represents the stoichiometric relationship between the reactants and products involved in the reaction.
The coefficients of the balanced equation represent the number of moles of each reactant and product involved in the reaction.
In this case, the equation is:
2 NaCl + MgO → \(Na_{2} O\) + \(MgCl_{2}\)
From the equation, we can see that 2 moles of NaCl are required to produce 1 mole of . Therefore, to produce 5.4 moles of \(Na_{2} O\), we need to use stoichiometry to determine the amount of NaCl required.
We can use the following formula to calculate the number of moles of NaCl required:
moles of NaCl = moles of \(Na_{2} O\) × (2 moles NaCl / 1 mole \(Na_{2} O\))
Substituting the given values, we get:
moles of NaCl = 5.4 moles \(Na_{2} O\) × (2 moles NaCl / 1 mole \(Na_{2} O\)) = 10.8 moles NaCl
Therefore, we need 10.8 moles of NaCl to produce 5.4 moles of \(Na_{2} O\) in the given reaction.
To learn more about moles here
brainly.com/question/26416088
#SPJ1
Cassini has a mass of 2523 kg, and Saturn
has a mass of 5.68 x 1026 kg. Saturn's radius
is 54,364 km. If Cassini feels a gravitational
force of 2.980 x 104 N, how high above
Saturn's surface is it?
Rearrange F gravity Gm,m₂/r2
to solve this problem
In 10 words or fewer, how high above Saturn's surface is the Cassini
satellite?
Answers
The F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and height is 108,728 km.
What is gravity?Gravity is the amount of force that is produced by the earth to attract the object toward the surface and it doubles if the mass is double.
The height of Saturn is the duble of the radius of the given radius of 54,364 km of the planet Saturn which is 108,728 km.
Therefore, F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and hight is 108,728 km.
Learn more about gravity, here:
https://brainly.com/question/4783082
#SPJ1
How many moles of SO3 are produced when 1.5 mol of O2 react with SO2?
Answers
Answer:
3 mole O2
Explanation:
Need balanced equation first: O2 + 2SO2 --> 2SO3
assuming SO2 is in excess,
1.5 mol O2 (2moles SO3/1mole O2) = 3 mole O2
explain the relationship (linear or exponential) between rate and concentration including what order the iodate ion would be in.
CONCENTRATIONS
EXP. 1: 0.020
EXP 2: 0.019
EXP 3: 0.017
EXP 4: 0.016
EXP 5: 0.014
EXP 6: 0.013
EXP 7: 0.011
EXP 8: 0.01
EXP 9: 8.6x10^-3
EXP 10: 7.1x10^-3
EXP 11: 5.7x10^-3
EXP 12: 4.3x10^-3
RATE (s^-1):
EXP 1: 0.283
EXP 2: 0.1972
EXP 3: 0.2353
EXP 4: 0.2033
EXP 5: 0.1701
EXP 6: 0.133
EXP 7: 0.10
EXP 8: 0.1234
EXP 9: 0.077
EXP 10: 0.07380
EXP 11: 0.05102
EXP 12: 0.03883
By looking at the reaction mechanism, propose a Rate Law (WITHOUT the value of K). Explain the exponents for each reactant. Also, how does the rate law proposed compared to the relationship between rate and iodate concentration observed in the Rate law question?
Discuss, with respect to collision theory, the changes in the rates result from the changing concentrations of the iodate ion. What would you predict if we repeated these reactions at higher temperatures? Explain using collision theory.

Answers
Based on the given data, the relationship between rate and concentration is exponential.
A proposed rate law for the reaction based on the given data is:
Rate = k[IO3⁻]²[H+]What is the collision theory?Collision theory suggests that the rate of a chemical reaction is proportional to the frequency and energy of collisions between the reactant molecules.
As the concentration of iodate ions decreases, the frequency of collisions between reactant molecules decreases, which leads to a decrease in the rate of the reaction.
At higher temperatures, the kinetic energy of the reactant molecules increases, which increases the frequency and energy of collisions between reactant molecules.
Learn more about collision theory at: https://brainly.com/question/20628781
#SPJ1
Use the ball and stick models above. If you had a gram of water and a gram of
oxygen, which substance would you have more particles of? Why? (Right or Wrong)
Answers
Answer:
When the weather is nice, many people begin to work on their yards and homes. For many projects, sand is needed as a foundation for a walk or to add to other materials. You could order up twenty million grains of sand and have people really stare at you. You could order by the pound, but that takes a lot of time weighing out. The best bet is to order by the yard, meaning a cubic yard. The loader can easily scoop up what you need and put it directly in your truck.
Avogadro’s Number
It certainly is easy to count bananas or to count elephants (as long as you stay out of their way). However, you would be counting grains of sugar from your sugar canister for a long, long time. Atoms and molecules are extremely small – far, far smaller than grains of sugar. Counting atoms or molecules is not only unwise, it is absolutely impossible. One drop of water contains about 10 22 molecules of water. If you counted 10 molecules every second for 50 years without stopping you would have counted only 1.6 × 10 10 molecules. Put another way, at that counting rate, it would take you over 30 trillion years to count the water molecules in one tiny drop.
Explanation:
1. How would an element be classified?a. homogeneous mixtureb. pure substancec. solutiond. heterogeneous mixture
Answers
Hello
To solve this question, we should understand that an element is is the base form in which a particule exit. However, it can either be in a molecule, atom or in a compound. Elements are pure substance which form up the molecule, atom or compounds. An element can said to be an identity of a substance. Elements are generally classified using the periodic table into metals, non-metals, metalliods.
In this question, the most possibe defination is that an element is a pure substance
A homogenous mixture is a mixture that is said to have one phase of substance throughout. I.e the comprising substance are all in a single phase.
A solution is the mixture of a solute that is completely dissolved in a solvent.
A heterogeneous mixture is a solution that the substance are not in a single phase.
The answer to this question is option B
List 5 spheres and give an example of something you would find in each sphere . Ill give 50 points
Answers
Hydrosphere: Liquid, vapour or ice.
Atmosphere: Oxygen.
Geosphere: Minerals.
Biosphere: Ecosystem.
Anthrosphere: Human habitats
chocolate sphere - chocolate
icecream sphere - icecream
sun - plasma
tire - air
A cube of iron and a cube of wood, each having a volume of V = 3.25 10-4 m3, are each placed in a large beaker of water. The density of the wood is rhowood = 3.73 102 kg/m3 and the density of the iron is rhoiron = 7.86 103 kg/m3. Calculate the buoyant force on each. (The cube of wood is allowed to float at the surface.)
Answers
Answer:
anor277
Nov 17, 2017
Well, by definition,
Molarity
≡
Moles of solute
Volume of solution
Explanation:
And thus...
moles of solute
=
molarity
×
volume
We use a molar quantity of
80.3
⋅
g
174.01
⋅
g
⋅
m
o
l
−
1
=
0.461
⋅
m
o
l
with respect to
potassium sulfate
And thus
volume
=
moles of solute
molarity
80.3
⋅
g
174.01
⋅
g
⋅
m
o
l
−
1
3.12
⋅
m
o
l
⋅
L
−
1
=
0.148
⋅
L
.
The buoyant force is one that acts against the weight of an object suspended in a fluid. The value of the buoyant force on each are:
i. Buoyant force on the cube of iron = 25.0 N
ii. Buoyant force on the cube of wood = 1.20 N
Upthrust is a force which acts against the direction of the weight of an object when suspended in a fluid. It acts majorly upwards against gravity, and it can be referred to as a buoyant force.
The amount of upthrust on object in a fluid sometimes depends on the density, volume and gravity acting on the object.
So that;
Upthrust = density x volume x gravity
Thus from the given question;
i. The buoyant force on the cube of iron can be determined by:
Upthrust = 7.86 x \(10^{3}\) * 3.25 x \(10^{-4}\) * 9.8
= 25.034
Upthrust = 25.0 N
Therefore, the buoyant force on the cube of iron is 25.0 N.
ii. The buoyant force on the cube of wood can be determined by:
Upthrust = 3.73 x \(10^{2}\) * 3.25 x \(10^{-4}\) * 9.8
= 1.1880
Upthrust = 1.20 N
Therefore, the buoyant force on the cube of wood is 1.20 N.
Visit: https://brainly.com/question/11884584
Describe why the atoms Na and Cl would form an ionic bond
Answers
Answer:
• This is because Sodium is a metal so it loses its one outermost electron to chlorine which is a non metal
Explanation:
An ionic bond is a bond formed between a metal and a non metal after sharing electrons in order to gain stability.
\(.\)
Answer:
Sodium is metal and Chlorine is a non-metal
Explanation:
An ionic bond is a bond between a metal and a non-metal and also when Na combines with Cl it forms a complete duplex structure making the compound NaCl stable
Which of these qualities most likely helped Kepler construct the scientific explanation of the solar system?
a generosity and helpfulness
b igenuity and creativity
c assertiveness and flexibility
d consideration and compassion
Answers
what is the double bond of XeF4
Answers
What is The charge of PbI2
Answers
Answer:
Lead iodide (II) is an inorganic compound, lead salt and hydrogen acid, with the PbI2 formula.
The fossilized remains of a plant were found at a construction site. The fossilized remains contain 1/32 the amount of carbon-14 that is present in a living plant.
Determine the approximate age of these fossilized remains.
Answers
Answer:
28645 years
Explanation:
Given the formula;
0.693/t1/2 = 2.303/t log (No/N)
Given that N = 1/32 No
Note;
t1/2 = half life of Carbon-14
t = time required for N amount of carbon -14 to remain= 5,730 years
No= amount of carbon 14 initially present
N = amount of carbon-14 after time t
Substituting values;
0.693/5,730 = 2.303/t log (No/1/32No)
0.693/5,730 = 2.303/t log 32
1.21 * 10^-4 = 3.466/t
t = 3.466/1.21 * 10^-4
t = 28645 years
Any preserved imprints, remains and traces of once a living organism from history are called fossils. By utilizing the radioactive property of organic compounds one can determine the age of an object.
The approximate age of the fossilizes remain is 28645 years.
This can be estimated as:
The formula used will be:
\(\dfrac{0.693}{\dfrac{t1}{2}}= \dfrac{2.303}{t} log (\dfrac{No}{N})\)
Where,
Half-life of Carbon-14 =\(\dfrac{t1}{2}\) Time required (t) for N amount of carbon -14 to remain= 5,730 yearsAmount of carbon 14 initially present = \(N_{o}\)Amount of carbon-14 after time t = NGiven,
N = \({\dfrac{1}{32} No\)
Replacing values in formula:
\(\dfrac{0.693}{5,730} & = \dfrac{2.30}{t} log \:({{\dfrac{No}{\dfrac{1}{32} No}})\)
\(\dfrac{0.693}{5,730} & = \dfrac{2.30}{t} \;log 32\)
\(1.21 \times 10^{-4} = \dfrac{3.466}{t}\)
\(t = \dfrac{3.466}{1.21} \times 10^{-4}\)
\(t = 28645 \:\text{years}\)
Therefore, the approximate age of the fossil is 28645 years.
To learn more about fossils and their age follow the link:
https://brainly.com/question/8917701
When 7.59 grams of sodium hydroxide (NaOH) are dissolved in 80.0 grams of water at 25.0 °C in an insulated container, the temperature of the water increases to 48.0 °C. Assuming that the specific heat of the solution is 4.184 J/(g °C) and that no heat is gained or lost by the container, what is the ∆H of solution of NaOH in kJ/mol?
Answers
The ∆H of solution of NaOH is 46.8 kJ/mol.
First, we need to calculate the amount of heat absorbed by the solution:
q = m × c × ∆T
where q is the heat absorbed (in Joules), m is the mass of the solution (in grams), c is the specific heat capacity of the solution (in J/(g °C)), and ∆T is the change in temperature (in °C).
In this case, the mass of the solution is the sum of the mass of NaOH and the mass of water:
m = 7.59 g + 80.0 g = 87.59 g
The change in temperature is:
∆T = 48.0 °C - 25.0 °C = 23.0 °C
Substituting the values, we get:
q = 87.59 g × 4.184 J/(g °C) × 23.0 °C = 8,878 J
Next, we need to convert the heat absorbed into the enthalpy change of solution (∆H). The enthalpy change of solution is the heat absorbed per mole of solute. The number of moles of NaOH is:
n = m/M
where M is the molar mass of NaOH, which is 40.00 g/mol.
n = 7.59 g / 40.00 g/mol = 0.1898 mol
Therefore, the enthalpy change of solution is:
∆H = q/n = 8,878 J / 0.1898 mol = 46,780 J/mol = 46.78 kJ/mol
The H of a NaOH solution, rounded to three significant numbers, is 46.8 kJ/mol.
To know more about the Temperature, here
https://brainly.com/question/30411639
#SPJ1
A student makes a model of a mineral crystal. The scale is 1:24. The crystal in the model is 5 cm long. How long Is the real-life crystal. A. 120cm B. 0.96cm C. 4.8cm D. 29cm
Answers
The length of the real-life crystal is 120 cm which is 24 times longer than the length of the crystal in the model.
How long Is the real-life mineral crystal?Given that, a student makes a model of a mineral crystal.
The scale is 1:24. The crystal in the model is 5 cm long.
We are given a scale of 1:24, which means that every unit in the model represents 24 units in real life.
To find the length of the real-life crystal, we need to use the scale factor of 24.
We can set up a proportion to solve for the real-life length of the crystal:
1 unit in the model : 24 units in real life
5 cm in the model : x cm in real life
Using cross-multiplication, we get:
1 × x = 24 × 5
x = 24 × 5
x = 120cm
Therefore, the real-life crystal measures 120cm.
Option A) 120cm is the correct answer.
Learn more about scale factor here: https://brainly.com/question/29464385
#SPJ1
Which of the following best describes the reaction seen below?
2H2(g) + O2(g) → 2H2O(l)
a.
Two grams of hydrogen gas combine with 1 gram of oxygen gas to form 2 grams of water.
b.
Two molecules of hydrogen gas combine with 1 molecule of oxygen gas to form 2 molecules of water.
c.
Two liters of hydrogen gas combine with 2 liters of oxygen gas to form 2 liters of water.
d.
Two hydrogen atoms combine with 1 oxygen atom to form 2 water molecules.
Answers
Two molecules of hydrogen gas combine with 1 molecule of oxygen gas to form 2 molecules of water.
What is a word equation?A word equation is one in which the equation that is shown in the reaction is written in words rather than by the use of the chemical symbols of the elements that are involved.
We have the reaction; \(2H_{2} (g) + O_{2} (g) ---- > 2H_{2} O(l)\), in order for this reaction to be written in the word equation form we have; Two molecules of hydrogen gas combine with 1 molecule of oxygen gas to form 2 molecules of water.
Learn more about word equation:https://brainly.com/question/15423342
#SPJ1
What is the molarity of a 3.00 L solution that contains 0.400 mol FeCl3?
1.20 M
0.266 M
7.50 M
0.133 M
Answers
Answer: 0.133 mol/l
Explanation: molality = concentration = 0.400 mol/3.0 l
Which of these elements could be used in industry to control electron flow?
Select one :
a . sulfur
b . antimony
c . titanium
d. bromine
e. calcium
Answers
Answer:
the answer is calcium
Explanation:
There are various kind of elements that are present in periodic table. Some elements are harmful, some are radioactive, some are noble gases. Option E is correct option.
What is periodic table?Periodic table is a table in which we find elements with properties like metals, non metals, metalloids and radioactive element arranges in increasing atomic number.
Periodic table help a scientist to know what are the different types of elements are present in periodic table so that they can discover the new elements that are not being discovered yet. Out of given options, calcium element could be used in industry to control electron flow. Calcium belongs to alkaline earth metals that is group 2 of periodic table.
Therefore, the correct option is option E.
Learn more about periodic table, here:
https://brainly.com/question/11155928
#SPJ2
The sulfuric acid–catalyzed reaction of isopentyl alcohol with acetic acid to form isopentyl acetate is performed. This reaction does not proceed to completion. After the reaction is heated under reflux for an hour, the product mixture is washed with aqueous sodium bicarbonate solution, and the organic layer is thoroughly dried. A distillation of the organic layer is then performed. Given the boiling points listed below, select the compound that will distill first.
Compound Boiling point, ℃
acetic acid 118
isopentyl acetate 142
isopentyl alcohol 130
sulfuric acid water 290
Answers
Answer:
The correct option is acetic acid
Explanation:
Distillation is the process of separating a mixture of substances based on differences in boiling points. During distillation, the compound with the lowest/least boiling point is distilled and collected first and then the one with the next least boiling point and it goes on like that.
From the explanation above, acetic acid has the least boiling point (in the organic layer) with 118°C and thus will distill first. This is then followed by isopentyl alcohol (130°C) and then isopentyl acetate (142°C) and finally sulfuric acid water (290°C).
13
A Christmas tree lights use what type of energy?
(1 Point)
Mechanical
Thermal
Electrical
Answers
Answer:
Electrical
Explanation:
Describes the chemical reaction (s) that produce AMD. Equations
are balanced and formatted to show subscripts.
Pls help I’m so confused
Answers
FeS2 + 7O2 + H2O → Fe2+ + 2SO4^2- + 2H+
This reaction is an oxidation reaction, where the sulfide mineral is oxidized to sulfate ions and ferrous ions are released. The ferrous ions can then react with water and oxygen to form ferric hydroxide (Fe(OH)3), which is a yellow-orange solid that contributes to the characteristic color of AMD.
The overall reaction can be written as:
4FeS2 + 15O2 + 14H2O → 4Fe(OH)3 + 8SO4^2- + 16H+
This reaction shows that four molecules of pyrite react with 15 molecules of oxygen and 14 molecules of water to produce four molecules of ferric hydroxide, eight molecules of sulfate ions, and 16 molecules of hydrogen ions. The reaction is balanced to ensure that the number of atoms of each element is the same on both sides of the equation.
The passage's author most vividly conveys the sense that Plumpp's poetry is like music when he
O uses words like "swing," "dance," and "sway" to characterize phrases in Plumpp's poems
O defines Plumpp as "the poet laureate of Chicago jazz and blues"
explains how long Plumpp has been writing about "Chicago jazz giants"
urges people to read Plumpp's poems and listen to the music Plumpp "immortalizes in print"
Answers
It is an amine, and it has less polar nitrogen-hydrogen and oxygen-hydrogen bonds.
A compound's boiling point is a physical characteristic. These intramolecular linkages between the molecules that make up a chemical affect these physical characteristics.
Alcohols and amino acids have the same kind of intermolecular linkages. The hydrogen bond is the name of this kind of bond.
The electrical attraction between a hydrogen atom from one molecule and an electronegative atom from a nearby molecule is known as a hydrogen bond.
The strength of the bond is in the following order: H.....F > H.....O > H......N
The H....N hydrogen bonds exist in amines, whereas the H....O hydrogen bonds exist in alcohols.
Consequently, the alcohol's hydrogen bonds are stronger and it will impart a higher boiling point on the compound.
Learn more about hydrogen bonds here-
https://brainly.com/question/10904296
#SPJ9
Answer:uses world like
Explanation:
I need your help with this question
No need explaining just say the answer thanks
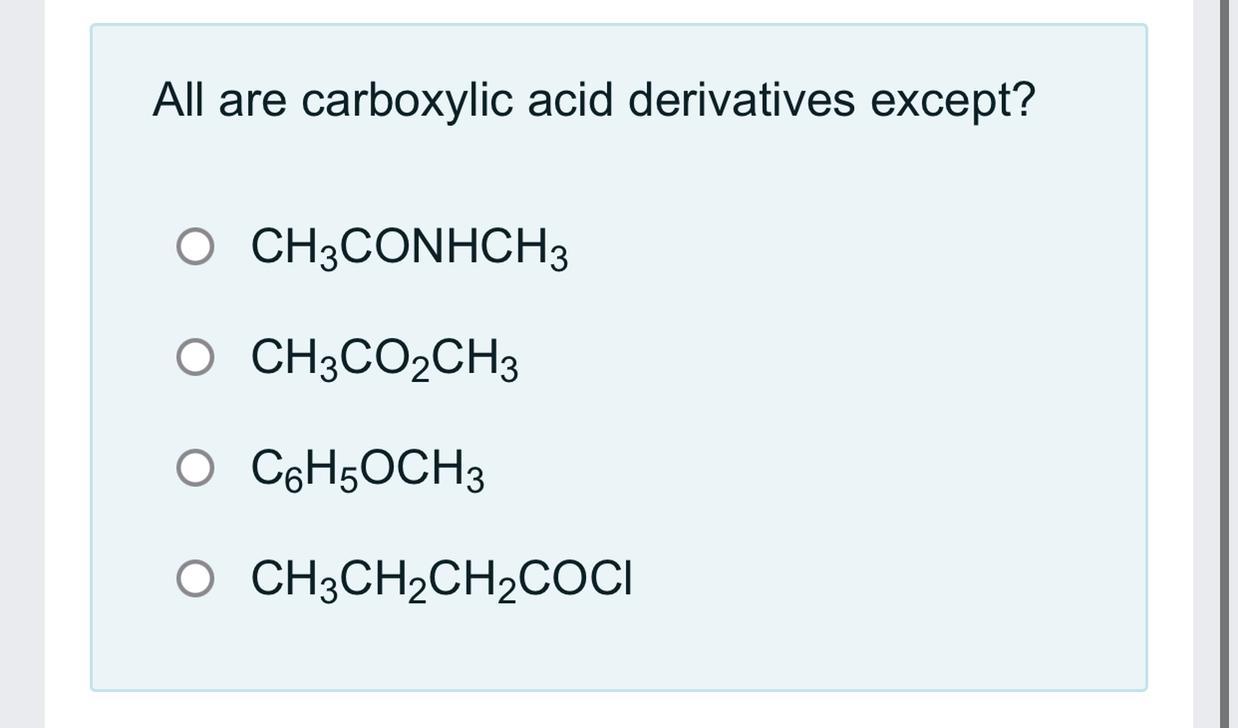
Answers
Answer: CHOCH
The third one
Explanation:
What is the IUPAC name for the compound below?

Answers
IUPAC name for the compound is 5,6 Dimethyl Heptene.
IUPAC naming / Nomenclature?This refers to a naming based on the highest length of the carbon molecule- chains that are joined by either a single bond, continuous chain, or ring.
In IUPAC naming / Nomenclature, multiple bonds or atoms other than carbon and hydrogen are indicated by suffixes and prefixes with respect to a particular set of priorities.
The general formula for hydrocarbons;Alkane= CnH2n+2
Alkene= CnH2n
Alkyne= CnH2n-2
From the question:C - Chain =7
H- Chain =14
CH₃ - bond= 2
Therefore,
n= 7
2n=14
It is an alkene hydrocarbon. The methyl group is present at the 5th and 6 chain.
Hence, the IUPAC name of the compound is 5,6 Dimethyl Heptene.
Learn more about IUPAC naming on
https://brainly.com/question/26247669
#SPJ1
what element is the chemical symbol K?
Answers
Answer:
It's Potassium My G
Answer:
Potassium
Explanation:
what is the function of eyepiece lens
Answers
Answer:
Eyepiece: The lens the viewer looks through to see the specimen. The eyepiece usually contains a 10X or 15X power lens. Diopter Adjustment: Useful as a means to change focus on one eyepiece so as to correct for any difference in vision between your two eyes.
Explanation:
plzzzzzz give brainly cuz
Answer:
some of them help with your vision and some help with eye problems like irritated
How many short columns are on the periodic table?
Answers
Answer:
They are 4 short column on the periodic table
