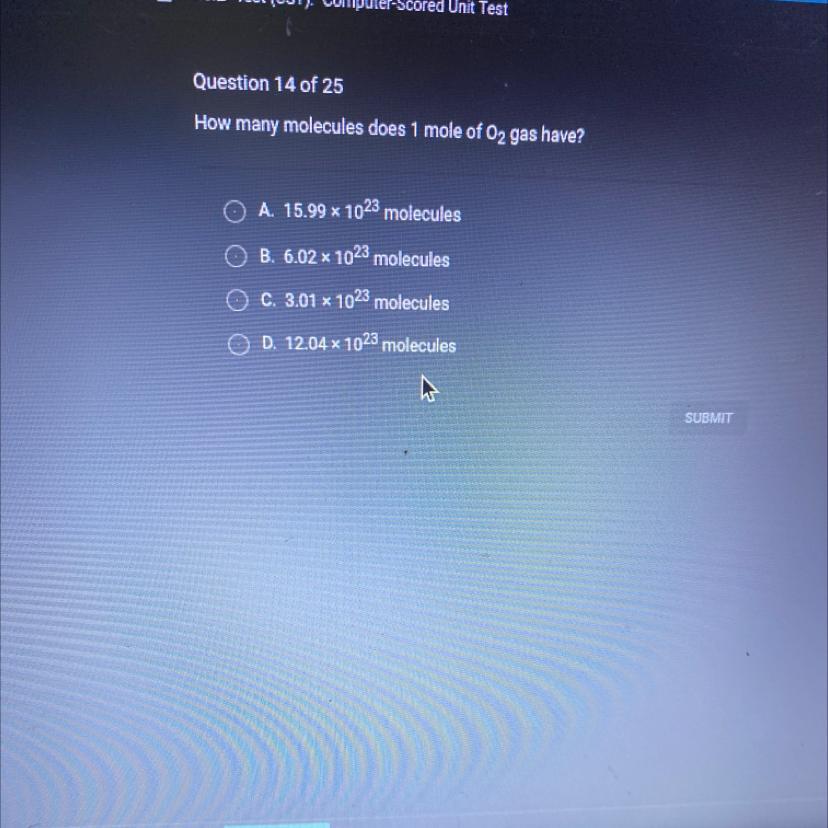
Answers
awnser : 6.02×1023 molcules
Related Questions
c) latent heat is the energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. based on this and what you know about evaporation, attempt to explain what is going on between 20º n and 40º n.
Answers
Latent heat refers to energy absorbed or released during a change in physical state without a temperature change. Between 20ºN and 40ºN, the increased temperature may enhance evaporation from bodies of water, allowing more water molecules to transition from liquid to gas.
Based on the given information, latent heat refers to the energy absorbed or released by a substance during a change in its physical state without a change in temperature. In the context of evaporation, the process involves the transformation of a liquid into a gas state.
Between 20ºN and 40ºN, it appears that we are referring to a geographic region or latitude range. The specific conditions or context of this region are not mentioned, so it is unclear how it directly relates to latent heat or evaporation.
However, if we consider the general principles of evaporation, we can infer that in this temperature range, there may be a significant amount of liquid water present, such as oceans, lakes, or bodies of water. The increase in temperature can lead to an increase in the rate of evaporation.
As the temperature rises, the kinetic energy of water molecules increases, causing them to move faster. This increased movement and energy can overcome the intermolecular forces holding the liquid together, allowing more water molecules to escape the liquid surface and become water vapor in the atmosphere. This process is known as evaporation.
In summary, between 20ºN and 40ºN, the increase in temperature may result in an increased rate of evaporation from bodies of water due to the greater availability of thermal energy for water molecules to transition from the liquid to the gas phase.
To know more about Latent heat refer here :
https://brainly.com/question/28044951#
#SPJ11
Identify the conjugate acid for each base.
conjugate acid of H2PO−4 :
inconjugate acid of SO2−4 :
conjugate acid of NH3 :
Answers
the conjugate acid for each base is conjugate acid of H2PO−4 : HPO4^2-, conjugate acid of SO2−4 : HSO4-, conjugate acid of NH3 : NH4+
A conjugate acid-base pair is a pair of substances that differ by only a proton. An acid is a substance that donates a proton (H+) in a chemical reaction, while a base is a substance that accepts a proton. When an acid loses a proton, it becomes its conjugate base, and when a base gains a proton, it becomes its conjugate acid.For example, the conjugate acid of the base NH3 is NH4+ and the conjugate acid of the base H2O is H3O+. The conjugate acid and the base are in equilibrium with each other, and the acidity constant (pKb) of the base is related to the basicity constant (pKa) of the conjugate acid. In acid-base reactions, the acid donates a proton to form its conjugate base, and the base accepts a proton to form its conjugate acid.
Learn more about acid-base pair here:
https://brainly.com/question/15579188
#SPJ4
Express the measurement using the appropriate SI prefix. 2.50 x 10-3g
Answers
What is the molarity of 2 mol of Kl dissolved in 1 L water?
A. 0.5 M
B. 3 M
C. 1M
D. 2 M
Answers
Answer:
d
Explanation:
How many formula units are there in 4.80x10^-3 mol of NaI?
Answers
The number of formula units present in 4.80×10⁻³ mole of NaI is 2.89×10²¹ formula units
How do i determine the formula units present?From Avogadro's hypothesis, we understood that 1 mole of NaI contains 6.022×10²³ formula units as shown below
1 mole of NaI = 6.022×10²³ formula units
Therefore, we can obtain the formula units in 4.80×10⁻³ mole of NaI as follow:
Mole of NaI = 4.80×10⁻³ moleFormula units =?From Avogadro's hypothesis,
1 mole of NaI = 6.022×10²³ formula units
Therefore
4.80×10⁻³ mole of NaI = 4.80×10⁻³ × 6.022×10²³
4.80×10⁻³ mole of NaI = 2.89×10²¹ formula units
Thus, the formula units present is 2.89×10²¹ formula units
Learn more about mole and formula units:
https://brainly.com/question/29159043
#SPJ1
How does radioactivity make it possible to understand how Earth can be so old and still have a hot interior?
A. Radioactive elements trap heat from underground magmd chambers.
B. Radioactive elements absorb heat from the atmosphere and release it underground.
C. Radioactive elements near the core release heat as theirnuclei decay.
D. Radioactive elements absorb the cold temperatures underground.
Answers
Answer:
Radioactive elements near the core release heat as their nuclei decay. ... Radioactive elements absorb the cold temperatures underground
Answer:
the answer is c
Explanation:
How many moles are there in 45. 0 grams of sulfuric acid.
Answers
We are given 45 grams of sulfuric acid and we need to calculate the number of moles in it. The number of moles can be found using the formula: N=m/M Where N is the number of moles, m is the mass of the substance and M is the molar mass of the substance. There are 0.4595 moles of sulfuric acid in 45.0 grams of it.
Sulfuric acid has a molecular formula H2SO4. Its molar mass can be calculated by adding the atomic masses of all the elements present in it. Molar mass of H2SO4 = 2(1.008) + 32.06 + 4(16.00) = 98.08 g/mol Now, we can calculate the number of moles using the formula: N=m/MN = 45.0/98.08N = 0.4595 moles. Therefore, there are 0.4595 moles of sulfuric acid in 45.0 grams of it.
To know more moles visit :
https://brainly.com/question/15209553
#SPJ11
Rechargeable batteries use a _________________medium to convert electrical current to a form of chemical energy which can be stored for future use.
Answers
Rechargeable batteries use a reversible reaction medium to convert electrical current to a form of chemical energy which can be stored for future use.
What is a rechargeable battery?A rechargeable battery is a type of battery that can be charged many times by passing electric current through the cells in a reversible reaction.
How does recahargeable battery store energy?When electrical energy from an outside source is applied to a secondary cell (reachargeable battery), the negative to positive electron flow that occurs during discharge is reversed, and the cell's charge is restored. This process is called reversible reaction.
Thus, rechargeable batteries use a reversible reaction medium to convert electrical current to a form of chemical energy which can be stored for future use.
Learn more about reversible reaction here: https://brainly.com/question/11412193
Look at the diagram below, which shows an atom of an element. How many valence electrons does it have? Based on this, would the atom be reactive or unreactive?

Answers
1. The number of valence electrons the atom have is seven (7).
2. The atom is reactive
1. How do i determine the number of valence electron in the atom?
Valence electron is simply defined as the number of electron(s) present in the outermost shell of an atom.
Considering the diagram given from the question, the number of electrons in the outermost shell is seven (7).
Thus, we can conclude that the valence electron the atom have is seven (7)
How do i know if the atom is reactive or not?From the diagram given, we determine that the number of valence electrons present is 7. This means that the atom required 1 more electron to complete its octet configuration.
Thus, the atom will be very reactive in order to quickly attain a stable configuration.
Learn more about valence electron:
https://brainly.com/question/30436806
#SPJ1
A mixture of three gases, A, B and C, is at a total pressure of 6.11 atm. The partial
pressure of gas A is 1.68 atm; that of gas B; is 3.89 atm. What is the partial pressure
of gas C?
2.13 atm
2.21 atm
5.57 atm
0.54 atm
Answers
The partial pressure of gas C is 0.54atm. The correct option is D.
What is partial pressure?The pressure that one of the gases in a mixture would exert if it were in the same volume on its own.
The total pressure of A, B and C is 6.11 atm.
The partial pressure of A is 1.68 atm
The partial pressure of B is 3.89 atm.
Total pressure = sum of partial pressures of all the gasses in that mixture
6.11atm = 1.68atm + 3.89atm + Pc
Pc = 6.11atm - (1.68 + 3.89) = 0.54atm
Thus, the partial pressure of C is 0.54atm. The correct option is D.
To learn more about partial pressure, refer to the link:
https://brainly.com/question/13199169
#SPJ1
25. The two major signs of a scam are a request for personal information
and
Answers
Scammers often attempt to obtain personal information such as social security numbers, bank account details, or passwords, under the guise of legitimate organizations. scammers often make enticing promises that are unrealistic or too good to be true. These promises may include guaranteed high returns on investments, lottery winnings, or extravagant rewards for minimal effort.
The two major signs of a scam are a request for personal information and promises that seem too good to be true. Scammers often attempt to obtain personal information such as social security numbers, bank account details, or passwords, under the guise of legitimate organizations. They may use tactics like phishing emails, fake websites, or phone calls to deceive individuals into revealing sensitive information. It's important to remember that reputable organizations typically do not ask for personal information via unsolicited communication. Additionally, scammers often make enticing promises that are unrealistic or too good to be true. These promises may include guaranteed high returns on investments, lottery winnings, or extravagant rewards for minimal effort. Such offers are designed to lure unsuspecting individuals into providing money or personal information. Being cautious and skeptical, avoiding sharing personal information without verifying the legitimacy of the request, and conducting thorough research can help protect against falling victim to scams.
For more question on organizations
https://brainly.com/question/21011563
#SPJ11
The pH of a solution of Ca(OH)2 is 8.57. Find the [Ca(OH)2]. Be careful, the fact that this base produces 2 OH- is important!
Answers
The concentration of Ca(OH)2 in the solution is approximately 1.33 x 10^(-6) M.
To find the concentration of Ca(OH)2 in a solution with a pH of 8.57, we need to use the concept of pOH, which is the negative logarithm of the hydroxide ion concentration ([OH-]). The pOH can be calculated by subtracting the pH from 14, which gives us 14 - 8.57 = 5.43.
Since Ca(OH)2 produces two OH- ions for every molecule of Ca(OH)2 that dissolves, the concentration of OH- ions will be twice the concentration of Ca(OH)2. Thus, we have [OH-] = 2x, where x represents the concentration of Ca(OH)2.
Taking the antilogarithm of the pOH, we find that [OH-] = 10^(-pOH) = 10^(-5.43).
Since [OH-] = 2x, we can write 2x = 10^(-5.43) and solve for x.
x = (10^(-5.43))/2 ≈ 1.33 x 10^(-6) M
For more such questions on Ca(OH)2
https://brainly.com/question/31035177
#SPJ8
If 10.0 liters of oxygen at STP are heated to 512 C, what will be the new volume of gas if the pressure is also increased to 1520. 0 mmHg?
Answers
Therefore, the new volume of gas is 14.5 L when 10.0 liters of oxygen at STP are heated to 512 C and the pressure is increased to 1520.0 mmHg the new volume of the gas will be approximately 28.8 liters.
To solve this problem, we can use the combined gas law, which relates the pressure, volume, and temperature of a gas. The formula for the combined gas law is:
(P1 x V1)/T1 = (P2 x V2)/T2
where P1, V1, and T1 are the initial pressure, volume, and temperature, and P2, V2, and T2 are the final pressure, volume, and temperature.
Given:
- P1 = 1 atm (STP)
- V1 = 10.0 L
- T1 = 273 K (STP)
- T2 = 512 C = 785 K
- P2 = 1520.0 mmHg
We need to convert the pressure units to atm, so we divide by 760 mmHg/atm:
- P2 = 1520.0 mmHg / 760 mmHg/atm = 2 atm
Now we can plug in the values and solve for V2:
- (1 atm x 10.0 L) / 273 K = (2 atm x V2) / 785 K
- V2 = (1 atm x 10.0 L x 785 K) / (2 atm x 273 K)
- V2 = 14.5 L (rounded to two significant figures)
To know more about gas law visit:-
https://brainly.com/question/12669509
#SPJ11
The Venn diagram shown below compares the nuclear reactions in the sun and nuclear power plants.
Picture shows two ovals which overlap in the middle. The oval on the left has the label 'Sun' on top and 'A' within it. The oval on the right has the label 'Nuclear power plants' on top and 'Splitting of atoms' within it. The overlapping portion has the label 'Release of energy' within it.
What process is best described by A?
Destruction of atoms
Fission of atoms
Fusion of atoms
Repulsion of atoms
Answers
The process which is best described by "A" in this Venn diagram is: C. fusion of atoms.
What is nuclear fusion?Nuclear fusion can be defined as a type of nuclear reaction that involves joining or combining two (2) smaller nuclei of atoms, so as to form a single heavier nucleus accompanied with the release of energy.
Based on the Venn diagram in the image attached below, we can logically deduce that the process which is best described by "A" is fusion of atoms.
Read more on nuclear fusion here: https://brainly.com/question/9903602
#SPJ1

1. Use Models: How does the GPS determine its distance from each satellite?
Answers
What is the formula for Iron(IV) phosphite
Answers
Answer:
The answer is Iron phosphide (Fe2P)
If a group of 100 exposed individuals are followed for three years to measure the frequency of chronic disease, and no individuals die or are lost to follow-up, then the cumulative incidence of disease for the three-year period will equal the prevalence of disease measured on the last day of the study period. True or False
Answers
The given statement is False. The cumulative incidence of disease for the three-year period will not necessarily equal the prevalence of disease measured on the last day of the study period.
Cumulative incidence measures the number of new cases of a disease that occur within a specific time period in a population initially free of the disease.
On the other hand, prevalence measures the proportion of individuals in a population who have the disease at a particular point in time, regardless of when they developed it.
Prevalence is influenced by both the incidence and duration of the disease, while cumulative incidence only considers the occurrence of new cases. Therefore, the two measures can be different, and the statement is false.
To know more about cumulative incidence, refer here:
https://brainly.com/question/31493651#
#SPJ11
At midday (12pm, Noon), what is happening in the leaf of a plant?
A) Cellular Respiration
B) Photosynthesis
C) Mainly photosynthesis and some cellular respiration
D) None of the above
Answers
Answer:
C
Explanation:
Noon is the peak amount of sun shining. Therefore the plant will be carrying out a lot of photosynthesis using the light. Cellular respiration happens regularly as well
At midday, what is happening in the leaf of a plant would be photosynthesis and cellular respiration. The correct option would, therefore, be C.
Photosynthesis has two reactions:
Light dependent reactionLight independent reactionThe light dependent reaction is rate determining step of photosynthesis. Hence, photosynthesis proceeds at normal rate during the day.
Cellular respiration, on the other hand, takes place with or without light.
Hence, at 12 pm, Noon, both photosynthesis and cellular respiration will be taking place simultaneously in the leaf of plants.
More on photosynthesis and cellular respiration can be found here: https://brainly.com/question/18728019
chlorine is an active non metal
Answers
Chlorine in group 17 in the periodic table and includes halogens (also fluorine, bromine, and iodine)
Electron configuration of Cl : [Ne] 3s² 3p⁵
From this configuration, it can be seen that to obtain stability like the noble gas Ar - [Ne] 3s² 3p⁶, Chlorine will need one more electron so that its configuration is the same as Ar( become anion-Cl⁻)
Then it is said that Cl is reactive
And the Cl element is included in the non-metal group because it attracts electrons (electronegative)
The elements that include non-metals are halogens, noble gases, and 7 elements H, C, N, O, P, S, Se.
What does each row (across) have in common?
Answers
(e) (i) What are the number average (Mn) and weight average (Mw) molecular weights of a polymer with equal number of chains with molecular weights of 2100, 6600 and 12000 mixed together? (ii) What is the answer if "equal number of chains" is replaced by "equal weights of chains"? (iii) What is the degree of polymerization (DP) of the polymer in the first question, if the repeating units of the three different chains have molecular weights of 126, 324 and 300?
Answers
(e) (i) Number average (Mn) = 6,900g/mol
(ii) Mn will remain the same in this case as it does not depend on weight.= 6,900g/mol
(iii) The degree of polymerization (DP) is 4492.
(i) Number average (Mn)
The number average molecular weight (Mn) is the sum of the molecular weights of all polymer chains divided by the total number of polymer chains.
Weight average (Mw)
The weight average molecular weight (Mw) is the sum of the product of the molecular weight and the fraction of the total polymer chains that have that molecular weight.
Using the given formula, let's first calculate the Mn:
(2100 + 6600 + 12000) / 3 = 6,900g/mol
Let's now calculate the Mw:
[(2100 x 2100) + (6600 x 6600) + (12000 x 12000)] / (2100 + 6600 + 12000)= 9966.67g/mol
(ii) Equal weights of chains
Mn will remain the same in this case as it does not depend on weight.
Mw, on the other hand, will change.
The following formula will be used:
2100 x (1/3) + 6600 x (1/3) + 12000 x (1/3) = 6,900g/mol
(iii) The degree of polymerization (DP)
DP refers to the number of repeating units in the polymer chain.
We can calculate the DP using the following formula:
DP = (Mn / M) * NA where M is the molar mass of the repeating unit, and NA is Avogadro's number.
Using the Mn value from part (i) and the given molecular weights for the repeating units, we can calculate the DP:
DP = (6,900 / ((126 + 324 + 300) / 3)) * 6.02 × 1023= 4492.45 (approximately 4492)
Therefore, the degree of polymerization is 4492.
To know more about polymerization, visit:
https://brainly.com/question/27354910
#SPJ11
The weight average molecular weight (Mw) of the polymer will be \($$M_w=\frac{1\times2100^2+1\times6600^2+1\times12000^2{1\times2100+1\times6600+1\times12000}=8,580\;g/mol$$\).
Weight average molecular weight (Mw) of the polymer will be \($$M_w=\frac{1.0587\times2100^2+1.0587\times6600^2+1.0587\times12000^2{1.0587\times2100+1.0587\times6600+1.0587\times12000}=8,825\;g/mol$$\).
The degree of polymerization of the polymer in the first question is 25.01.
Number average (Mn) and weight average (Mw) molecular weights of a polymer are calculated using the following formula:
\($$M_n = \frac{\sum N_iM_i}{\sum N_i}$$\)
and
\($$M_w = \frac{\sum N_iM_i^2}{\sum N_iM_i}$$\)
where Ni is the number of chains with molecular weight Mi.
(i) When the number of chains with molecular weights of 2100, 6600, and 12000 are mixed together:
Molecular weight (M) Number of chains (N) 2100 1 6600 1 12000 1
Total 3
The number average molecular weight (Mn) of the polymer will be:
\($$M_n=\frac{1\times2100+1\times6600+1\times12000}{1+1+1}= 6,900\;g/mol$$\)
The weight average molecular weight (Mw) of the polymer will be:
\($$M_w=\frac{1\times2100^2+1\times6600^2+1\times12000^2}{1\times2100+1\times6600+1\times12000}=8,580\;g/mol$$\)
(ii) When the equal weights of chains are mixed together:
The total weight of the chains is:
2100 + 6600 + 12000 = 20,700 g
Number of chains with molecular weight 2100 = 2100 x n1
Number of chains with molecular weight 6600 = 6600 x n2
Number of chains with molecular weight 12000 = 12000 x n3
So, the total weight of each group of chains will be
n1M1 + n2M2 + n3M3.
Now, we can calculate the values of n1, n2, and n3 as follows:
n1 = 6600 x n2n2 = 12000 x n3M1 + M2 + M3 = 2100 + 6600 + 12000 = 20700n1M1 + n2M2 + n3M3 = 20700
Equating the value of n1 in terms of n2 and n3 and substituting it in the equation above:
n1 = 6600 x n2
\($$\frac{6600}{n1}=n2$$\)
\($$\frac{12000}{n2}=n3$$\)
\($$n1=\frac{20700}{2100+6600+12000}=0.1739$$\)
n2 = 0.0208, n3 = 0.0052
Therefore, the number of chains with molecular weight 2100 = 0.1739 x 2100 / 6600 x 0.1739 x 6600 = 0.0208 x 12000 / 6600 x 0.0208 x 6600 = 0.0052 x 2100 / 6600 x 0.0052 x 12000 ≈ 1.0587
Number average molecular weight (Mn) will be:
\($$M_n=\frac{1.0587\times2100+1.0587\times6600+1.0587\times12000}{1.0587+1.0587+1.0587}= 7,170\;g/mol$$\)
Weight average molecular weight (Mw) of the polymer will be:
\($$M_w=\frac{1.0587\times2100^2+1.0587\times6600^2+1.0587\times12000^2}{1.0587\times2100+1.0587\times6600+1.0587\times12000}=8,825\;g/mol$$\)
(iii) The degree of polymerization (DP) of the polymer in the first question will be:
Number of chains with molecular weight 2100 = 1
Number of chains with molecular weight 6600 = 1
Number of chains with molecular weight 12000 = 1
The molecular weight of the repeating units of the three different chains have molecular weights of 126, 324, and 300 respectively.
Therefore, the degree of polymerization (DP) of the polymer in the first question will be:For 2100 chain,
\($$n_1=\frac{2100}{126}=16.67$$\)
For 6600 chain,
\($$n_2=\frac{6600}{324}=20.37$$\)
For 12000 chain,
\($$n_3=\frac{12000}{300}=40.00$$\)
So, the average degree of polymerization (DP) is:
\($$DP=\frac{1\times16.67+1\times20.37+1\times40.00}{1+1+1}=25.01$$\)
Therefore, the degree of polymerization of the polymer in the first question is 25.01.
To know more about molecular weight, visit:
https://brainly.com/question/20380323
#SPJ11
How many moles of gas are in a 30 liter compressed air tank if the
temperature of the tank is 300K and the pressure is 200 atmospheres? *
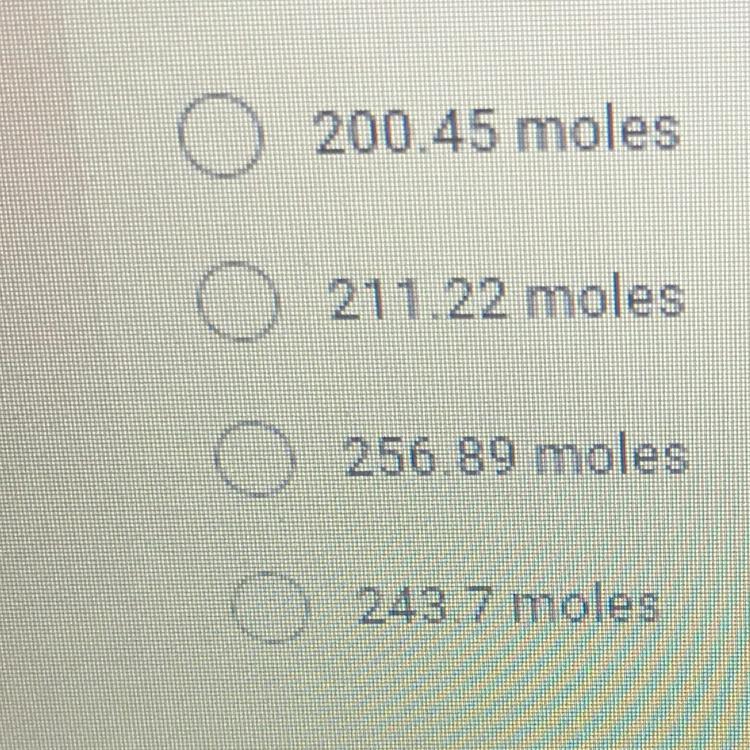
Answers
Answer:
B
Explanation:
What do you think happens to the bonds between atoms when substances melt?
Answers
Answer:
As a substance melts, and goes from a solid to a liquid state, the kinetic energy of the molecules increases, and the molecules move faster, and they separate further and further away from each other. The intermolecluar forces holding the molecules together become weaker. This is why a liquid can take fill the shape of its container, whereas a solid has a fixed shape.
Explanation:
take your notes man
What is the complete ionic equation for the reaction between Na 2 SO 4 and CaCl 2 ?
Answers
Answer:
Na2SO4 + CaCl2 → 2Na+ SO4 2- + Ca2+ 2Cl-
Hope this helps.
Answer:
2Na+(aq)+SO42-(aq)+Ca2+(aq)+2Cl-(aq)->CaSO4(s)+2Na+(aq)+2Cl-(aq)
Explanation:
Write the nuclear equation for the beta decay Ni-60
Answers
\(^6^0_2_7CO\;\rightarrow\;^6^0_2_8Ni\;+\;^0_-1e\) is the nuclear equation for the beta decay Ni-60.
What is beta decay?Beta-decay occurs when, in a nucleus with too many protons or too many neutrons, one of the protons or neutrons is transformed into the other.
The nuclear equation for the beta decay Ni-60
\(^6^0_2_7CO\;\rightarrow\;^6^0_2_8Ni\;+\;^0_-1e\)
In beta minus (β−) decay, a neutron is converted to a proton, and the process creates an electron and an electron antineutrino; while in beta plus (β+) decay, a proton is converted to a neutron and the process creates a positron and an electron neutrino. β+ decay is also known as positron emission.
Hence, \(^6^0_2_7CO\;\rightarrow\;^6^0_2_8Ni\;+\;^0_-1e\) is the nuclear equation for the beta decay Ni-60.
Learn more about the beta decay here:
https://brainly.com/question/25455333
#SPJ1
if there is 16.66 g p4 and excess cl2 present, the reaction yields 54.8 g pcl3. calculate the percent yield for the reaction.
Answers
The percent yield of a reaction is a measure of how efficiently the reaction proceeds, calculated by comparing the actual yield to the theoretical yield. In this case, the reaction involves 16.66 g of phosphorus (P4) and excess chlorine (Cl2), resulting in the production of 54.8 g of phosphorus trichloride (PCl3). To calculate the percent yield, we need to determine the theoretical yield first. The percent yield for the reaction is approximately 74.3%.
The molar mass of P4 is 123.88 g/mol, while the molar mass of PCl3 is 137.33 g/mol. Based on the balanced chemical equation, 1 mol of P4 reacts with 6 mol of Cl2 to produce 4 mol of PCl3. Therefore, the molar ratio between P4 and PCl3 is 1:4.
To calculate the theoretical yield, we convert the given mass of P4 into moles using its molar mass:
16.66 g P4 * (1 mol P4 / 123.88 g P4) = 0.1343 mol P4
Using the molar ratio, we can determine the moles of PCl3 that should be produced:
0.1343 mol P4 * (4 mol PCl3 / 1 mol P4) = 0.5372 mol PCl3
Finally, we convert the moles of PCl3 into grams using its molar mass:
0.5372 mol PCl3 * (137.33 g PCl3 / 1 mol PCl3) = 73.84 g PCl3
The theoretical yield of PCl3 is calculated to be 73.84 g. To determine the percent yield, we divide the actual yield (54.8 g) by the theoretical yield (73.84 g) and multiply by 100:
Percent Yield = (54.8 g / 73.84 g) * 100 = 74.3%
Therefore, the percent yield for the reaction is approximately 74.3%. This value indicates that the reaction produced 74.3% of the expected amount of PCl3 based on the given amount of P4. The lower percent yield suggests that there may have been some inefficiencies or losses during the reaction, resulting in a reduced yield of the desired product.
Learn more about theoretical yield here:
brainly.com/question/33781695
#SPJ11
Complete this sentence. If mass remains the same while the volume of a substance ________, the density of the substance will_______________.
(NOT GIVING BRAINLIEST, JUST ANSWERING A QUESTION MOST PEOPLE GOT WRONG ON)
A. decreases, decrease
B. increases, decrease
C. increases, stay the same
D. decreases, stay the same
**It's D**
Answers
Answer:
B. Increases, Decreases
Explanation:
I majored in Chemistry
A sample of coffee grounds is measured to have a mass of 1400 g. Express the
mass of the coffee grounds in decigrams (dg) and in kilograms ( kg).
Answers
1kg = 1000g
So,
1400 grams equals 140 decigrams and 1.4 kg
The half-life of C-14 is 5,730 years. How much of a 50.0-gram sample of C-14 will remain after 28,650 years?
Answers
Answer:
1.5625
Explanation:
the half life of an isotope is the amount of time it takes for one half of the isotope to decay
28650 is 5 half lives (28650÷5730=5)
after one half life the mass will be 25 grams
after two half lives the mass will be 12.5 grams
after 3 half lives the mass will be 6.25 grams
after 4 half lives the mass will be 3.125 grams
after 5 half lives the mass will be 1.5625 grams
a sample of unknown material weighs 500 n in air and 200 n when immesersed in alcholol with a specfic gravity of 0.7 what is the mass density
Answers
Answer: The mass density is 1166.36 \(kg/m^{3}\).
Explanation:
Given: Weight of sample in air \((F_{air})\) = 500 N
Weight of sample in alcohol \((F_{alc})\) = 200 N
Specific gravity = 0.7 = \(0.7 \times 1000 = 700 kg/m^{3}\)
Formula used to calculate Buoyant force is as follows.
\(F_{B} = F_{air} - F_{alc}\\= 500 - 200 \\= 300 N\)
Hence, volume of the material is calculated as follows.
\(V = \frac{F_{B}}{\rho \times g}\)
where,
\(F_{B}\) = Buoyant force
\(\rho\) = specific gravity
g = acceleration due to gravity = 9.81
Substitute the values into above formula.
\(V = \frac{F_{B}}{\rho \times g}\\= \frac{300}{700 \times 9.81}\\= \frac{300}{6867}\\= 0.0437 m^{3}\)
Now, mass of the material is calculated as follows.
\(mass = \frac{F_{air}}{g}\\= \frac{500 N}{9.81}\\= 50.97 kg\)
Therefore, density of the material or mass density is as follows.
\(Density = \frac{mass}{volume}\\= \frac{50.97 kg}{0.0437 m^{3}}\\= 1166.36 kg/m^{3}\)
Thus, we can conclude that the mass density is 1166.36 \(kg/m^{3}\).