How many molecules of water are needed to completely hydrolyze a polymer that is 10 monomers long.
Answers
To completely hydrolyze a polymer that is 10 monomers long, nine molecules of water are needed.
Polymerization is a process by which smaller organic molecules, referred to as monomers, are linked together to form a more complex organic molecule known as a polymer. The bonds between the monomers are covalent bonds, which necessitate the consumption of energy to break them down.
In a reverse process known as hydrolysis, water is added to break the covalent bonds that connect the monomers and return the polymer to its constituent monomers. To completely hydrolyze a polymer that is 10 monomers long, nine molecules of water are required. This is because to separate the polymer back into monomers, nine covalent bonds between the monomers must be broken. Each bond requires a molecule of water to break it down, which means nine molecules of water are needed to hydrolyze a 10-monomer polymer completely.
Learn more about monomers here:
https://brainly.com/question/18784783
#SPJ11
Related Questions
Help I’ll give Brainliest
A typical galaxy is a collection of about a hundred billion stars spanning a diameter of about -
A. 1 AU
B. 3DO AU
c. 1 light-year
D. 100,000 light-years
Answers
Answer:
D
Explanation:
ive watched this on a national geo show. But remind me again what is 1 Au and 3DO AU i forgot...
A researcher grew a plant in an illuminated chamber with 18o-radiolabeled co2. After allowing time for photosynthesis, where will the radiolabeled 18o be found?.
Answers
After allowing time for photosynthesis the ¹⁸O-radiolabeled will be found in the plants in the form of C₆H₁₂O₆.
A researcher grew a plant in an illuminated chamber with ¹⁸O-radiolabeled CO₂. After allowing the time for photosynthesis, the ¹⁸O-radiolabeled will be found in C₆H₁₂O₆ in the plant.
Photosynthesis is a process in which plants produces their food by using sunlight, carbon dioxide and water to produce their food with the help of the a green colored pigment called chlorophyll.
It does not matter which isotope of oxygen we are using for photosynthesis, the amount of the food produced will be more or less same in case of every isotope.
The oxygen isotope will get mixed in the soil of the plants and after the whole photosynthesis process is done. the oxygen is constituted in the plant as C₆H₁₂O₆ will would be further broken down into the form of o₂ and H₂O. These H₂O and O₂ will be released in the atmosphere as a final product of the complete photosynthesis.
To know more about Photosynthesis, visit,
https://brainly.com/question/26568636
#SPJ9
how much is 3.5 gallons in cups
Answers
Answer:
3.6 gallons are equal to 56 cups
Hydrogen gas was collected in a burette in a water bath with a water height difference of 15. 0 cm. Please calculate the pressure in the burette if the atmospheric pressure in the room at that time was 765. 0 torr.
Answers
The pressure in the burette if the atmospheric pressure in the room at that time was 765.0 torr is 915 torr
How do I determine the pressure in the burette?The pressure in the burette can be obtained by using the following formula:
Pressure of gas = Atmospheric presure + pressure due to height
With the above formula, we can determine the pressure in the burette. This is illustrated below:
Pressure due to height = 15 cmHg = 150 mmHg = 150 torrAtmospheric pressure = 765.0 torrPressure in burette =?Pressure in burette = Atmospheric presure + pressure due to height
Pressure in burette = 765.0 + 150
Pressure in burette = 915 torr
Thus, the pressure in burette is 915 torr
Learn more about pressure:
https://brainly.com/question/11742398
#SPJ1
Consider the molecular orbital model of benzene. In the ground state, how many molecular orbital's are filled with electrons? Select one:
A. 1
B. 2
C. 3
D. 4
E. 5
F. 6
G. 7
H. 8
Answers
Consider the molecular orbital model of benzene. In the ground state, how many molecular orbitals are filled with electrons is C. 3
In the molecular orbital model of benzene, the ground state refers to the lowest energy state of the molecule. Benzene has 12 electrons, with six of these electrons forming a delocalized π-system that contributes to its aromatic properties.
To determine the number of molecular orbitals filled with electrons, we can look at the molecular orbitals formed by the π-system. In benzene, there are six π molecular orbitals created by the overlapping of the p-orbitals from each of the six carbon atoms. These π molecular orbitals can be classified into three bonding (lower energy) and three antibonding (higher energy) orbitals.
In the ground state, the electrons fill the molecular orbitals from the lowest energy level to the highest. The six π electrons in benzene fill the three lower energy-bonding molecular orbitals, with each orbital containing two electrons. Therefore, there are 3 molecular orbitals filled with electrons in the ground state of benzene. Therefore the correct option is C.
Know more about molecular orbitals here:
https://brainly.com/question/17371976
#SPJ11
Hypothesis: If you can measure the pH of a range of acids and bases using a universal pH indicator, then you can use those values to calibrate a cabbage pH indicator. To determine the pH of a solution using a pH indicator paper, you need a .
Answers
To determine the pH of a solution using a pH indicator paper, you need a color chart or a color scale that corresponds to different pH values.
This color chart or scale is used to compare the color of the pH indicator paper after it has been immersed in the solution. The pH indicator paper is impregnated with a universal pH indicator, which is a chemical compound that changes color depending on the acidity or alkalinity of the solution.
The indicator undergoes a chemical reaction with the hydrogen ions (H+) or hydroxide ions (OH-) present in the solution, resulting in a color change.
By comparing the color of the pH indicator paper with the color chart or scale, you can determine the approximate pH of the solution. The color chart usually provides a range of colors corresponding to different pH values, allowing you to match the observed color to the nearest pH value.
In the hypothesis mentioned, the aim is to calibrate a cabbage pH indicator using the pH values obtained from a universal pH indicator. Therefore, in addition to the pH indicator paper and color chart, you would also need a range of solutions with known pH values to establish a calibration curve specific to the cabbage pH indicator.
In summary, to determine the pH of a solution using a pH indicator paper, you need a color chart or scale that correlates the observed color of the pH indicator paper with different pH values. This chart or scale serves as a reference for interpreting the color change and determining the pH of the solution.
Know more about hypothesis here:
https://brainly.com/question/31293943
#SPJ8
Answer: COLOR KEY
Explanation: CS
he long run equilibrium condition for perfect competition is:
a. P=AVC=MR=MC.
b. Q=AVC=MR=MC.
c. Q=ATC=MR=MC.
d. P=ATC=MR=MC.
Answers
Option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
The long-run equilibrium condition for perfect competition is that price (P) is equal to average total cost (ATC), which is also equal to marginal cost (MC), and marginal revenue (MR).
Option (d), P=ATC=MR=MC, best represents the long-run equilibrium condition for perfect competition. In perfect competition, firms operate at the minimum point of their average total cost curve, where price equals both average total cost and marginal cost. This condition ensures that firms are earning zero economic profit and are producing at an efficient level.
In the long run, if firms are earning economic profit, new firms will enter the market, increasing competition and driving prices down. Conversely, if firms are experiencing losses, some firms may exit the market, reducing competition and causing prices to rise. This process continues until firms reach a state where price equals average total cost, marginal cost, and marginal revenue, ensuring a long-run equilibrium.
Therefore, option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
Know more about Equilibrium here:
https://brainly.com/question/30694482
#SPJ11
How is the process of natural selection different from the process of selective breeding?
Answers
Answer:
Explanation:
The difference between the two is that natural selection happens naturally, but selective breeding only occurs when humans intervene. For this reason selective breeding is sometimes called artificial selection. Different varieties of plants and animals with desired characteristics can be developed by selective breeding.
Calculate the average atomic mass of chromium, given the following percent abundances and isotope masses: 4.350 % 49.946 amu; 83.790% 51.941 amu; 9.500% 52.941 amu and 2.360% 53.939 amu
Answers
Asnwer: average atomic mass of chromium is 52amu
Calculations:
49.946amu: 4.350%= 0.0435
51.941amu: 83.790%= 0.8379
52.941amu: 9.500%= 0.095
53.939amu: 2.360%= 0.0236
Average atomic mass of chromium = 0.0435(49.946) + 0.8379(51.941) + 0.095(52.941) + 0.0236(53.939)
= 51.9963703amu
= 52 amu
A medallion was dropped into 20.0 mL of water. The water level rose to 25.3 mL. What is the volume of the medallion? (Vobj = Vf - Vi)
Answers
Answer:
5.3 mL
Explanation:
We solve this problem using Archimede's principle, which states that the volume displaced by a submerged object is equal to the volume of the object.
With that in mind we calculate the volume of water displaced by the medallion:
25.3 mL - 20.0 mL = 5.3 mLAs the medallion displaced 5.3 mL of water when submerged, the volume of the medallion is also 5.3 mL.
Bases are sharp and sweet in taste.
TRUE
FALSE
Answers
What is the expected markovnikov addition product from the addition of hi to 2-methyl-2-butene?.
Answers
Markovnikov addition product from the addition of hi to 2-methyl-2-butene is 2-iodo-2-mehtylbutane.
What is Markonikov rule?
In natural science, Markovnikov's standard or Markownikoff's standard portrays the result of some expansion responses. The standard was formed by Russian scientist Vladimir Markovnikov in 1870.Markovnikov's standard is an exact rule used to foresee regioselectivity of electrophilic expansion responses of alkenes and alkynes.Markovnikov predicts the results of an electrophilic expansion of hilter kilter reagents (for example hydrogen halides, water and alcohols) to hilter kilter alkenes.To learn more about Markonikov rule from the given link
https://brainly.in/question/232893
#SPJ4
When white light strikes this object, the light is completely absorbed, with none of it transmitted or reflected. Which type of object
could this be? (1 point)
O a black piece of paper
O a white sheet of plastic
O a green long-sleeved shirt
O a clear windowpane
Answers
The type of object that completely absorbs white light would be a black piece of paper. First option.
What are black bodies?Black bodies are objects that completely absorb all the components of white light.
White light is a mixture of different colors of light, each with a different wavelength and frequency.
When white light strikes a black body, the black body absorbs a large fraction of all the different colors, regardless of their wavelength. This absorption causes the light to be transformed into thermal energy, which increases the temperature of the black body.
More on black bodies can be found here: https://brainly.com/question/28271975
#SPJ1
the more particles a substance has at a given temperature the more thermal energy it has true or false
Answers
Answer:
\(\huge\boxed{\sf True}\)
Explanation:
Temperature and thermal energy are in a direct proportion which means that if temperature of a substance increases, its thermal energy also increases and vice versa.
\(\rule[225]{225}{2}\)
Hope this helped!
~AH18073 things to say when your girlfriend is sad?
Answers
Answer:
don't be sad you have a nice peach
babe you got that wap
don't be sad go get a tattoo
Answer:
Netflix and chill | do you need d!c rn ? | spoil her
Explanation:
how many fluid ounces can i take on a plane united
Answers
Liquid or gel food items larger than 3.4 oz are not allowed in carry-on bags and should be placed in your checked bags if possible. TSA officers may instruct travelers to separate items from carry-on bags such as foods, powders, and any materials that can clutter bags and obstruct clear images on the X-ray machine.
Is adding manganese dioxide to hydrogen peroxide, a chemical or physical change?
Answers
Answer: chemical change as a new product is formed
What is the molarity of a solution in which 25.3 grams of potassium bromide is dissolved in 150. mL of solution?
Answers
Answer:
~1.417M
Explanation:
Molarity=(number of moles of solute)/(litres of solution)
In this case, we need to find moles of potassium bromide.
Mass=25.3g
Molar mass= 119g/mol
moles=(mass/molar mass)
=(25.3)/(119)
=0.2126moles of potassium bromide
Molarity=(0.2126)/(150/1000)
~1.417M
Hope this helps:)
Answer:
\(\boxed {\boxed {\sf molarity \approx 1.42 \ M \ KBr}}\)
Explanation:
Molarity is a measure of concentration in moles per liter.
\(molarity= \frac{moles \ of \ solute}{ liters \ of \ solution}}\)
1. Find Formula for CompoundWe have the compound potassium bromide. Potassium (K) has an oxidation state of +1 and bromine (Br) has -1. They bond in a 1:1 ratio, so the formula is KBr.
2. Convert Grams to MolesWe are given the amount of solute in grams, but we need moles. To convert, we use the molar mass. These values are found on the Periodic Table. They are the same as the atomic masses, but the units are grams per moles (g/mol) instead of atomic mass units (amu).
Look up the individual element's molar mass.
Potassium: 39.098 g/molBromine: 79.90 g/molThe formula of KBr contains no subscripts, so we can add the molar masses.
KBr: 39.098+ 79.90 =118.99 g/molUse the molar mass as a ratio.
\(\frac {118.998 \ g\ KBr}{ 1 \ mol \ KBr}\)
We want to convert 25.3 grams, so we multiply by that value.
\(25.3 \ g\ KBr*\frac {118.998 \ g\ KBr}{ 1 \ mol \ KBr}\)
Flip the ratio so the units of grams of KBr cancel.
\(25.3 \ g\ KBr*\frac{ 1 \ mol \ KBr}{118.998 \ g\ KBr}\)
\(25.3*\frac{ 1 \ mol \ KBr}{118.998} = 0.2126086153\ mol \ KBr\)
3. Convert Milliliters to LitersMolarity uses liters, so we must convert the 150 milliliters. 1 liter contains 1000 milliliters.
\(\frac{1 \ L }{1000 \ ml}\)
\(150 \ mL *\frac{1 \ L }{1000 \ ml}\)
\(150 *\frac{1 \ L }{1000}= 0.150 \ L\)
4. Calculate MolarityNow we have the moles of solute and liters of solution, so we can find molarity.
\(molarity= \frac{ moles \ of \ solute}{liters \ of \ solution}\)
\(molarity= \frac{0.2126086153 \ mol \ KBr}{ 0.150 \ L}\)
\(molarity = 1.417390768\ mol KBr/L\)
The original measurements had 3 significant figures, so our answer must have the same. For the number we found, that is the hundredth place. The 7 in the thousandth place tells us to round the 1 to a 2.
\(molarity \approx 1.42 \ mol KBr/L\)
1 mole per liter is equal to 1 Molar (M), so we must convert the units.
\(molarity \approx 1.42 \ M \ KBr\)
solid sodium chloride is dissolved in water. the sodium chloride solution is electrolysed in the apparatus. state why sodium chloride solution , rather than solid chloride must be used in this experiment.
Answers
Answer: water solutions conducts charge
Explanation: solid sodium chloride is n insulator.NaCl is dissolved in water
In ionic forms. In solution there are Na+ and Cl- ions which can transfer charge.
A molecule that contains 6 carbon atoms with a single functional group that is an alcohol
Answers
The molecule that contains 6 atoms comprising a single functional group is Hexanol, under the condition that the given molecule is that of an alcohol.
Its molecules contain 6 carbon atoms. The finishing -ol states an alcohol (the OH functional group), and the hex- stem presents that there are six carbon atoms in the LCC. The OH group is assembled to the second carbon atom.
Functional groups are considered as specified groups of atoms within molecules that are the reason for characteristic chemical reactions of those molecules . Some examples of functional groups include alcohols, aldehydes, ketones, carboxylic acids, esters, ethers, halogens, amines and amides.
To learn more about functional groups
https://brainly.com/question/30483921
#SPJ4
The complete question
Name a molecule that contains 6 carbon atoms with a single functional group that is an alcohol
What effect does distance have on the force of gravity? (2 points) a Increasing the distance between two objects increases the gravitational force. b Decreasing the distance between two objects decreases the gravitational force. c Increasing the distance between two objects decreases the gravitational force. d Distance is not a factor in determining the force of gravity.
Answers
Answer:
The answer is increasing the distance between two objects decreases the gravitational force. which is C
Explanation:
HELP MEEE PLEASEEEEEEEEEEEEEE
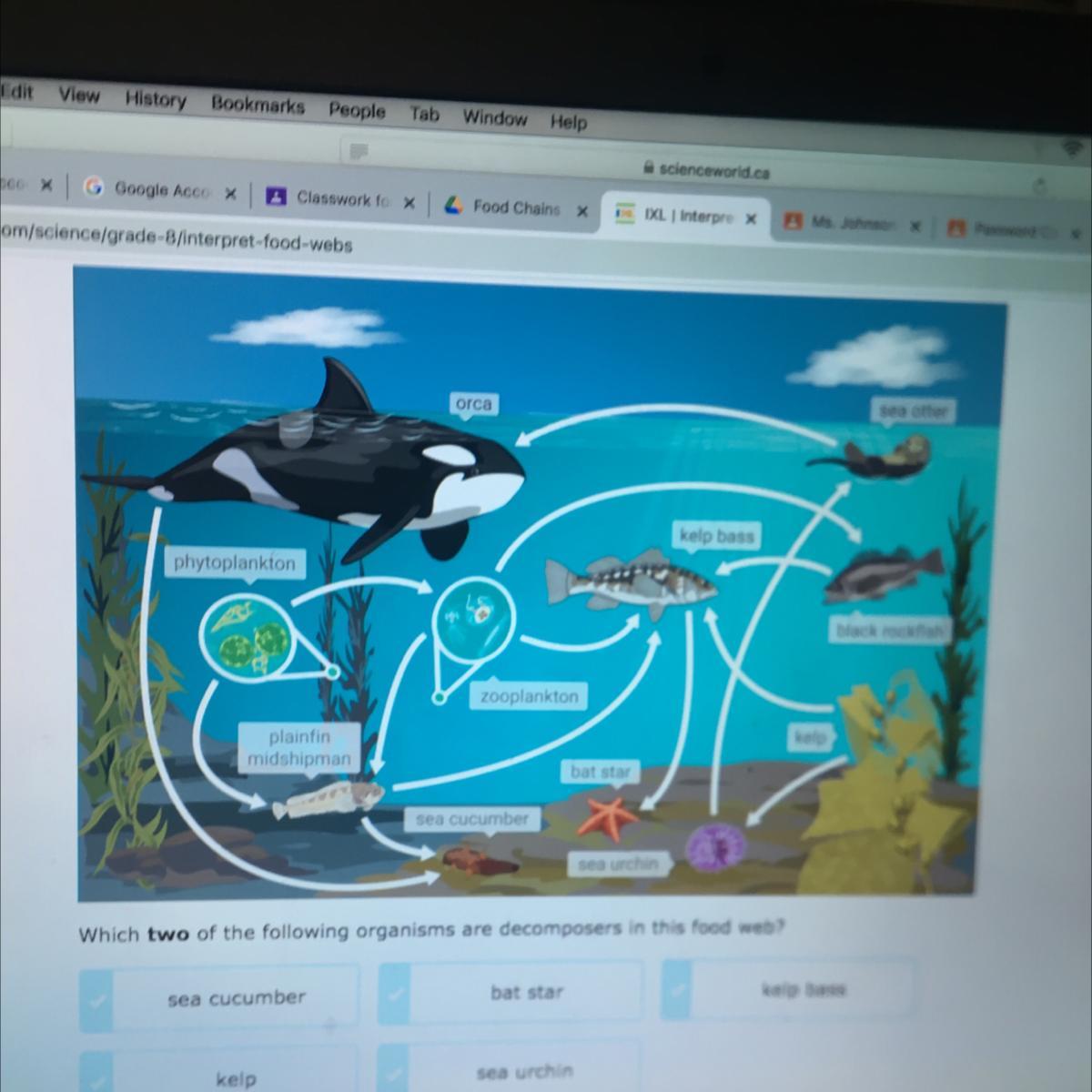
Answers
I think its the sea cucumber and sea urchin
but it could be the star too
How many moles are in a sample of H2SO4 with a mass of 54.7?
Answers
Answer: The molar mass of sulfuric acid is 98.07848 g mol.
Explanation:
which of the following elements would decrease in size when it became an ion
Answers
Answer:
nickel, cobalt would decrease in size when it became an iron
Use the electron-transfer method to balance the equation involving the reaction of lithium with water to produce lithium hydroxide and hydrogen gas.
Answers
Answer:
Explanation:
The equation for the reaction of lithium with water to produce lithium hydroxide and hydrogen gas is:
Li + H2O -> LiOH + H2
To balance this equation using the electron-transfer method, we first need to determine the oxidation states of each element in the reactants and products.
In Li, the oxidation state of lithium is +1. In H2O, the oxidation state of hydrogen is +1 and oxygen is -2.
In LiOH, the oxidation state of lithium is +1, oxygen is -2 and hydrogen is +1.
In H2, the oxidation state of hydrogen is +1.
It's clear that lithium loses 1 electron to become Li+ and hydrogen gains 1 electron to become H-.
So, we have to add an electron on the right side of the equation to balance the electrons.
Li + H2O -> LiOH + H2 + e-
Now, the number of electrons on both sides of the equation is the same and the equation is balanced.
Alternatively, we can use the oxidation number method, where we balance the charge of the reactant and product side of the equation. The sum of the oxidation numbers of all atoms on the reactant side must equal the sum of the oxidation numbers of all atoms on the products side. In this case, the oxidation state of lithium is +1, hydrogen is +1 and oxygen is -2.
Li + H2O -> LiOH + H2
+1 +1 -2 = +1 +1 +1 -2
The equation is already balanced with respect to oxidation numbers
In what circumstance will a flash of lightning travel through the sky and touch the ground?
Answers
Seawater is 3.5% Sodium Chloride by mass. Sea Salt can contain about 10% water. How much water is removed from every Kilogram of Commercial Sea Salt?
Answers
The amount of water removed from every kilogram of commercial sea salt would be 27.57 kg.
Dimensional analysisSeawater contains 3.5% sodium chloride by mass. This means every 1 kg of seawater contains 0.035 kg of sodium chloride.
Sea salt contains about 10% water. This means every 1 kg of sea salt contains 0.1 kg of water.
In order to calculate the amount of water removed from every kilogram of commercial sea salt produced, we need to know the amount of seawater that will give 1 kilogram of sea salt.
We said every 1 kg of seawater contains 0.035 kg of salt. How many 0.035 kg can be found in 1kg?
1/0.035 = 28.57
This means 28.57 kg of seawater would need to be processed in order to have 1 kg of salt. Thus, the amount of water removed can be calculated as:
28.57 - 1 = 27.57 kg
In other words, about 27.57 kg of water is removed for every kilogram of commercial salt produced.
More on dimensional analysis can be found here: https://brainly.com/question/13078117
#SPJ1
In the diagram below, what will allow more solute to be dissolved in the
solvent?
Answers
Answer:
missin a diagram buddy
Explanation:
I have little or no rainfall for long periods of time. I cause death to all living things because of lack of water. What am I?
Answers
Answer:
a drought !
- little to no water causes it
- it causes for organisms to die and organisms need water !
from the choices below, choose the major force controling tertiary protein structure. O hydrogen bonding O disulfide bonds O ion pairs hydrophobic effect O inorganic ions
Answers
From the choices below, the major force controlling tertiary protein structure are hydrogen bonding, disulfide bonds and ion pairs hydrophobic effect.
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways.
Important to tertiary structure are hydrophobic interactions, in which amino acids with nonpolar, hydrophobic R groups cluster together on the inside of the protein, leaving hydrophilic amino acids on the outside to interact with surrounding water molecules.
Therefore, From the choices below, the major force controlling tertiary protein structure are hydrogen bonding, disulfide bonds and ion pairs hydrophobic effect.
Learn more about tertiary protein structure, here:
https://brainly.com/question/14652022
#SPJ1