How many minutes are In 3,417 seconds?
Answers
Answer:
57 minutes and 35 seconds, or 56.95
Explanation:
Answer:
56.95 minutes
Explanation:
hope it helps
Related Questions
Explain why solids and liquids are difficult to compress
whereas gases can easily be compressed.
Answers
Answer:
Because they take up a certain amount of volume because there is no space in-between the particles, a gases can be compressed because there is alot of space in-between each particle
Explanation:
In permanent waving, panels of hair are divided into smaller subsections called _____.
Select one:
a. outer sections
b. rod sections
c. placements
d. base sections
Answers
In permanent waving, panels of hair are divided into smaller subsections called base sections.
Base sections are the smaller subsections into which the hair is divided before being wound onto perm rods during a permanent waving treatment. The size and shape of the base sections, as well as the size and type of perm rods used, will determine the final curl pattern achieved. The base section is the starting point of the perm wrap and helps to anchor the hair onto the perm rod. The tension and direction in which the hair is wrapped around the perm rod will also affect the final result. The size of the base section can vary depending on the desired result, hair length, and density. The stylist will choose the appropriate base section size and perm rod based on the client's hair and desired outcome.
To learn more about subsections, Click here; brainly.com/question/28433464
#SPJ11
What substance is present if the FeCl3 test gives a purple color? Which sample is the most impure?
Answers
FeCl_{3} is a chemical reagent used to detect the presence of phenols in a sample. When FeCl3 reacts with phenols, it forms a purple color complex.
This complex is due to the formation of an iron-phenol complex, which has a distinct purple color. Therefore, if the FeCl_{3} test gives a purple color, it indicates the presence of phenols in the sample. The sample that gives the darkest purple color is likely to be the most impure. This is because a higher concentration of phenols in the sample will result in a stronger reaction with FeCl_{3} , leading to a more intense purple color. However, it is important to note that the intensity of the purple color may not always correlate with the degree of impurity, as other factors such as the concentration of the sample and the reaction conditions may also affect the color intensity. In summary, the FeCl_{3} test is a useful tool for detecting the presence of phenols in a sample, and the intensity of the purple color can provide an indication of the degree of impurity in the sample.
learn more about chemical reagent Refer: https://brainly.com/question/30256313
#SPJ11
A company is trying to design new carbon monoxide detectors that register carbon monoxide at a level of 5 parts per million (ppm). The company engineers decide that the detector needs improvement and start designing new versions. They develop each new version based on new ideas and the features that worked in the previous one. They continue testing each new version in the process. Which process does this best describe?
Answers
The question is incomplete; the complete question is;
A company is trying to design new carbon monoxide detectors that register carbon monoxide at a level of 5 parts per million (ppm). The company engineers decide that the detector needs improvement and start designing new versions. They develop each new version based on new ideas and the features that worked in the previous one. They continue testing each new version in the process. Which process does this best describe?
making trade-offs
the iterative design process
process and system optimization
evaluating advantages and disadvantages
Answer:
the iterative design process
Explanation:
The Iterative design is a circular design process which comprises of models that are regularly evaluated and improved based on the results of testing of previous models or versions.
Hence, when the company is involved in developing a new version of the carbon monoxide detector by evaluating the older versions and continue testing each new version, they are involved in the circular process of iterative design.
True or false; A solution always contains only one solvent.
Answers
A solution is defined as a mixture of two or more substances, usually, a solute and a solvent, and the difference between these two are in quantity, solute represents the smallest amount and solvent will represent the highest amount, and while you can have more than one solute, you can only have one solvent for a solution. Therefore the statement is true
9. During a titration, 50.0 ml of 0.2M NaOH were required to neutralize 50.0ml of H_{3}*P * O_{4} What's the concentration of the H_{3}*P * O_{4} solution?
Answers
Answer:
0.067M H3PO4
Explanation:
H3PO4 reacts with NaOH as follows:
H3PO4 + 3NaOH → 3H2O + Na3PO4
Where 1 mole of H3PO4 reacts with 3 moles of NaOH
To solve trhis question we need to find the moles of NaOH required. With the chemical equation we can find the moles of H3PO4 and its concentration as follows:
Moles NaOH:
50.0mL = 0.0500L * (0.20moles /L) = 0.0100 moles NaOH
Moles H3PO4:
0.0100 moles NaOH * (1mol H3PO4 / 3mol NaOH) = 0.00333 moles H3PO4
Concentration:
0.00333 moles H3PO4 / 0.0500L = 0.067M H3PO4
The equilibrium constant for the formation of hydrogen iodide from hydrogen and iodine is 45 at a certain temperature. H2(g)+I2(s) <---> 2HI(g)
Which of the following is true regarding this equilibrium?
Answers
The reaction favors the formation of HI(g) (product) over the presence of H2(g) and I2(s) (reactants) at equilibrium, as indicated by the equilibrium constant (K) of 45.
Based on the given equilibrium reaction:
H2(g) + I2(s) ⇌ 2HI(g)
The equilibrium constant (K) for this reaction is given as 45.
The equilibrium constant provides information about the relative concentrations of the reactants and products at equilibrium. It can also indicate the extent to which the reaction favors the formation of products.
Regarding the given equilibrium, the following statements can be made:
1. If the value of the equilibrium constant (K) is greater than 1 (which is the case here with K = 45), it indicates that the reaction favors the formation of products. In this case, the formation of HI(g) is favored over the presence of H2(g) and I2(s) at equilibrium.
2. The stoichiometric coefficient of each species in the balanced equation relates to their powers in the expression of the equilibrium constant. Since the stoichiometric coefficient of HI(g) is 2, it means that the concentration of HI(g) is squared in the equilibrium expression.
3. The equilibrium constant is a dimensionless quantity and does not depend on the absolute values of concentrations or pressures. It only depends on the ratio of the concentrations of products to reactants at equilibrium.
Therefore, the correct statement regarding this equilibrium is:
- The reaction favors the formation of HI(g) (product) over the presence of H2(g) and I2(s) (reactants) at equilibrium, as indicated by the equilibrium constant (K) of 45.
To know more about Equation related question visit:
https://brainly.com/question/28774287
#SPJ11
Can someone please help

Answers
Answer:
1. c) shiny
2) True. Reactivity is a chemical property.
1. A metal is shiny
2. yes
that's all
Which of the following options correctly describe enolate formation for a ketone such as the one shown? Select all that apply.
a. Deprotonation of the alpha carbon
b. Formation of a double bond between the alpha carbon and carbonyl carbon
c. Loss of a proton from the carbonyl oxygen
d. Formation of a nucleophile
Answers
The following options correctly describe enolate formation for a ketone such as the one shown are:
a. Deprotonation of the alpha carbon
d. Formation of a nucleophile
For a ketone, the process of enolate formation involves the following options:
a. Deprotonation of the alpha carbon: This option is correct. Enolate formation involves the removal of a proton (deprotonation) from the alpha carbon adjacent to the carbonyl group. The resulting species is called an enolate.
b. Formation of a double bond between the alpha carbon and carbonyl carbon: This option is incorrect. Enolate formation does not involve the direct formation of a double bond between the alpha carbon and the carbonyl carbon. Instead, the enolate is formed as an intermediate, which may subsequently tautomerize to form an enol, where the double bond forms between the alpha carbon and the carbonyl carbon.
c. Loss of a proton from the carbonyl oxygen: This option is incorrect. In the process of enolate formation, a proton is not lost from the carbonyl oxygen. Instead, a proton is removed from the alpha carbon, resulting in the formation of the enolate.
d. Formation of a nucleophile: This option is correct. Enolate formation leads to the creation of a nucleophilic species. The negative charge that develops on the oxygen of the carbonyl group makes it nucleophilic, and the alpha carbon becomes electron-rich and capable of nucleophilic attack.
Therefore, correct options are:
a. Deprotonation of the alpha carbon
d. Formation of a nucleophile
To know more about enolate formation here
https://brainly.com/question/31558549
#SPJ4
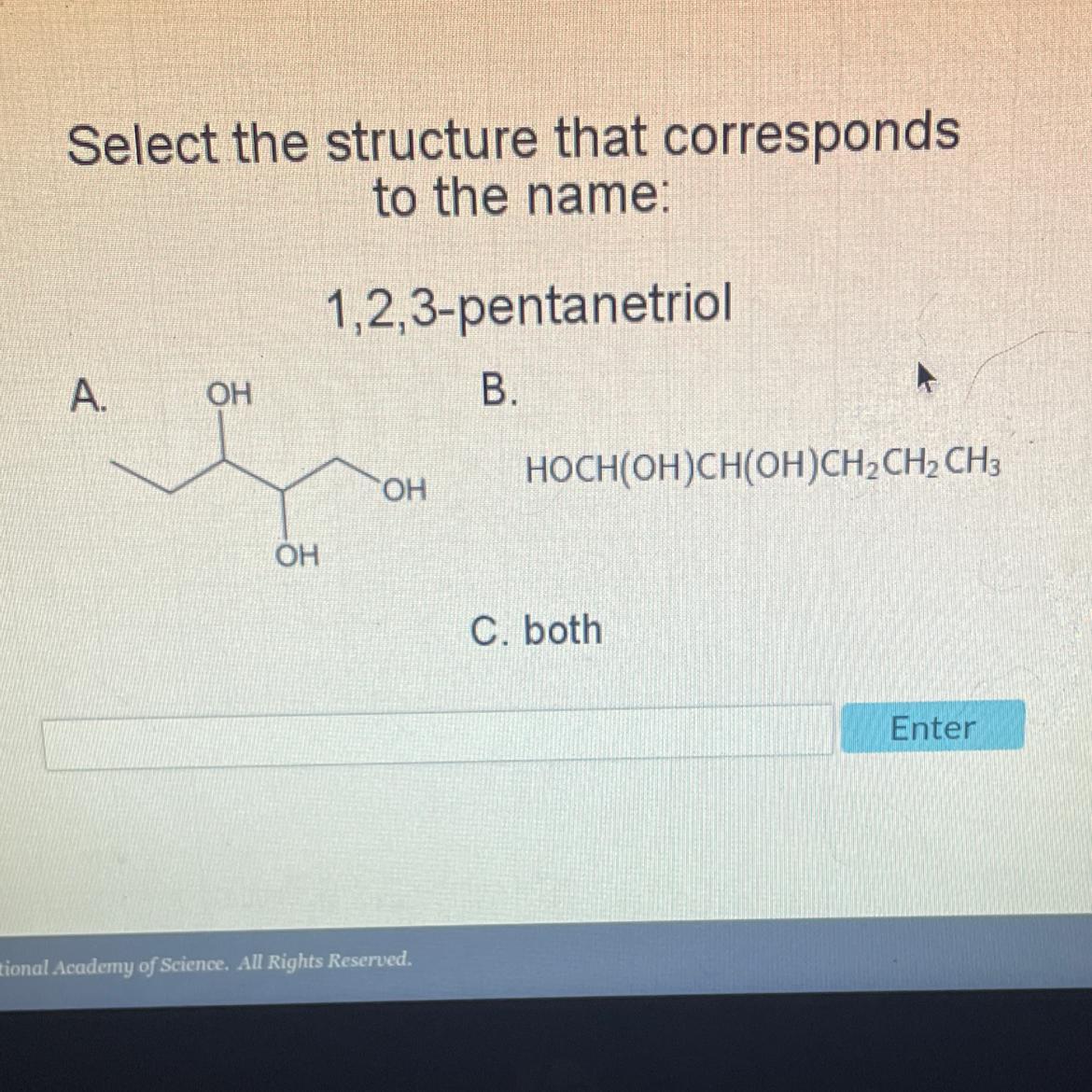
Select the structure that corresponds
to the name:
A.
OH
OH
1,2,3-pentanetriol
B.
OH
HOCH(OH)CH(OH)CH2 CH₂ CH3
C. both
Enter
Answers
The structure that corresponds to the name 1,2,3-pentanetriol is structure A. Hence, option A is correct.
Structure A shows a molecule with three hydroxyl (-OH) functional groups attached to a pentane chain. The prefix "pent-" indicates that the chain has five carbon atoms, while the suffix "-triol" indicates that there are three hydroxyl groups present in the molecule.
In the name "1,2,3-pentanetriol", the numbers indicate the positions of the hydroxyl groups on the pentane chain. The hydroxyl groups are located on the first, second, and third carbon atoms, respectively.
The structure in option A matches this description, with three hydroxyl groups located on the first, second, and third carbon atoms of the pentane chain.
To learn more about the structural formula, follow the link:
https://brainly.com/question/2120948
#SPJ1
How many moles of NH3 can you make from 6.20 moles of N2?
Answers
$ hope it welp
The term mole concept is used here to determine the moles of ammonia. The number of moles of ammonia which can be make from 6.20 moles of N₂ is 12.4.
What is a mole?One mole of a substance is defined as that quantity of it which contains as many entities as there are atoms exactly in 12 g of carbon - 12. The formula used to calculate the number of moles is:
Number of moles = Given mass / Molar mass
You need one nitrogen atom to produce ammonia. Here we can see that there are two nitrogen atoms in N₂.
One mole of any substance contains Avogadro number of molecules. A mole is defined as the mass of the substance which consists of the equal quantity of basic units.
The number of moles of ammonia from 6.20 moles of N₂ is:
6.20 × 2 = 12.4
Thus the number of moles is 12.4.
To know more about mole concept, visit;
https://brainly.com/question/19730733
#SPJ2
Select the net ionic equation for the reaction that occurs when sodium hydroxide and sulfuric acid are mixed. o No reaction occurs o 2Na+ (aq) + 2OH(aq) + 2H(aq) + SO2 (aq) → 2Na+ (aq) + SO, (aq) + 2 H2O(g) o H(aq) + OH(aq) → H2O
o H+(aq) + HSO4(aq) + 2OH- (aq) → 2 H2O(l) + SO4- (aq) o 2 Na+ (aq) +20H(aq) + 2H+ (aq) + S0,2-(aq) → 2 Na+ (aq) + SO42+ (aq) + 2 H2O(l) o 2 Na+ (aq) + SO42-(aq) → Na2SO4 (g)
Answers
The correct net ionic equation for the reaction that occurs when sodium hydroxide and sulfuric acid are mixed is H⁺(aq) + OH⁻(aq) → H₂O(l).
When sodium hydroxide (NaOH) and sulfuric acid (H₂SO₄) are mixed, they react to form water (H₂O) and sodium sulfate (Na₂SO₄). The balanced chemical equation for this reaction is:
2 NaOH(aq) + H₂SO₄(aq) → 2 H₂O(l) + Na₂SO₄(aq)
To find the net ionic equation, we need to separate all the aqueous species into their respective ions:
2 Na⁺(aq) + 2 OH⁻(aq) + 2 H⁻(aq) + SO₄²⁻(aq) → 2 H₂O(l) + 2 Na⁺(aq) + SO₄²⁻(aq)
Next, we can cancel out any spectator ions that appear on both sides of the equation. In this case, the sodium ions (Na⁺) and the sulfate ions (SO₄²⁻) are spectator ions. After canceling out the spectator ions, we are left with the net ionic equation:
2 OH⁻(aq) + 2 H⁻(aq) → 2 H₂O(l)
Simplifying the equation, we get:
H⁺(aq) + OH⁻(aq) → H₂O(l).
This is the correct net ionic equation for the reaction that occurs when sodium hydroxide and sulfuric acid are mixed.
Learn more about net ionic equation here: https://brainly.com/question/25604204.
#SPJ11
If you made a three-dimensional model of an atom and its nucleus, how would you represent the atom? how would you represent nucleus?.
Answers
To represent an atom, you could use a sphere to represent the electron cloud, with the nucleus in the center.
What is atom?
Each atom is made up of a nucleus one and or more electrons that are bound to it. One or even more protons and a significant number of neutrons make up the nucleus. Only the most prevalent type of hydrogen is neutron-free. Atoms that are neutral or ionised make up every solid, liquid, gas, as well as form of plasma. Atoms are incredibly tiny, measuring typically 100 picometers across. Due to quantum effects, they are so small that it is impossible to predict their behaviour with sufficient accuracy using classical physics, as would be the case, for example, if they were tennis balls.
The nucleus could be represented by a smaller sphere or a cube, depending on the size you want to make it. The nucleus is composed of protons and neutrons, so the cube or sphere could be made to represent these particles, with each particle being a different color or size. This way, it would be easier to distinguish between the electron cloud and the nucleus.
To learn more about atom
https://brainly.com/question/26952570
#SPJ4
which of these is a redox process? i. rusting of iron ii. respiration iii. burning hydrocarbons group of answer choices i only i, ii, and iii i and ii i and iii iii only
Answers
The redox process involves both reduction (gain of electrons) and oxidation (loss of electrons). The correct answer is i and iii - rusting of iron and burning hydrocarbons are both redox processes. Respiration is also a redox process, but it was not included as an option in the answer choices.
The redox process involves both reduction (gain of electrons) and oxidation (loss of electrons). Among the given options, rusting of iron, respiration, and burning hydrocarbons are all redox processes. So, the correct answer is: i, ii, and iii. Respiration is also a redox process, but it was not included as an option in the answer choices.
To know more about redox process visit:
https://brainly.com/question/29969536
#SPJ11
How many hydrogens are in 4H207?
Answers
Hope that helped
It’s 8 because you mulitiply 4x2 so if you had to do oxide(oxygen) it would be 4x7 cuss you multiply the number added before H2O7 and the small numbers already there (deside it) is multiplied
Hope this helps you
What Celsius temp, T2, is required to change the volume of the gas sample in part A (T1=43 degrees Celsius, V1 = 1.65x 10^3 L) to a volume of 3.30x10^3L? Assume no change in pressure or amount of gas in the balloon.

Answers
The temperature, T2, is 359°C.
1st) We need to identify the volume and the temperature of the gas in the point A (initial state of gas) and point B (final state of gas):
- Point A:
V1= 1.65x10^3 L
T1= 43°C (316K)
-Point B:
V2= 3.30x10^3 L
T2= unknown
2nd) With the Ideal Gas Law and assuming there is no change in pressure or amount of gas in the balloon, we calculate the temperature in point B with the formula that relates temperature and volume:
\(\begin{gathered} \frac{V_1}{T_1}=\frac{V_2}{T_2} \\ \frac{1.65x10^3L_{}}{316K}=\frac{3.30x10^3L}{T_2} \\ T_2\cdot1.65x10^3L=3.30x10^3L\cdot316K \\ T_2=\frac{3.30x10^3L\cdot316K}{1.65x10^3L} \\ T_2=632K \end{gathered}\)It is important to use the units of the ideal gas constant, so the units must be in Kelvin (K) and liters (L). That's why the temperature (T2) it is 316 K.
3rd) Finally, it is necessary convert the Kelvin unit into Celsiud degrees:
\(\begin{gathered} T_2=632K-273 \\ T_2=359^oC \end{gathered}\)So, the temperature, T2, is 359°C.
What is physical weathering?
the building up of rock that has been brought from other places
the breaking of rock into smaller pieces from chemical processes
the breaking of rock into smaller pieces from nonchemical processes
the moving of rock from one place to another
Answers
Physical weathering is caused by physical processes such as changes in temperature, freezing and thawing, and the effects of wind, rain and waves.
hope this helped
Which element is placed in the same period as ruthenium but has a higher atomic number than it?
A.bismuth
B.osmium
C.silver
D.zirconium
Answers
Answer:
The part that is put in the same time as ruthenium, but has a greater number of atoms than silver.
Answer:
The answer is silver.
Explanation:
edge 2022, trust
What is the intermolecular force of h2co.
Answers
The intermolecular force of H₂CO is dipole-dipole force.
Dipole-dipole force as an intermolecular forceDipole-dipole forces are attractive intermolecular forces that exist between the positive end of one polar molecule and the negative end of another polar molecule.
In order for dipole-dipole forces to be present, a molecule must be polar. The positive end of one molecule is attracted to the partial negative end of another H₂CO as a polar moleculeH₂CO is a polar molecule due to the differences in the electronegativity of carbon and oxygen.
Oxygen is more electronegative than carbon, hence, some negative charge is induced on the oxygen atom and a partial positive charge is induced on the carbon atom.
The induced positive and negative charges generates the dipole moment directed from carbon to oxygen atom.
Therefore, molecules of H₂CO are held together by dipole-dipole attractions between the oxygen and carbon atoms.
Learn more about intermolecular forces at: https://brainly.com/question/13588164
Use properties of exponents to simplify the given expression. Express the answer in exponential form. (3^(7))/(3^(3))
Answers
Expressing the answer in exponential form we get 3⁴.
To simplify the expression (3⁷/(3³), we can apply the properties of exponents. When dividing two exponential expressions with the same base, we subtract the exponents.
In this case, we have 3⁷ divided by 3³, which can be simplified as:
3⁽⁷⁻³⁾
3⁴
Therefore, the simplified expression is 3⁴.
To understand why we subtract the exponents when dividing, we can break down the steps.
The expression 3⁷ represents 3 multiplied by itself seven times:
3 × 3 × 3 × 3 × 3 × 3 × 3.
The expression 3³ represents 3 multiplied by itself three times:
3 × 3 × 3.
When dividing these two expressions, we can cancel out common factors by subtracting the exponents:
(3 × 3 × 3 × 3 × 3 × 3 × 3) / (3 × 3 × 3)
This simplifies to:
3 × 3 × 3 × 3
Which is equivalent to 3⁴.
Thus, the answer in exponential form is 3⁴
Learn more about properties -
brainly.com/question/29547278
#SPJ11
4. What coefficients do you need to balance the following equation?
CH4 + O2 + CO2 + H2O
O 1, 2, 1, 2
O 2, 1, 1, 1
O 1, 1, 1, 1
O 2, 1, 2, 2
Answers
Answer:
option first is the right answer
a reaction takes place in a flexible container initially at 298 k and volume of 3.00 liters. a reaction takes place causes the container to decrease in volume to 2.50 liters and its temperature becomes 273 k. what is the sign of the enthalpy change involved in this reaction and what is the sign of the work being done?
Answers
The sign of the enthalpy change involved in the reaction is negative and the sign of work done is also negative.
A reaction takes place in a flexible container initially at 298 K and volume 3L .then reaction takes place causes decrease in the volume to 2.5L and its temperature becomes 273 K.
T1 = 298 K
V1 = 3 L
V2 = 2.5 L
T2 = 273 K
The Enthalpy change is the name given to the amount of heat evolved or absorbed in a reaction carried out at constant pressure. It is given the symbol ΔH.
H = U+PV
sign of enthalpy change involved in the reaction,
Volume decreases --> PV will decrease
T decreases --> change in energy decreases
so the enthalpy change must be negative, since the change or content of energy is getting lower and same in work done.
To know more about enthalpy change please visit:
https://brainly.com/question/16387742
#SPJ4
Which answer choice correctly lists only elements?
A
Cl, Na, CH3, Ne
B
Mg, NO2, Mg, B
C
H2O, Li, N, I2
D
N2, O2, Al, Zn
Group of answer choices
A
Answers
Answer:
D
Explanation:
Nitrogen^2, Oxygen^2, Aluminum, Zinc
the others have combination
what are examples of things that are not solids liquids or gas
Answers
Answer:
Plasma which is the 4th element, examples are lightning,
aurorae, solar wind, and many more.
Explanation:
Just look up examples of plasma.
What is the Sl unit for energy? Be sure to give the full name for the unit
Answers
Answer:
Joules
Explanation:
when a solution evaporates, what is the sign of δg?
Answers
The system's increased disorder is balanced by the energy needed for vaporization. Due to the fact that δg = 0, the liquid and vapor are in an equilibrium, which is true for any liquid at its boiling point under normal circumstances.
The increase in systemic disorder is balanced by the energy needed for the vaporization process. Therefore, δg is equal to 0, which indicates that the liquid and vapor are in equilibrium. Under normal circumstances, any liquid at its boiling point.
A chemical reaction's general direction is determined by the sign of δg, which also indicates whether or not a reaction is spontaneous. When δg is equal to 0, it is clear that the system is in a state of general equilibrium. None of the reactions show a net change in either the forward or reverse direction.
Learn more about δg
brainly.com/question/10948314
#SPJ4
What is the percent yield for a certain reaction when 56.8 g of product was
obtained in the lab, but the theoretical yield was 64.4g?
Answers
Answer:
88.20%
Explanation:
% yields =( experimental yield /theoretical yield )x100%
= ( 56.8 ÷ 64.4 ) x100%
= 88.20%
The percent yield for the given reaction where in the product obtained is 56.8 g is 88.19%.
What is percent yield?Percent yield is defined as the ratio of actual yield to the theoretical yield multiplied by 100. If the actual and theoretical yield are same then the percent yield is 100%.If actual yield is less than the theoretical yield then the percent yield is less than 100%.Reason of this condition arising is the incompletion of reaction or loss of sample during recovery process.
In cases where percent yield is over 100% it indicates that more sample is recovered than the predicted amount.This condition arises when there are other simultaneous reactions taking place leading to the formation of product. It can also arise if there is incomplete removal of impurities from the sample .In the given example , percent yield=56.8/64.4×100=88.19%.
Thus, the percent yield of reaction is 88.19 %.
Learn more about percent yield,here:
https://brainly.com/question/2506978
#SPJ2
Compound A is heated with silver Powder and give compound B. Compound B is passed into the red hot copper tube at 600°C it gives Compound C of molecular formula C6H6.
i)identify Compound A and B with IUPAC name.
ii) How do you prove that the acidic nature of compound B?
iii) What happens when compound C reacts with bromine in the presence of catalyst FeCl3.
iv) Convert Compound C into Toulene.
Answers
Compound A is likely an organic halide, Compound B is a derivative of benzene, Compound C is benzene itself, and Compound C can be converted into toluene through a Friedel-Crafts alkylation reaction.
i) Compound A is an alkene.
When heated with silver powder, it undergoes oxidative cleavage to produce Compound B which is an aldehyde.
So the IUPAC names of Compound A and Compound B are ethene and ethanal, respectively.
ii) The acidic nature of Compound B can be proved by treating it with sodium hydrogen carbonate. If effervescence occurs, it is due to the evolution of carbon dioxide gas.
This indicates that Compound B is acidic in nature and reacts with a base to form salt and water.
iii) When Compound C (Benzene) reacts with bromine in the presence of catalyst FeCl3, Bromine water is decolorized to form a colorless solution.
This is an addition reaction that occurs due to the presence of an electron-rich benzene ring.
iv) Compound C (Benzene) can be converted into Toluene (Methylbenzene) through a process known as Friedel-Crafts Alkylation, where Benzene is allowed to react with Chloromethane (Methyl chloride) in the presence of Lewis acid catalyst, Aluminum chloride (AlCl3).
The resulting product is then heated to obtain Toluene (Methylbenzene).
The chemical reaction for the conversion of Benzene to Toluene is given below:C6H6 + CH3Cl → C6H5CH3 + HCl
For such more questions on Friedel-Crafts
https://brainly.com/question/30900581
#SPJ8
A particle accelerates from rest with an acceleration of 8.0m/s2. Calculate its velocity after 150s. What kinematic formula must be used to solve this problem?
Sample answer format: c=a+b (no spaces in between). If subscript is needed type it as it is , for example cf=ai+b.
Answers
Answer:
Final velocity: 1200 m/s
Explanation:
Formula: \(\overline{a} = \frac{v - v_0}{t} = \frac{\Delta v}{\Delta t}\)
Where:
a = average acceleration
v = final velocity
vo = starting velocity
t = elapsed time
Here the final velocity is unknown which we have to find. The initial is 0 m/s
as it was at rest. The time taken: 150 seconds. Acceleration given 8.0 m/s²
using the formula:
\(\overline{a} = \frac{v - v_0}{t}\)
\(8 = \frac{v - 0}{150}\)
\(v = 1200\) m/s
Please help! Worth 20 points and I’ll give brainliest.
