how many molecules are there in 125 grams of sugar, C12H22O11
Answers
Answer:
A molecule of sucrose (C12H22O11) has 12 carbon atoms, 22 hydrogen atoms and 11 oxygen atoms. The subscripts also indicate the ratios of the elements.
Explanation:
This is because it has the abbreviation of the Carbon atoms, Hydrogen atoms, and Oxygen atoms are right before the number.
The number of molecules of sugar is required.
The number of molecules is \(2.2\times 10^{23}\ \text{molecules}\)
m = Mass of sugar = 125 g
\(N_A\) = Avogadro's number = \(6.022\times 10^{23}\ \text{mol}^{-1}\)
Molar mass of sugar
\(M=C_{12}H_{22}O_{11}=12\times 12+1\times 22+16\times 11\\\Rightarrow M=342\ \text{g/mol}\)
Number of moles is
\(n=\dfrac{m}{M}\\\Rightarrow n=\dfrac{125}{342}\\\Rightarrow n=0.365\ \text{mole}\)
Number of molecules is
\(N=N_An\\\Rightarrow N=6.022\times 10^{23}\times 0.365\\\Rightarrow N=2.2\times 10^{23}\ \text{molecules}\)
The number of molecules is \(2.2\times 10^{23}\ \text{molecules}\)
Learn more:
https://brainly.com/question/20333160?referrer=searchResults
Related Questions
The measurement 0.01011 km has how many significant figures?
Answers
Answer: 4
Explanation:
Sig Figs
4
Decimals
5
Scientific Notation
1.011 × 10-2
Words
zero point zero one zero one one
what is required to initiate an energy-releasing reaction like the combustion of methane?
Answers
Answer:
Activation.
Explanation:
Activation Energy is the minimum amount of energy needed to start a chemical reaction.
The requirement to initiate an energy-releasing reaction like the combustion of methane is; attaining the Activation energy.
Discussion;
Although, exothermic reactions release energy upon completion; the commencement of such reactions still depends on attaining the Activation energy of the reaction.
The activation energy of a reaction is the minimum quantity of energy required for the commencement of the reaction.
This activation energy can however be lowered by the use of Catalysts that function to lower the Activation energy accordingly.
Read more;
https://brainly.com/question/7639475
number of o atoms in 4.25×10−3 mol al(no3)3.
Answers
There are 3.2 × \(10^-5\) mol of oxygen atoms in \(4.25×10^-3\)mol Al(NO₃)₃.
To determine the number of oxygen atoms in 4.25×10⁻³ mol Al(NO₃)₃, we need to first convert the mass of the Al(NO₃)₃ into moles.
The mass of Al(NO₃)₃ can be calculated using the molecular formula Al(NO3)3, which is 18 + 16 + 15 = 49 atomic mass units (amu). The molar mass of Al(NO₃)₃ is therefore 49 g/mol.
To convert the mass of Al(NO3)3 into moles, we can divide the mass by the molar mass:
mass of Al(NO3)3 = moles of Al(NO₃)₃ × molar mass of Al(NO₃)₃
mass of Al(NO3)3 = 4.9 × 10⁻³ mol × 49 g/mol
mass of Al(NO3)3 = 0.0002 mol
To determine the number of oxygen atoms in 4.25×10⁻³ mol Al(NO₃)₃, we need to consider the molecular formula of Al(NO₃)₃. The molecular formula indicates the number of atoms of each element in the molecule.
The molecular formula of Al(NO₃)₃ is Al(NO₃)₃, which means that it contains one atom of aluminum, one atom of nitrogen, and three atoms of oxygen. Therefore, there are 1 atom of Al, 1 atom of N, and 3 atoms of O in 4.25×10⁻³ mol Al(NO₃)₃.
The number of oxygen atoms can be calculated as follows:
number of oxygen atoms = number of moles of Al(NO₃)₃ × molecular mass of O
number of oxygen atoms = 0.0002 mol × 16 g/mol
number of oxygen atoms = 0.000032 g
number of oxygen atoms = 3.2 × 10⁻⁵ mol
Therefore, there are 3.2 × \(10^-5\) mol of oxygen atoms in \(4.25×10^-3\)mol Al(NO₃)₃.
Learn more about oxygen atoms
https://brainly.com/question/12442489
#SPJ4
how many ml of 0.871 m koh is required to reach the endpoint (equivalence point) in a titration with 25.0 ml of 0.449 m hch3co2? express your answer in ml.
Answers
12.9 ml ml of 0.871 M KOH are required to reach the end point in a titration with 25.0 ml of 0.449 M HCl
Neutralization reaction is defined as a chemical reaction in which an acid and a base reacts with each other. Basically it is explained as when a strong acid reacts with a strong base the resultant salt is neither acidic nor basic in nature which is called neutral. The balanced chemical equation for the neutralization reaction between KOH and HCl can be written as,
KOH +HCl → KCl + H₂O
The equivalence point of a chemical reaction is defined as the point at which chemically equivalent quantities of reactants of the reaction have been mixed together. For an neutralization reaction the equivalence point is where the moles of acid and the moles of base would neutralize each other as per the chemical reaction.
Moles of KOH is equals to moles of HCL.
Equivalent point of KOH = 0.449 M × 25.0 ml / 0.871 M
= 12.9 ml
To know more about equivalence point here
https://brainly.com/question/31359634
#SPJ4
The complete question is
how many ml of 0.871 m koh is required to reach the endpoint (equivalence point) in a titration with 25.0 ml of 0.449 m HCl ? express your answer in ml.
Help me with this pls

Answers
In 1 angle AOD is an obtuse angle and is 110° and angle GOD is an acute angle and it is 70°.
What is an acute angle and an obtuse angle?90 degrees is the standard for a right angle. Any angle that is less than 90 degrees is called acute, and any angle that is larger than the 90 degrees is called obtuse.
How to use a Protractor for acute angle?Follow the instructions below to use a protractor to measure an angle. Align the angle's vertex with the protractor's dot in the center. Set the protractor such that one angle's side is parallel to 0 degrees. Find the point where the angle's opposite side crosses the number scale by reading the protractor.
To know more about angles visit
https://brainly.com/question/28451077
#SPJ1
Two bottles are connected with a valve. In bottle A, there are 2.4 L of nitrogen gas at 1.8 atm. In bottle B, there are 4.8 L of nitrogen gas at 1.8 atm.
After opening the valve, what is the pressure of nitrogen gas?
Please answer in atmospheres.
I will give brainliest please answer quickly!
My
Answers
Answer:
1.8 atm
Explanation:
For bottle "A"
P1 = 1.8 atm V1 = 2.4L, P2 = ?, V2 = 2.4 + 4.8 L = 7.2 L,
Thus using P1•V1 = P2•V2, we have;
P2 = (1.8 × 2.4)/7.2 = 0.6 atm
For bottle "B"
P1 = 1.8 atm, V1 = 4.8L, P2 = ?, V2 = 7.2L,
P2 = (1.8 × 4.8)/7.2 = 1.2 atm
We will now add the individual pressures of each bottle;
Total pressure = P(A) + P(B)
Total pressure = 0.6 + 1.2
Total pressure = 1.8 atm.
How can a balance be used to measure the amount of gas being produced? Choose the correct answer
Answers
A balance can be used to measure the amount of gas being produced by measuring the change in mass before and after the gas is released.
How to measure the amount of gas being produced?To measure the amount of gas produced, a closed container or reaction vessel is placed on the balance. The initial mass of the container, including the reactants, is recorded. As the reaction proceeds and gas is produced, the total mass inside the container decreases.
By monitoring the change in mass over time, one can determine the amount of gas being produced. The mass difference is attributed to the mass of the gas generated during the reaction.
It is essential to ensure that the reaction vessel is airtight and that no gas escapes during the process. By using a precise and sensitive balance, even small changes in mass can be accurately measured.
This method is commonly used in experiments where the gas produced is difficult to capture directly or when determining the stoichiometry of a reaction involving gases. The balance provides a direct and quantitative measurement of the gas production by monitoring the change in mass.
To know more about stoichiometry, refer here:
https://brainly.com/question/28780091#
#SPJ4
A student titrated 60 mL of 0.2 M HCl solution
with 15 mL of Mg(OH)2 solution. What is the
concentration of the base solution?
Answers
Answer:
The concentration is 0.2 M HCI / 15mL 62g
Explanation:
----->
the force attraction between the particles are ______in solid _____in liquid and______ in gas
Answers
Explanation:
greatest , greater and last
hope it helps
does free energy decreases and the stability of a system increases during which types of reactions?
Answers
Free energy declines as a system's stability rises during a spontaneous transition.
The greatest amount of work performed in a thermodynamic system at constant temperature and pressure is measured using the Gibbs free energy.
Free energy is released during spontaneous reactions, hence the sign of G must be negative. Gibbs free energy varies as a result of exergonic and endergonic reactions. In an exergonic reaction, the products' free energy is lower than the reactants', whereas in an endergonic reaction, the products' free energy is higher than the reactants'.
The reactants of an exergonic reaction have a higher free energy level than the products (reaction goes energetically downhill).
To know more about Gibss free energy, visit,
https://brainly.com/question/14415025#:~:text=Expert%2DVerified%20Answer&text=The%20value%20of%20Gibbs%20free,be%20formed%20and%20occur%20naturally.
#SPJ4
Helpppp what do i do the assisnment is
Data
Record the distance traveled for each of the trials for both floor surfaces in the table below.
Floor Surface
Data Analysis
Trial 1
Trial 2
Trial 3

Answers
Answer:
Everyday Examples of Convection
boiling water - When water boils, the heat passes from the burner into the pot, heating the water at the bottom. This hot water rises and cooler water moves down to replace it, causing a circular motion.
Explanation:
Chromatogram results are considered to have better resolution if the RF values of the components being separated or further apart rather than closer together
Answers
Answer:
False
Explanation:
False : It is better they are closer together
The RF ( retention factor ) is calculated with this formula
= distance travelled / distance travelled by solvent
And the larger the RF factor of a compound the less polar the compound ( i.e. it will not stick to the stationary phase ) hence it is better the RF values of the components to be closer
Answer:
True
Explanation:
The resulting Rf factor, which has no units, is dependent upon the specific stationary and mobile phase used. Chromatogram results are considered to have better resolution if the Rf values of the components being separated are farther apart (e.g. 0.3 and 0.7) rather than closer together (e.g. 0.4 and 0.5). The distance the compound moves relative to the distance the solvent (or mobile phase or eluent) moves is a characteristic of that compound. (where Rf is equal to distance compound moved divided by solvent front distance)
Make a flow chart on uses of metals and non metals
you have to make flow chart with explanation
Answers
Answer:
Metals:
1. Metals are strong than non metals
2. Metals can be formed in wires, thin sheets, utensils and any other heavy meachinary for industries.
3. Metals are used for building construction and used in many other manufacturers
Non Metals:
1. Non metals are less stronger than metals.
2. Non metals can be used for manufacturing bottles, toys, bags, files etc.
3. Non metals are used in making of bottles, jars, chairs etc.
Thank you.....
Have a good day.....
Name the following compound
CH 3 – CH 2 – CH = CH – CH 2 – CH 3
Answers
Answer:
hex-3-ene
remember the least number rule and since the double bond lies at 3rd C-atom from either ways, the compound is named hex (six carbon atoms) -3(position of double bond)-ene (primary suffix for unsaturated organic compound where any two carbons have double bond). hex-3-ene.
if you have any doubt, please feel free to ask :-))
What do you understand by the terms radial node and nodal plane, as applied to AO wavefunctions? Illustrate your answer using the 2s and 2p AOs. Explain why radial nodes arise from the radial part of the wavefunction, whereas nodal planes arise from the angular part of the wavefunction
Answers
In the context of atomic orbital (AO) wavefunctions, the terms "radial node" and "nodal plane" refer to different aspects of the wavefunction's behavior.
A radial node is a region in the AO wavefunction where the probability of finding an electron is zero along the radial direction. In other words, it represents a spherical shell where the electron is unlikely to be found. The number of radial nodes is determined by the principal quantum number (n) of the orbital. For example, the 2s orbital has one radial node, while the 2p orbital has no radial nodes.
On the other hand, a nodal plane is a flat plane within the AO wavefunction where the probability of finding an electron is zero along a particular direction. It represents a surface that divides the orbital into two regions of opposite phases. The number of nodal planes is determined by the angular quantum numbers (l and m) of the orbital. For example, the 2s orbital has no nodal planes, while the 2p orbital has one nodal plane (the xz or yz plane).
Radial nodes arise from the radial part of the wavefunction because they depend on the distance from the nucleus. The radial part determines the distribution of the electron density as a function of distance, and the nodes correspond to regions where the density drops to zero.
On the other hand, nodal planes arise from the angular part of the wavefunction because they depend on the orientation and shape of the orbital. The angular part describes the angular distribution of the electron density around the nucleus, and the nodal planes correspond to regions where the phase of the wavefunction changes sign.
In summary, radial nodes are related to the distance from the nucleus and arise from the radial part of the wavefunction, while nodal planes are related to the orientation and shape of the orbital and arise from the angular part of the wavefunction. The 2s orbital has one radial node and no nodal planes, while the 2p orbital has no radial nodes and one nodal plane.
learn more about radial node here
https://brainly.com/question/31829965
#SPJ11
What is carrying capacity?
Define population.
What environmental problems are
associated with human population
growth?
What events influenced human
population growth?
Answers
Answer:
1) Carrying capacity is the maximum number of individuals of a species that an environment can support.
2) Population - all the inhabitants of a particular town, area, or country.
3) An increase in population will inevitably create pressures leading to more deforestation, decreased biodiversity, and spikes in pollution and emissions, which will exacerbate climate change.
4) The three leading causes of population growth are births, deaths, and migration. Births and deaths are seen as natural causes of population change.
which of the air mass polygons in the source regions folder would most likely be characterized as a continental tropical (ct) air mass?
Answers
To determine which air mass polygon in the source regions folder would most likely be characterized as a continental tropical (ct) air mass, we need to consider the characteristics of this type of air mass.
Continental tropical air masses originate in hot and dry regions, typically over deserts or semi-arid areas. As they move away from their source region, they can become very warm and dry, and are often associated with clear skies and high temperatures.
Based on these characteristics, the air mass polygon in the source regions folder that is most likely to be characterized as a continental tropical air mass would be the one covering the southwestern United States and northern Mexico. This region is known for its hot and dry climate, with temperatures often reaching well above 100 degrees Fahrenheit in the summer months. The air mass that originates from this region would be very warm and dry, and would likely move northward into the central and eastern United States, bringing with it hot and dry conditions.
It is important to note that air masses can change and evolve as they move away from their source region, so it is possible for a continental tropical air mass to become modified or transformed as it moves into different areas. However, based on the characteristics of the source region and the expected behavior of a continental tropical air mass, the southwestern United States and northern Mexico would be the most likely source region for this type of air mass.
for more such question on mass.
https://brainly.com/question/837939
#SPJ11
Choose the situation below that would result in an exothermic ΔHsolution:
a. When |∆Hmx | > |(∆Ho + ∆Ho)|
b. When |∆Hmx | < |(∆Ho + ∆Ho)|
c. When|∆Hmx | is close to |(∆Ho + ∆Ho)|
d. When >>
e. There isn’t enough information to determine.
Answers
The energy released during mixing is greater than the energy required to overcome the forces between solute and solvent particles.
The exothermic or endothermic nature of a solution process can be determined by comparing the magnitude. If the magnitude is smaller than the combined magnitude it means that the energy required to overcome the forces between solute and solvent particles is larger than the energy released during mixing. In this case, the excess energy is released to the surroundings, resulting in an exothermic solution process.
the solution process is exothermic because energy is being released to the surroundings. This indicates that the formation of solute-solvent interactions releases more energy than is required to separate the solute and solvent particles. As a result, heat is released, and the overall process is exothermic.
Therefore, the situation described in option b, is the condition that would result in an exothermic solution.
To learn more about solvent, click here: brainly.com/question/2166468
#SPJ11
describe the relationship between the mass volume and density of material
Answers
Answer: For a given mass and volume, how much physical space a material takes up, of an object or substance, the density remains constant at a given temperature and pressure. The equation for this relationship is ρ = m / V in which ρ (rho) is density, m is mass and V is volume, making the density unit kg/m3.
Explanation:
The pH of a solution is 3.17, what is the (H3O+)Group of answer choices6.8 X 10 minus 4 Molar3.5 X 10 minus 6 Molar3.5 X 10 plus 6 Molar6.8 X 10 plus 4 Molar
Answers
we are given the pH of the solution as 3.17 are we are required to find the [H₃O+]
we know that :
pH = - log[H₃O+]
-pH = log[H₃O+]
10⁻ᵖᴴ = 10ˡᵒᵍ[ᴴ₃ᴼ⁺]
10⁻ᵖᴴ = [H₃O+]
therefore:
10⁻³.¹⁷ = [H₃O+]
6.8x10⁻⁴ M = [H₃O+]
therefore the [H₃O+] is 6.8x10 minus 4 Molar
Why must the final product of a decay series be stable?
a. The daughter isotope of any radioactive decay is stable.
b. No decay chain can emit more than two particles.
c. Each decay results in a net loss of particles.
d. If the daughter isotope is unstable, it will decay.
Answers
Answer:
I think its d
Explanation:
Because isotopes decay due to being unstable so if the daughter isotope is unstable it will just decay till it produces a stable isotope
Answer:
d. If the daughter isotope is unstable, it will decay
Explanation:
got it correct on quiz
a letter stating three things that need urgent fixing in your school
Answers
Answer and Explanation:
Computers ( The computers are very old and very slow, and we have to notice that almost everyone are using computers at the same time at the school, which makes it even harder for it to load up assignments. )The Rick Rolling ( Everyone keeps sending links to teachers and students saying that it is part of some assignment but then you have to listen to Rick Astley, they should really block these links. )The lockers ( The lockers are also very old and they are breaking down and rusting a lot from the moisture in the hallways. One of the lockers even broke down today!!! I hope they can fix this so no one else gets hit with a locker door. )Hope this helps! ;)
If it took 25.0 mL of 0.108 M Ag to reach the equivalence point in this titration, what is the mass percentage of arsenic in the pesticide
Answers
To find the mass percentage of arsenic in the pesticide, we can use the following formula:
mass percentage of arsenic = (mass of arsenic / total mass of pesticide) * 100%
where the mass of arsenic is the mass of arsenic in the solution that was used to reach the equivalence point, and the total mass of pesticide is the total mass of pesticide in the sample.
We are given the following information:
The titration solution contained 0.108 M Ag.
It took 25.0 mL of the titration solution to reach the equivalence point.
Using these values, we can calculate the mass of arsenic in the solution as follows:
mass of arsenic = volume of solution * molar mass of Ag
mass of arsenic = 25.0 mL * 107.87 g/mol = 275.75 g
We can then use the formula above to find the mass percentage of arsenic in the pesticide:
mass percentage of arsenic = (mass of arsenic / total mass of pesticide) * 100%
mass percentage of arsenic = (275.75 g / total mass of pesticide) * 100%
where the total mass of pesticide is not given in the problem.
We will need to use another piece of information from the problem to find the total mass of pesticide:
The pesticide was diluted to 0.10 M.
Using this information, we can calculate the total mass of pesticide as follows:
total mass of pesticide = volume of pesticide * molar mass of pesticide
total mass of pesticide = 100 mL * 5.25 g/mL * 1.0 g/mol = 525 g
Substituting the values into the formula above, we get:
mass percentage of arsenic = (275.75 g / 525 g) * 100% = 53.48%
Therefore, the mass percentage of arsenic in the pesticide is approximately 53.48%.
Learn more about pesticide Visit : brainly.com/question/6589507
#SPJ11
Aqueous sodium carbonate reacts with aqueous iron (II) chloride to produce solid iron (II) carbonate and aqueous sodium chloride with a yield of 92.5 %. Calculate the mass (in grams) of solid copper (II) carbonate produced if 94.7 mL of 0.121 M iron (II) chloride reacts with 2.56 x1021 formula units of sodium phosphate.
Answers
In the given chemical reaction 455 grams of solid iron (II) carbonate will be formed.
The balanced chemical equation for the reaction is:
Na2CO3 + FeCl2 ---> FeCO3 + 2NaCl
From the equation, we can see that the stoichiometry of Na2CO3 to FeCO3 is 1:1. So, the moles of FeCO3 produced will be the same as the moles of Na2CO3 used.
First, let's calculate the moles of FeCl2:
moles of FeCl2 = concentration x volume
moles of FeCl2 = 0.121 mol/L x 0.0947 L
moles of FeCl2 = 0.0115 mol
The reaction yield is 92.5%, so the actual moles of FeCO3 produced will be:
moles of FeCO3 = yield x moles of Na2CO3
moles of FeCO3 = 0.925 x (2.56 x 10^21 / Avogadro's number)
moles of FeCO3 = 0.925 x 4.25 mol
moles of FeCO3 = 3.93 mol
Therefore, the mass of FeCO3 produced can be calculated using its molar mass:
mass of FeCO3 = moles of FeCO3 x molar mass
mass of FeCO3 = 3.93 mol x 115.86 g/mol
mass of FeCO3 = 455 g
Therefore, it can be inferred that 455 grams of solid iron (II) carbonate will be produced.
Learn more about balanced chemical equation :
https://brainly.com/question/28294176
#SPJ4
Show a numerical setup for calculating the quantity of heat in joules required to completely vaporize 102.3 grams of H2O(l) at 100°C and 1.0 atm.
Answers
Answer:
The heat required to vaporize 102.3 grams of H₂O(l) is 231198 J.
Explanation:
The heat required to vaporize 102.3 g of H₂O(l) can be calculated as follows:
\( q = m*\Delta H_{v} \)
Where:
q: is the heat
ΔHv: is the heat of vaporization of water = 2260 J/g
m: is the mass = 102.3
\(q = m*\Delta H_{v} = 102.3 g*2260 J/g = 231198 J\)
Therefore, the heat required to vaporize 102.3 grams of H₂O(l) is 231198 J.
I hope it helps you!
The heat required to vaporize 102.3 grams of H₂O(l) is 231198 J.
The heat required to vaporize 102.3 g of H₂O(l) can be calculated as follows:
\(q=m\times H_v\).....(1)
Here, q is the heat
\(H_{v}\): is the heat of vaporization of water = 2260 J/g
m: is the mass = 102.3 g
Substitute the value in equation (1) as follows:-
\(q=102.3\ g\times2260\ J/g\\\\=231198\ J\)
Therefore, the heat required to vaporize 102.3 grams of H₂O(l) is 231198 J.
To know more about:-
brainly.com/question/12625048
Write the balanced chemical equation for this reaction. Phases are optional. Lead(IV) oxide decomposes to yield lead(II) oxide and a colorless gas
Answers
The balanced chemical equation for the given reaction can be written as:
PbO2 → PbO + O2
This equation indicates that lead(IV) oxide decomposes to yield lead(II) oxide and a colorless gas, which in this case is oxygen. The balanced equation shows that for every one molecule of PbO2 that decomposes, one molecule of PbO and one molecule of O2 are produced. The chemical equation is balanced because the number of atoms of each element is the same on both sides of the equation.
It is important to note that the state of the reactants and products is optional, and may or may not be included in the equation. In this case, the states are not specified, so we can assume that they are in their standard states.
Overall, the balanced chemical equation for the decomposition of lead(IV) oxide helps us to understand the stoichiometry of the reaction and the amounts of reactants and products involved.
To learn more about chemical click here: brainly.com/question/29240183
#SPJ11
consider the reaction when aqueous solutions of barium hydroxide and cobalt(ii) bromide are combined. the net ionic equation for this reaction is:
Answers
The net ionic equation is;
Co^2+(aq) + OH^-(aq) -----> Co(OH)2(s)
What is the net ionic reaction?The net ionic reaction is a chemical reaction that describes the species involved in a reaction that undergo a change (i.e., reactants and products) and exclude spectator ions, which do not participate in the reaction and remain in their original form. The net ionic equation only includes ions that are changed in the reaction.
The molecular reaction equation is;
Ba(OH)2 + CoBr2 → BaBr2 + Co(OH)2
The net ionic equation is;
Co^2+(aq) + OH^-(aq) -----> Co(OH)2(s)
Learn more about ionic equation:https://brainly.com/question/29299745
#SPJ1
please balance the equation i really need it quick 50 points and marked brainliest answer
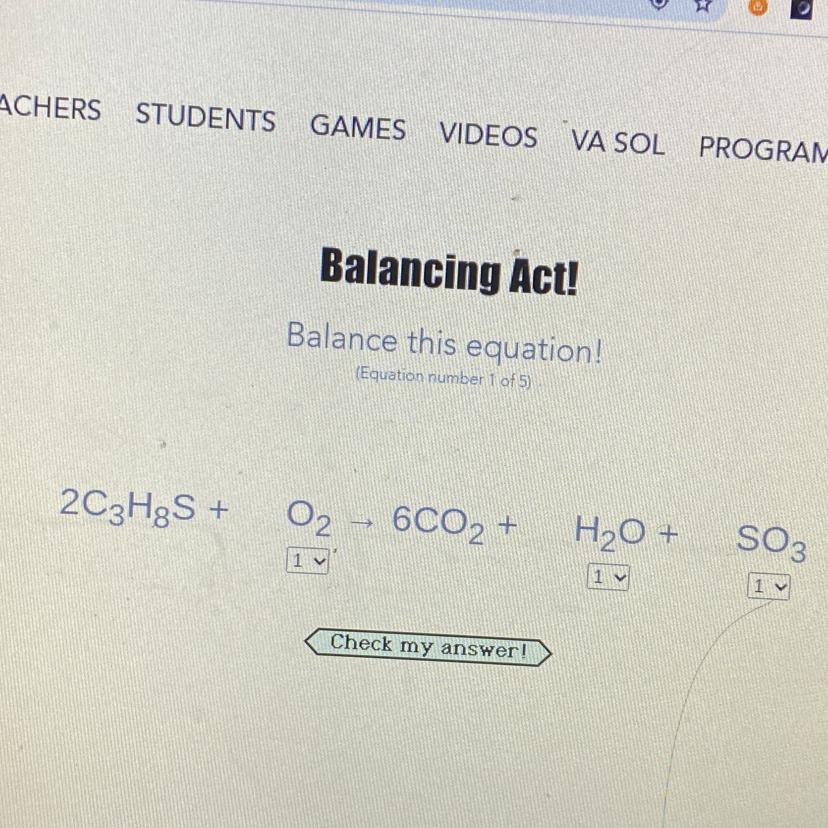
Answers
Answer:
2C3H8S +13O2 =6CO2 +8H20 +2SO3
Answer:
get from (4,1) to (2,9) go up 8 and over 2 left (-2) - (4 - 2, 1 + 8), do this again (2 - 2, 9+8) and end up at (0,17) which is the y how do you change 7x+3y=3 into slope intercept form.
get from (4,1) to (2,9) go up 8 and over 2 left (-2) - (4 - 2, 1 + 8), do this again (2 - 2, 9+8) and end up at (0,17) which is the y how do you change 7x+3y=3 into slope intercept form.
get from (4,1) to (2,9) go up 8 and over 2 left (-2) - (4 - 2, 1 + 8), do this again (2 - 2, 9+8) and end up at (0,17) which is the y how do you change 7x+3y=3 into slope intercept form.
get from (4,1) to (2,9) go up 8 and over 2 left (-2) - (4 - 2, 1 + 8), do this again (2 - 2, 9+8) and end up at (0,17) which is the y how do you change 7x+3y=3 into slope intercept form.
how much energy should be transferred when 18.2 g of ammonia is formed during the reaction of hydrogen gas with nitrogen gas? add the energy term to the correct side of the equation.
Answers
The formation of 18.2 g of ammonia (NH3) from the reaction of hydrogen gas (H2) with nitrogen gas (N2) involves the release or absorption of energy.
To determine the amount of energy transferred, we need the enthalpy change per mole of ammonia formed, which is typically expressed as ΔHf (the enthalpy of formation). The balanced equation for the reaction is:
N2(g) + 3H2(g) → 2NH3(g)
The energy term should be added to the correct side of the equation based on whether the reaction is exothermic (energy released) or endothermic (energy absorbed). Without the specific value for the enthalpy of formation (ΔHf) of ammonia, we cannot calculate the exact amount of energy transferred.
In the reaction, if the enthalpy change (ΔH) is negative (exothermic), it means energy is released during the formation of ammonia. If ΔH is positive (endothermic), it means energy is absorbed. To determine the energy transferred, we would multiply the amount of ammonia formed (18.2 g) by the enthalpy change per mole (ΔHf) of ammonia.
In summary, to calculate the amount of energy transferred during the formation of 18.2 g of ammonia, we need the specific enthalpy of formation (ΔHf) of ammonia, which is not provided. The energy term should be added to the correct side of the equation based on whether the reaction is exothermic or endothermic.
Learn more about ammonia here:
https://brainly.com/question/29519032
#SPJ11
the question is~ Where does the heat come from for geothermal energy?

Answers
Answer:
The Earth
Explanation:
Answer:
The Earth
Explanation:
Geothermal energy comes from the sub surface of the Earth. It is contained in the rocks and fluids beneath the earth's crust and can be found as far down to the earth's hot molten rock, magma.