Answers
Answer: 7.505
×
10
22
atoms. (rounded to third place of decimal)
Explanation:
Related Questions
• How does the name of the salt tell us that:
a) there is just one other element combined with the metal?
b) there is oxygen present in the salt?
Answers
The name of the salt tells us that:
a) there is just one other element combined with the metal by looking at the suffix of the salt's name.
b) the presence of oxygen in a salt can be indicated by the name of the salt.
a) The name of a salt can tell us that there is just one other element combined with the metal by looking at the suffix of the salt's name. If the salt name ends in "-ide," it indicates that the salt is composed of a metal and a single non-metal element.
For example, sodium chloride (NaCl) and potassium bromide (KBr) are salts where the metal (sodium and potassium) is combined with a single non-metal element (chlorine and bromine, respectively). The "-ide" suffix suggests the presence of only one other element in the salt.
b) The presence of oxygen in a salt can be indicated by the name of the salt. If the salt name includes the element oxygen, it suggests that oxygen is present in the salt compound.
For example, sodium carbonate (Na₂CO₃) and calcium sulfate (CaSO₄) contain the element oxygen in their chemical formulas. The presence of oxygen in the salt is implied by the name and the combination of elements in the compound.
Therefore, the name of salt tells us that there is just one other element combined with the metal and there is oxygen present in the salt
Learn more about salt here:
https://brainly.com/question/31814919
#SPJ 1
determine the pressure in kpa of hydrogen gas produced when 29.51g of aluminum reacts with excess sodium hydroxide and water if the temperature is 25.67c and the volume is 14.75L?
Answers
The pressure of hydrogen gas is 276.25 Kpa .
Given,
Mass of aluminum = 29.51g
Temperature (T) = 25.67 degC =298.67 K
Volume (V) = 14.75 L
The required equation when aluminum reacts with excess sodium hydroxide and water is given by ,
2Al + 2NaOH + 2H2O ==>2NaAlO2 + 3H2
molecular mass of aluminum =26.98 g
1 mole of aluminum= 26.98g
2 moles of aluminum = 53.96g
2 mole of aluminum produces = 3 moles of hydrogen gas
53.96 g of aluminum produces = 6 g of hydrogen gas
29.51 g of aluminum produces = 6*(29.51) /53.96 =3.28 g of hydrogen gas
Thus ,
2 g of hydrogen gas = 1 mole of hydrogen
3.28 g of hydrogen gas = 3.28/2 mole =1.64 mol of hydrogen
Thus , n = 1.64 mol
According to ideal gas equation ,
PV=nRT
P=nRT/V
P = 1.64 * (0.0821L atm K^-1mol^-1 ) *(298.67 K)/14.75L
P=2.726 atm
P= 276.25 Kpa
Hence the pressure of hydrogen gas is 276.25 Kpa .
Learn more about hydrogen gas here :
brainly.com/question/19813237
#SPJ9
How many molecules are in 3 grams of NO2?
Answers
Answer:
0.06520959450500399
Explanation:
Answer:
46.0055
Explanation:
what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.
A nitrogen-containing compound shows no absorption band at ∼3400cm−1 and no absorption bands between ∼1700cm−1 and ∼1600cm−1. what class of compound is it
Answers
Explanation:
A nitrogen-containing compound that shows no absorption band at around 3400 cm^−1 and no absorption bands between approximately 1700 cm^−1 and 1600 cm^−1 is likely an amide compound.
Amides typically exhibit a characteristic absorption band in the region of 3200-3500 cm^−1 due to the N-H stretching vibration. The absence of this absorption band suggests the absence of N-H bonds, which rules out compounds like primary or secondary amines.
The absence of absorption bands between 1700 cm^−1 and 1600 cm^−1 eliminates functional groups such as carbonyl compounds (e.g., aldehydes, ketones, carboxylic acids, esters) and imines, which typically exhibit absorption in this region.
Therefore, based on the given information, it can be inferred that the compound is likely not an amine, carbonyl compound, or imine. Other classes of compounds that do not possess these characteristic absorption bands would need to be considered.
How many formula units make up 24.2 g of magnesium chloride (MgCl2)?
Help!!
Answers
Answer:
Approximately \(1.53 \times 10^{23}\) formula units (\(0.254\; \rm mol\)).
Explanation:
Refer to a modern periodic table for the relative atomic mass of magnesium (\(\rm Mg\)) and chlorine (\(\rm Cl\)):
\(\rm Mg\): \(24.305\).\(\rm Cl\): \(35.45\).In other words, the mass of \(1\; \rm mol\) of \(\rm Mg\) atoms would be (approximately) \(24.305\; \rm g\).
Likewise, the mass of \(1\; \rm mol\) of \(\rm Cl\) atoms would be approximately \(35.45\; \rm g\).
One formula unit of the ionic compound \(\rm MgCl_{2}\) includes exactly as many atoms as there are in the given formula. The formula mass of a compound is the mass of \(1\; \rm mol\) of the formula units of this compound.
The formula \(\rm MgCl_{2}\) includes one \(\rm Mg\) atom and two \(\rm Cl\) atoms.
Hence, every formula unit of \(\rm MgCl_{2} \!\) would include the same number of atoms: one \(\rm Mg\!\) atom and two \(\rm Cl\!\) atoms. There would be \(1\; \rm mol\) of \(\rm Mg\) atoms and \(2\; \rm mol\) of \(\rm Cl\) atoms in \(1\; \rm mol\!\) of \(\rm MgCl_{2}\) formula units.
Thus, the mass of \(1\; \rm mol\!\) of \(\rm MgCl_{2}\) formula units would be equal to the mass of \(1\; \rm mol\) of \(\rm Mg\) atoms plus the mass of \(2\; \rm mol\) of \(\rm Cl\) atoms. (The mass of \(1\; \rm mol\!\!\) of each atom could be found from the relative atomic mass of each element.)
\(\begin{aligned}& M({\rm MgCl_{2}}) \\ =\; & 24.305\; {\rm g \cdot mol^{-1}} + 2\times {\rm 35.45 \; \rm g \cdot mol^{-1}} \\ =\; & 95.205\; \rm g \cdot mol^{-1}\end{aligned}\).
In other words, the formula mass of \(\rm MgCl_{2}\) is \(95.205\; \rm g \cdot mol^{-1}\).
Therefore, the number of formula units in \(m = 24.2\; \rm g\) of \(\rm MgCl_{2}\) would be:
\(\begin{aligned}n &= \frac{m({\rm MgCl_{2}})}{M({\rm MgCl_{2}})} \\ &= \frac{24.2\; \rm g}{95.205\; \rm g\cdot mol^{-1}} \\ & \approx 0.254\; \rm mol\end{aligned}\).
Multiple \(n\) by Avogadro's Number \(N_{A} \approx 6.022 \times 10^{23}\; \rm mol^{-1}\) to estimate the number of formula units in \(0.254\; \rm mol\):
\(\begin{aligned}N &= n \cdot N_{A} \\ &\approx 0.254\; \rm mol \times 6.022 \times 10^{23}\; \rm mol^{-1} \\ &\approx 1.53\times 10^{23}\end{aligned}\).
Stamples of heterogeneous equilibria. FeO(s) + CO(g) = Fe(s) + CO₂(g) II. H₂(g) L₂(g) = 2HI(g) III. CO₂(g) + C(s) = 2CO(g) IV. N₂(g) 3H₂(g) + 2NH3(g) Identify I.
Answers
An example of heterogeneous equilibrium is:
I. FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g)What is heterogeneous equilibrium?Heterogeneous equilibrium refers to an equilibrium state in a chemical reaction where the reactants and products exist in different physical states or phases. It occurs when substances in different phases, such as solids, liquids, and gases, are involved in a chemical reaction.
Considering the given equations:
The equation I: FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g) represents a heterogeneous equilibrium.
This is because the reactants and products involve different phases (solid and gas). FeO is a solid (s), CO is a gas (g), Fe is a solid (s), and CO₂ is a gas (g). The reaction involves the conversion of a solid and a gas to another solid and a gas, and the equilibrium is established between these different phases.
Learn more about heterogenous equilibrium at: https://brainly.com/question/25257772
#SPJ1
Which of the following is an example of a problem that could be solved using the scientific method?
a. Tire is flat due to a nail
b. Grass is dead due to a drought
c. Water pressure is low due to a leak
d. Apple tree is not producing apples
Answers
A scientific method is a systematic approach to solving problems by collecting data, formulating hypotheses, and testing those hypotheses through experimentation.
Out of the options provided, (d) "Apple tree is not producing apples" is an example of a problem that could be solved using the scientific method.
To solve this problem, we can follow the scientific method by first making observations and gathering data on the apple tree's environment, such as the amount of sunlight, water, and nutrients it receives. Next, we can formulate a hypothesis, such as the lack of pollination or nutrient deficiency, and design experiments to test this hypothesis.
The experiments may involve changing the conditions of the apple tree's environment, such as adding nutrients or pollinating the tree, and observing the results. By collecting and analyzing the data from these experiments, we can come to a conclusion and make recommendations for how to improve the apple tree's productivity.
To know more about pollination, visit:
https://brainly.com/question/28301188
#SPJ1
A 300.0 mL quantity of hydrogen is collected over water at 19.5 C and a total atmospheric pressure of 750. mm Hg. The partial pressure of water at this temperature is 17.0 mm Hg
Answers
The partial pressure of hydrogen in the collected gas sample is 733.0 mm Hg (calculated by subtracting the partial pressure of water, 17.0 mm Hg, from the total atmospheric pressure, 750.0 mm Hg).
When a gas is collected over water, the presence of water vapor affects the total pressure observed. In this case, the total atmospheric pressure is given as 750.0 mm Hg, and the partial pressure of water vapor at 19.5°C is 17.0 mm Hg.
To determine the partial pressure of hydrogen, we need to subtract the partial pressure of water vapor from the total atmospheric pressure. Partial pressure refers to the pressure exerted by an individual gas component in a mixture. In this scenario, the collected gas is primarily hydrogen, with water vapor being the other component.
By subtracting the partial pressure of water vapor (17.0 mm Hg) from the total atmospheric pressure (750.0 mm Hg), we can find the partial pressure of hydrogen:
Partial pressure of hydrogen = Total atmospheric pressure - Partial pressure of water vapor
Partial pressure of hydrogen = 750.0 mm Hg - 17.0 mm Hg
Partial pressure of hydrogen = 733.0 mm Hg
Therefore, the partial pressure of hydrogen in the collected gas sample is 733.0 mm Hg.
Know more about hydrogen here:
https://brainly.com/question/24433860
#SPJ8
Fill in the Blank.
Mass - measured in Kilograms or _____. Tells the amount of matter in an object.
Answers
it's Gram because why not
Hello, Tia! Mass is measured in kilograms or grams. It tells the amount of matter in an object.
Please give me brainliest for more high quality answers! - longstretch
Select the correct structure that
corresponds to the name.
2,2,3-trichloro-3-fluorohexane
A.
B.
CI
CI
CI
CI
F
חד
CI
C. both

Answers
Answer:
C
Explanation:its right
The correct structure that corresponds to the name 2,2,3-trichloro-3-fluorohexane is both. Thus option C is correct.
What is chemical structure?Chemical structure is defined as the physical configuration of atoms within a molecule. The molecular geometry of the molecule is determined by its chemical structure. By illustrating how the atoms and chemical bonds are arranged in space within the molecule, chemical structure can be used to establish the molecular geometry of a product. Chemists can now see a crucial visual depiction of a chemical formula thanks to this.
2,2,3-trichloro-3-fluorohexane structure given is both are correct as one of the structure is trans form and the other is cis form of the structure. Option A is trans form while option B is cis form of structure.
Thus, the correct structure that corresponds to the name 2,2,3-trichloro-3-fluorohexane is both. Thus option C is correct.
To learn more about chemical structure, refer to the link below:
https://brainly.com/question/12166462
#SPJ2
Which of these is not used to make models of interstellar medium?
Material from meteorites.
Minerals.
Synthetic materials.
Burnt wood ash.
Answers
Material from meteorites are not the elements used to make models of interstellar medium. The correct option is A.
What is interstellar medium?The matter and radiation that prevail in the space between star systems in a galaxy are referred to as the interstellar medium in astronomy. This matter consists of gas including ionic, atomic, and molecular, dust, and cosmic rays.
Meteorite material is not used to create models of the interstellar medium.
Thus, the correct option is A.
For more details regarding interstellar medium, visit:
https://brainly.com/question/12497791
#SPJ1
Without doing a calculation, arrange the following group of molecules in order of decreasing standard molar entropy (S^o ):
hexane (C6H14), benzene (C6H6), cyclohexane (C6H12)
a. C6H14 > C6H6 > C6H12
b. C6H6 > C6H12 > C6H14
c. C6H12 > C6H14 > C6H6
d. C6H14 > C6H12 > C6H6
e. C6H12 > C6H6 > C6H14
f. C6H6 > C6H14 > C6H12
Answers
Answer:
d. C6H14 > C6H12 > C6H6
Explanation:
Standard molar entropy has to do with the number of atoms that are present in each of the species. The greater the number of atoms possessed by the species, the higher the value of the standard molar entropy due to a greater number of vibration modes.
Hexane (C6H14) has the highest number of atoms followed by cyclohexane (C6H12) and lastly benzene (C6H6).
Thus the order of decreasing molar entropy is; C6H14 > C6H12 > C6H6.
what element is 1s2 2s2 2p6 3s2 3p6 3d7 4s1
Answers
Answer:
The element is Iron
Explanation:
The single strand of nucleic acid shown is representative of
A). RNA
B). DNA
C). both RNA and DNA
D). protein

Answers
a. Using the Born-Mayer Equation, calculate the lattice enthalpy for sphalerite
(zinc blende), ZnS. You must look up the appropriate parameters for the equation.
b. Using the Born-Mayer Equation, calculate the lattice enthalpy for wurtzite, ZnS. You must
look up the appropriate parameters for the equation.
c. Which is thermodynamically stable at ambient conditions (25 °C, 1 bar)? Find a reference
with the T and P phase diagram for ZnS. Submit the pdf of the reference with your file . Also,
compare your answer to the standard enthalpies of formation for wurtzite compared to sphalerite.
Answers
ΔLatticeU = ΔLatticeH – pΔVm is the lattice energy of wurtzite. Ionic compounds often have flat surfaces that meet at distinctive angles and are stiff, brittle, crystalline materials.
Remember that when a metal reacts with a nonmetal, often an ionic compound results from the transfer of electrons form the metal (the reductant) towards the nonmetal (the oxidant). Ionic compounds often have flat surfaces that meet at distinctive angles and are stiff, brittle, crystalline materials. They melt at rather high temperatures and are not easily distorted. ΔLatticeU = ΔLatticeH – pΔVm is the lattice energy of wurtzite.
To know more about lattice energy, here:
https://brainly.com/question/29735933
#SPJ1
starting with lead ii oxide powder describe how lead ii oxide can be prepared. (preparation of salts)
Answers
The lead II carbonate is prepared from the lead II oxide and carbonic acid.
How can lead II carbonate be prepared?We know that salts can be prepared by some kind of chemical reactions. We also know that the salts of the metals are basic in nature. The meaning of this is that the oxide of the metal can be able to react with an acid so as to produce a salt.
In this case, we can be able to prepare the lead II carbonate when we add the lead II oxide to the carbonic acid, there is the production of the the required carbonate as a product of the reaction.
Learn more about reaction:https://brainly.com/question/28984750
#SPJ1
Missing parts;
Starting with lead ii oxide powder describe how lead ii carbonate can be prepared. (preparation of salts)
In the following reaction, 2H2(g) + O2(g) --> 2H2O(g), how many moles of oxygen would be required to produce 5.76 moles of water?
Answers
Answer: 2.88
Explanation:assume oxygen as O2, then 1 mole O2 = 2 moles H2O
so 2.88 moles O2
What are the weather conditions such as temperature clouds and precipitation for point A?
Explain reasoning
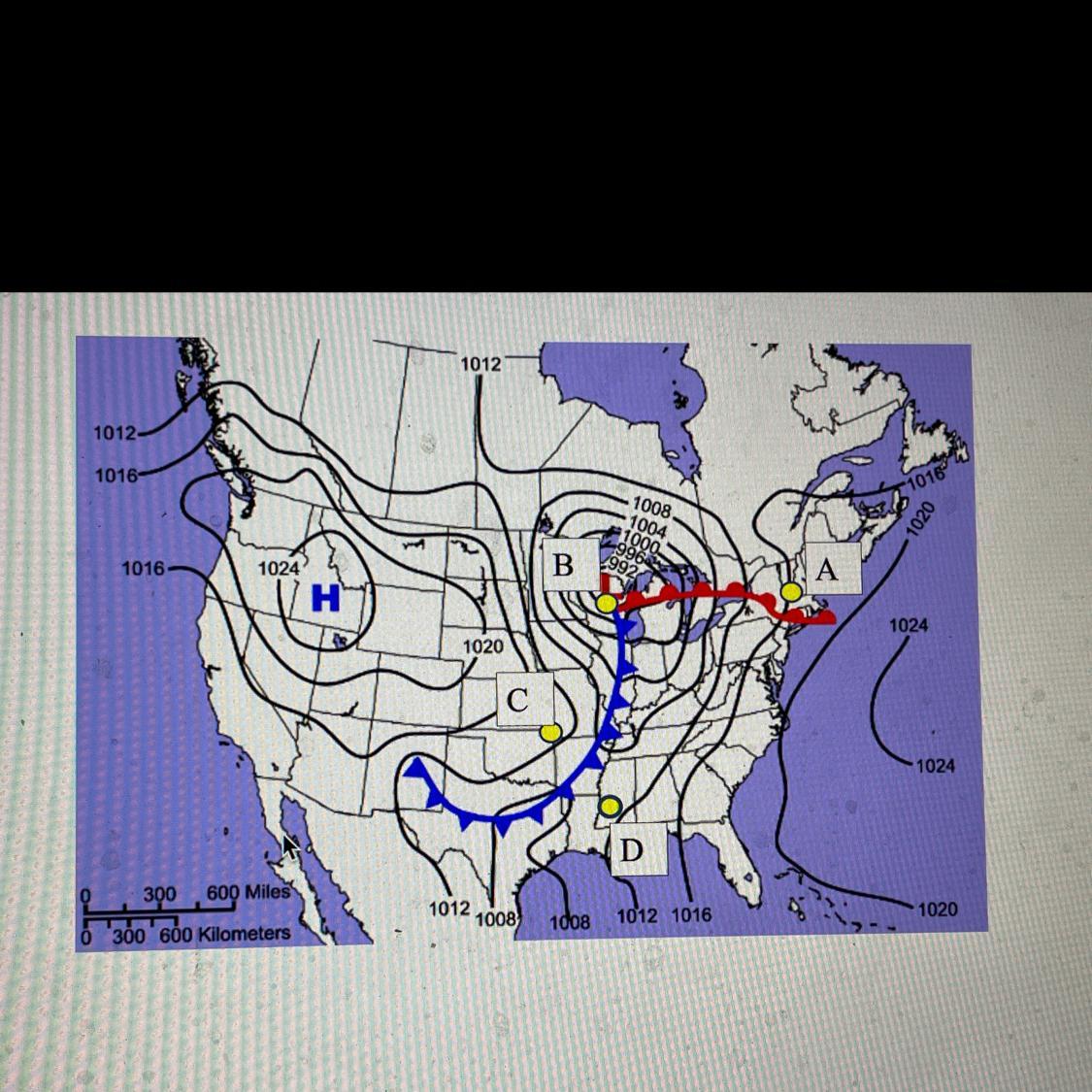
Answers
Any liquid or frozen water that condenses in the atmosphere and falls to the ground is referred to as precipitation. Rain, sleet, and snow are only a few of its various manifestations and evaporation.
Thus, Precipitation is one of the three main processes that make up the global water cycle, together with evaporation and condensation.
Water vapour in the clouds condenses into increasing-sized droplets of water, forming precipitation.
Particles of clean or smoke within the air are basic for precipitation. These particles, called “condensation nuclei,” give a surface for water vapor to condense upon.
Thus, Any liquid or frozen water that condenses in the atmosphere and falls to the ground is referred to as precipitation. Rain, sleet, and snow are only a few of its various manifestations and evaporation.
Learn more about Precipitation, refer to the link:
https://brainly.com/question/18109776
#SPJ1
how many mL of 0.1M HCl are required to react completely with 1 g mixture of \( Na_{2}CO_{3}\) and \( NaHCO_{3} \) containing equimolar amounts of both ?
ty! :)
Answers
Answer:
158 mL
158 mL of 0.1 M of HCl is required to react completely with 1 g mixture of Na2CO3 and NaHCO3, containing equimolar amounts of both
Explanation:
hope helpez -,-~
btw masoom bachi copied me~
Compare the relative strength of the two forces B and C. Explain how you determined this comparison by identifying the forces.
Answers
The intermolecular forces or the strength of C is more than that of B.
What are intermolecular forces?
Intermolecular forces are the forces that hold the molecules of any object in a particular state of matter.It exists between molecules.These forces are weak when compared with intramolecular forces.Hydrogen bonding, dipole-dipole, Vander Walls, and ion-dipole are the types of intermolecular forces.At a short distance, these forces are repulsive but at large distances they are attractive.Here, we can see that C has dipole-dipole interaction while B has none.
Therefore, it states that the intermolecular forces present in C are much more than that in B.
To learn more about intermolecular forces, visit: https://brainly.com/question/9007693
#SPJ9
Identify the reactants in the equation: 6C0, + 6H2O - CHUO+ 60,
Answers
Answer:
The correct answer is d. 6H20 + 6CO2.
The reactant in the chemical reaction 6H2O + 6CO2 ---> C6H12O6 + 6O2 is 6H20 + 6CO2. Remember that the reactant is always at the left side of the equation. So the correct answer is 6H20 + 6CO2 since it's in the left of the equation. I hope this answer helped you.
Explanation:
ose you has aniline as your unknown. What kind of solid derivative would be suitable to synthesize for aniline
Answers
Answer:
Amides are suitable to synthesize aniline
Explanation:
Amide are basically used to synthesize aniline.
The chemical formula of Aniline is C6H5NH2 while that of Amide is RCONH2
Amide are solids that can be easily converted to amines.
Duncan takes a break from studying and goes to the gym to swim laps if swimming burns, 615,000 cal per hour, how many kilojoules does swimming burn in the same amount of time?
Answers
Select the best answer for the question. 1. Mei is seated doing leg extensions and going through the full path of motion. What type of exercise is Mei doing? O A. Free-weight exercise B. Resistance exercise C. Machine exercise O D. Cable exercise
Answers
The correct answer is "C.
The type of exercise that Mei is doing is the "Machine exercise."
Machine exercise refers to a physical fitness training technique that allows the muscles to develop and strength through the use of machines that use hydraulic cylinders, weights, and cables to produce resistance. The machine exercises are generally performed in a seated position or lying down, and most often use a series of cables and weights that are adjusted to the user's specific body weight and desired level of resistance.Machine exercises can effectively target specific muscle groups and help strengthen them.Machine exercises can help you increase muscular endurance and improve your overall fitness level.Machine exercises are often safer and easier to perform than free-weight exercises.Machine exercises are generally easier on your joints and can help reduce the risk of injury.Machine exercises are also helpful for people with limited mobility or those recovering from an injury or surgery.For such more questions on Machine exercise
https://brainly.com/question/29402478
#SPJ8
Bluetooth devices operate in the 2.4GHz range. What is the wavelength and energy of a photo of this light ? What part of the spectrum does this fall in?
Answers
Hope this helps.
A mass of 6.005 g of carbon (atomic mass 12.010 amu) contains...? ty in advance
Answers
The mass of 6.005 g of carbon contains approximately 3.011 x 10²³ carbon atoms.
How much mass do six moles of carbon atoms have?We are aware that a mole is a grouping of 6.022 10²³ atoms. 6.0221023 carbon atoms make up a mole of carbon. As a result, we can estimate that 6.0221023 carbon atoms have a mass of 12 grammes.
There are: atoms of carbon in the sample.
The amount of carbon atoms in the sample may be determined using Avogadro's number (6.022 x 10²³ atoms per mole) and the molar mass of carbon (12.010 g/mol)
Number of moles of carbon = mass of carbon/molar mass of carbon
= 6.005 g / 12.010 g/mol
= 0.500 mol
Number of carbon atoms=number of moles of carbon x Avogadro's number
= 0.500 mol x 6.022 x 10²³ atoms/mol
= 3.011 x 10²³ atoms
To know more about carbon visit:-
https://brainly.com/question/22530423
#SPJ1
Explanation of the concept of percentage composition and empirical formula. Support the concept with;a real world example including its significance, relevant formula, calculations and ratios between reactants and products.
Answers
Percentage composition of a compound is the percentage by mass of each element presents in a compound. For instance is the percent by weight of the elements in a compound are given in percentage, they can be converted to masses.
These masses are converted to moles and its simplest multiple ratio is useful in determining the empirical formula of the molecule. The empirical formula is simply the simplest whole number ratio of atoms present in a compound.
The significance of empirical formula is that it helps us to determine the numbers and types of atoms composing a single molecule of the substance;
For instance, if a compound contains 5g of carbon, 2g of hydrogen and 12 grams of oxygen, the empirical formula is calculated using the steps below:
Step 1: Calculate the moles of the element
Mole = mass/molar mass
Moles of C = 5/12 = 0.42moles
Moles of H = 2/1 = 2moles
Moles of O = 12/16 = 0.75 moles
Step 2: Divide by the simplest mole ratio
For Carbon: 0.42/0.42 = 1(5) = 5
For hydrogen: 2/0.42 = 4.76(5) = 24
For Oxygen: 0.75/0.42 = 1.79(5) = 9
This shows that the empirical formula is C5H24O9
The general chemical reaction is expressed as:
\(C_xH_yO_z+yO_2\rightarrow xCO_2+yH_2O\)What is happening in the picture?
A)
Sunlight is reacting with waste gases produced by cellular respiration from the trees to produce chemical smog.
B)
Sunlight is reacting with waste gases produced by cellular respiration from the trees and by vehicles burning fossil fuels to produce chemical smog.
C)
Sunlight is reacting with water vapor produced by vehicles burning fossil fuels to produce photochemical smog.
D)
Sunlight is reacting with waste gases produced by vehicles burning fossil fuels to produce photochemical smog.

Answers
The most likely option based on common knowledge is sunlight is reacting with waste gases produced by vehicles burning fossil fuels to produce photochemical smog.
Photochemical smog is a type of air pollution that is formed when sunlight reacts with pollutants released from vehicle exhaust and other sources, such as industrial emissions. This reaction produces a mixture of harmful chemicals, including ground-level ozone and various secondary pollutants.
However, it's important to note that a definitive answer would require specific information about the picture in question, as different scenarios may lead to different outcomes.
Learn more about Photochemical smog on:
https://brainly.com/question/27960448
#SPJ1
Graphite and diamond are both solid forms of the element carbon. Which statement explains
the different properties of these two forms of carbon?
(1) Diamond has ionic bonding and graphite has metallic bonding.
(2) Diamond has metallic bonding and graphite has ionic bonding.
(3) Diamond has a different crystal structure from graphite.
(4) Diamond has carbon atoms with more valence electrons than graphite.
Answers
Answer: C diamond has a different crystal structure from graphite
Explanation:
The difference between the properties of two forms of carbon is that
Diamond has a crystal structure different from graphite.
Graphite and Diamond are known as allotropes of Carbon as they have the
same chemical properties but different crystal structure.
Diamonds form a 3 crystal lattice with no flexibility while Graphite are
bonded to sheets which makes them slide easily within one another and
gives it its soft texture.
Read more on https://brainly.com/question/23491824