How is raw steel made and how is it different from raw iron?
Answers
Related Questions
iven the biaryl product, select the two reactants that would give this product via a suzuki coupling. the unknown compound reacts with palladium tetrakis triphenyl phosphine, heat and sodium carbonate to give an alkene where each carbon is bonded to a benzene ring and a hydrogen and the stereochemistry is cis. select two reactants. an alkyne where carbon 1 is bonded to a benzene ring and carbon 2 is bonded to hydrogen. an alkene where carbon 1 is bonded to bromine and carbon 2 is bonded to benzene. the stereochemistry is cis. an alkene where carbon 1 is bonded to bromine and carbon 2 is bonded to benzene. the stereochemistry is trans. phenyl grignard tri butyl tin is bonded to benzene boronic acid is bonded to benzene an alkene is bonded to three hydrogens and one benzene ring.
Answers
Based on the given information, the two reactants that would give the described product via a Suzuki coupling are:
An alkyne where carbon 1 is bonded to a benzene ring and carbon 2 is bonded to hydrogen.
An alkene where carbon 1 is bonded to bromine and carbon 2 is bonded to benzene. The stereochemistry is cis.
The Suzuki coupling is a widely used cross-coupling reaction that involves the palladium-catalyzed cross-coupling of an organoboron compound (such as a boronic acid) with an organic halide or pseudo-halide. In this case, the reactants that can undergo the Suzuki coupling are the alkyne and the cis-alkene described above.
The alkene and alkyne provide the necessary carbon-carbon bond formation, while the benzene rings and hydrogens ensure the desired connectivity in the product. The palladium tetrakis triphenyl phosphine catalyst, heat, and sodium carbonate facilitate the coupling reaction, resulting in the formation of the biaryl product with the specified stereochemistry.
To know more about Suzuki coupling :
brainly.com/question/29857055
#SPJ11
The pressure in a tire is 101 kPa at 10.0°C, what will be the pressure of a tire at 45.0°C?
Answers
Answer:
P₂ = 113.49 kPa
Explanation:
Given that,
Initial pressure, P₁ = 101 kPa
Initial temperature, T₁ = 10.0°C = 283 K
Final temperature, T₂ = 45°C = 318 K
The relation between pressure and temperature is given by :
\(\dfrac{P_1}{T_1}=\dfrac{P_2}{T_2}\)
Where
P₂ is the new pressure
So,
\(P_2=\dfrac{P_1T_2}{T_1}\\\\P_2=\dfrac{101 \times 318}{283 }\\\\P_2=113.49\ kPa\)
So, the new pressure is equal to 113.49 kPa.
6. Choose a pair of words to make the statement correct. Energy transfers from
substances to ___substances.
A. cold to hot
B. hot to cold
C. hot to hot
D. cold to cold
Answers
Answer:
B. hot to cold
Explanation:
Energy wants to reach thermal equilibrium (which is basically just balanced temperature) so it will move from hot to cold to make the cold object warmer and the hot object cooler.
If a piece of metal is put into boiling water for five minutes, what will its temperature be? Explain your reasoning.
Answers
The slower-moving water molecules are hit by the faster-moving metal atoms when the hot metal washers are submerged in the room-temperature water, which causes the water molecules to travel a little more quickly.
Why does heat not raise the temperature of boiling water when added to it?The heat source is employed to convert liquid water to vapour, which is the cause. Latent heat of vaporisation is the name given to this heat.
Why does the temperature stay the same while water is boiling?Even if heat is continuously applied, the temperature doesn't change during boiling because all of the heat energy is expended in converting the liquid state of the water to the gaseous water vapour.
To know more about temperature visit:-
brainly.com/question/29072206
#SPJ1
How many protons, neutrons, and electrons are in an Fe atom?
Answers
Answer:
26 protons, 30 neutrons, and 26 electrons
Explanation:
There are total 26 protons, 30 neutrons and 26 electrons in an Fe atom.
What do you mean by electron, proton and neutron ?The electron has a negative charge. The symbol for electrons is (e–). An electron’s atomic mass unit is (5.45×10–4). The orbits of electrons are located outside the nucleus of the atoms.
A proton has a positive charge. (p+) is the symbol for protons. The proton’s atomic mass unit is 1. Protons are found within the nucleus of atoms. A proton has a mass of (1.672×10–27 kg).
A neutron is either neutrally charged or uncharged. The symbol for neutrons is (n⁰). The neutron’s atomic mass unit is one. Neutrons are also found inside the nucleus.
These all combinedly makes an atom, hence there are total 26 protons, 30 neutrons and 26 electrons in an Fe atom.
Learn more about electron, proton and neutron ,here:
https://brainly.com/question/13131235
#SPJ2
What is the wavelength (in nm) of an electron with the following kinetic energies? (a) 20.0 ev (no response) nm (b) 200 ev (no response) nm (c) 2.00 kev (no response) nm (d) 20.0 kev (no response) nm (e) 0.200 mev (no response) nm (f) 2.00 mev (no response) nm which of these energies are most suited for study of the nacl crystal structure? (select all that apply.) 20.0 ev 200 ev 2.00 kev 20.0 kev 0.200 mev 2.00 mev none of these
Answers
The wavelength of an electron can be calculated using the formula: wavelength = h / (mass of electron * velocity). Since kinetic energy is equal to the mass of the electron multiplied by the velocity squared, we can also calculate wavelength by using the formula: wavelength = h / sqrt(2mass of electron kinetic energy).
To convert the kinetic energies given in electron volts (eV) to Joules (J), you can use the formula: 1 eV = 1.6 x 10^-19 J
(a) 20.0 eV = 3.2 x 10^-18 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-18 J) = 2.4 x 10^-12 m or 2.4 pm (picometers)
(b) 200 eV = 3.2 x 10^-17 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-17 J) = 2.4 x 10^-11 m or 24 pm
(c) 2.00 keV = 3.2 x 10^-14 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-14 J) = 2.4 x 10^-8 m or 2.4 nm
(d) 20.0 keV = 3.2 x 10^-13 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-13 J) = 2.4 x 10^-7 m or 24 nm
(e) 0.200 MeV = 3.2 x 10^-11 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-11 J) = 2.4 x 10^-5 m or 0.24 nm
(f) 2.00 MeV = 3.2 x 10^-10 J, wavelength = h / sqrt(2mass of electron3.2 x 10^-10 J) = 2.4 x 10^-4 m or 2.4 nm
A lower energy electron will have a longer wavelength, while a higher energy electron will have a shorter wavelength. To study the crystal structure of NaCl, you would need to use a technique such as X-ray diffraction, which typically uses X-rays with energies in the range of a few keV to a few tens of keV. Based on this, 2.00 keV and 20.0 keV energies are most suited for study of the NaCl crystal structure.
To know more about wavelength of electrons visit :
https://brainly.com/question/17295250?referrer=searchResults
#SPJ4
What would be the direction of a reaction if the reaction quotient QC is greater than the equilibrium constant KEQ QC KC )?
Answers
The direction of a reaction if the reaction quotient QC is greater than the equilibrium constant Kc is in the direction of reactants.
The reaction quotient Qc can be used to explain which direction the reaction will be shift to reach the equilibrium. The Kc is the equilibrium constant.
If Kc > Qc, the reaction will be proceed the forward reaction, converting reactants in to the products.
If Kc < Qc, the reaction will be proceed in the reverse direction, it converting products in to the reactants.
If Qc = Kc then the system is already at the equilibrium.
The reaction quotient (Q) is measurement of the relative amounts of the products and the reactants present during the reaction at the particular point in the time.
To learn more about equilibrium constant here
https://brainly.com/question/17204020
#SPJ4
the mass of a subatomic particle compared to the mass of a proton
Answers
Answer:
neutron
Explanation:
Hopefully this will help you!!!!
lowest frequency?______
Answers
a 1.0 l balloon has a pressure of 2 atm. when the pressure increases to 2,000 kpa, what is the volume? responses 0.1 l 0.1 l 0.2 l 0.2 l 2.0 l 2.0 l 510 l 510 l
Answers
When the pressure of the 1.0 L balloon increases to 2,000 kPa, the volume will be 510 L.
The ideal gas law also states that if the temperature of the gas is increased while the pressure is held constant, the volume of the gas will increase. This is because the increased temperature will cause the gas particles to move faster, and the faster they move, the more space they will take up, resulting in an increase in volume.
Therefore, if the temperature of the 1.0 L balloon is increased while the pressure is held constant, the volume of the gas will increase.
Learn more about the pressure of the balloon:
https://brainly.com/question/12148102
#SPJ4
Can someone pleaseeee help if you’re correct I’ll give u brainlist

Answers
Answer:
I think it the second one adding external weight or the third one
Explanation:
I could never. im more like the last one
I'm really sorry if this was no help
The process of natural selection causes evolution, what is changing over time
Answers
hope it helps :-).
Electron transition can be likened to moving up and down a ladder. Which of the following statements best describes how an electron transitions in an electron cloud?
A. An electron can only gain or lose energy to move between quantized energy levels.
B. An electron can only gain or lose charge in integers when ions are formed.
C. An electron can only change its mass in quantized amounts when it gains or loses energy.
D. An electron can only change into certain types of particles when it gains or loses charge.
Answers
Answer:
C. An electron can only change its mass in quantized amounts when it gains or loses energy.
Explanation:
The electron mostly acts in a wave form and shales the orbit and in other worlds the electron transition to lower energy change its wave shape and sinks less but it does not leap or bounce back anywhere.3 waves are shown with a line through their center. The bottom of the first wave is labeled C. A bracket labeled D connects the bottom of the second and third waves. A line from the center to the top of the first wave is labeled A. The line leading to the top is labeled B.
Label the parts of this wave.
Answers
Answer:Label the parts of this wave.
A:
✔ crest
B:
✔ amplitude
C:
✔ trough
D:
✔ wavelength
Explanation:
Answer:
A- crest
B- Amplitude
C- Trough
D- wavelength
Explanation:
Just did it on Edge Trust me 2022
In the water cycle we can observe transformations:
a) Permanent
b) Temporary
c) Chemical
d) None of the above
Answers
Answer:
b
Explanation:
temporary bc it's have time so the water can cycle
Read the following reactions.
Reaction 1: NaCl(s) → Na+(aq) + Cl−
Reaction 2: CaCO3(s) → CaO(s) + CO2(g)
Which reaction leads to an increase in entropy?
a
Only Reaction 1
b
Only Reaction 2
c
Both Reaction 1 and 2
d
Neither Reaction 1 nor 2
Answers
Answer: C
Explanation:
Reaction 1 starts off with one mole of reactant and produces 2 moles of product. The increase in the number of moles, along with the fact that aqueous compounds are more disordered than solid compounds, increases the disorder and thus entropy.
Reaction 2 starts off with 1 mole of solid and produces 1 mole of solid and one mole of gas. The increase in the number of moles, along with the fact that gases are more free and disordered than solids, increases the disorder and thus entropy.
Explain how we know that charge is conserved in this
reaction: Li+ CI → Lici
Answers
Answer:
Charge is conserved due to the groups in which Lithium and Chlorine are located in the periodic table of the elements.
Explanation:
In the reaction \(Li + Cl - > LiCl\), we can examine the groups in which Li and Cl are found in the periodic table of the elements. Lithium appears in Group 1A, or the alkali metals group, indicating that it carries a charge of +1. Chlorine appears in Group 7A, or the halogen group, indicating that it carries a charge of -1. Because LiCl's constituent elements carry the same charges as previously mentioned, LiCl will have an overall charge of 0.
The chemical equation can then be rewritten as \(Li^{+} + Cl^{-} - > LiCl\), which, if looking at the individual charges of Li and Cl in lithium chloride, becomes \(Li^{+} + Cl^{-} - > Li^{+}Cl^{-}\). Adding the charges on the reactant and product sides of this chemical equation gives us zero in both locations, meaning that we have a charge of 0 on the reactant side and a charge of 0 on the product side. This indicates that charge is conserved in this reaction.
Another way to look at this is expressed in the valence electrons of Li and Cl. Li has an electron configuration of \(1s^{2}2s^{1}\), where the n = 2 electron shell has one of eight total electrons needed to fill the valence shell. This means that Li will easily lose one electron in order to have an electron configuration where the n = 1 electron shell is full, \(1s^{2}\), and become the \(Li^{+}\) ion. Similarly, Cl has an electron configuration of \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{5}\) (or \([Ne]3s^{2}3p^{5}\)), meaning that the n = 3 electron shell is one electron away from becoming complete. Cl will easily gain one electron to have the electron configuration \([Ne]3s^{2}3p^{6}\) (or \([Ar]\)) in order to have an electron configuration where the n = 3 electron shell is full, \(3s^{2}3p^{6}\), and become the \(Cl^{-}\) ion. Thus, when Li and Cl bond, Li will lose the electron \([1, 0, 0, +\frac{1}{2}]\) and transfer it to Cl, where it will become the electron \([3, 1, 1, -\frac{1}{2}]\), thus conserving charge, as there is an equal total number of electrons before and after the reaction.
What is the difference between a group and a row on the periodic table?
Answers
Answer: A row is horizontal and a group is vertical on the periodic table.
PH CHEM, PLEASE HELP QUICK! NO LINKS/VIRUSES PLEASE!
A solution is created by measuring 3.60 x 10-3 moles of NaOH and 5.95 x 10-4 moles of HCl into a container and then water is added until the final volume is 1.00 L. What is the pH of this solution?
Answers
Answer:
The pH of the solution is 11.48.
Explanation:
The reaction between NaOH and HCl is:
NaOH + HCl → H₂O + NaCl
From the reaction of 3.60x10⁻³ moles of NaOH and 5.95x10⁻⁴ moles of HCl we have that all the HCl will react and some of NaOH will be leftover:
\( n_{NaOH}} = n_{i_{NaOH}} - n_{HCl} = 3.60 \cdot 10^{-3} moles - 5.95 \cdot 10^{-4} moles = 3.01 \cdot 10^{-3} moles \)
Now, we need to find the concentration of the OH⁻ ions.
\( [OH^{-}] = \frac{n_{NaOH}}{V} \)
Where V is the volume of the solution = 1.00 L
\( [OH^{-}] = \frac{n_{NaOH}}{V} = \frac{3.01 \cdot 10^{-3} moles}{1.00 L} = 3.01 \cdot 10^{-3} mol/L \)
Finally, we can calculate the pH of the solution as follows:
\( pOH = -log([OH^{-}]) = -log(3.01 \cdot 10^{-3}) = 2.52 \)
\( pH + pOH = 14 \)
\( pH = 14 - pOH = 14 - 2.52 = 11.48 \)
Therefore, the pH of the solution is 11.48.
I hope it helps you!
1 An atom has 24 protons, 22 electrons, and 28 neutrons. What is the atomic number of the atom?
Answers
Answer:
24
Explanation:
A sample of oxygen gas has a volume of 453.7 mL when its pressure is 0.435 atm. What will the volume of the gas
be
at a pressure of 0.88 atmospheres, if the temperature remains constant?
Answers
Answer:
is you'r question correctly?
or not
What happened when you mixed the two substances together? The substances changed into different substances. The substances changed into different substances. The substances did not change into different substances. The substances did not change into different substances. I am not sure if the substances changed into different substances. I am not sure if the substances changed into different substances.
Answers
Answer:
is this a poem or something?
What is the molarity of the solution formed by dissolving 80. G of NAOH(s) into water to give a total volume of 4.00 l
Answers
Answer:
0.5 M
Explanation:
From the question given above, the following data were obtained:
Mass of NaOH = 80 g
Volume of solution = 4 L
Molarity =?
Next, we shall determine the number of mole in 80 g of NaOH. This can be obtained as follow:
Mass of NaOH = 80 g
Molar mass of NaOH = 23 + 16 + 1
= 40 g/mol
Mole of NaOH =?
Mole = mass / molar mass
Mole of NaOH = 80 / 40
Mole of NaOH = 2 moles
Finally, we shall determine the molarity of the solution. This can be obtained as follow:
Mole of NaOH = 2 moles
Volume of solution = 4 L
Molarity =?
Molarity = mole / Volume
Molarity = 2/4
Molarity = 0.5 M
Therefore, the molarity of the solution is 0.5 M.
The pressure on 30 milliliters of an ideal gas increases from
760 torr to 1520 torr at constant temperature. The new volume is

Answers
V1 = 30 mL
P1 = 760 torr
P2 = 1520 torr
V2 = ?
applying Boyle's Law
P1*V1 = P2*V2
760 torr * 30 mL = 1520 torr * V2
V2 = 760 torr * 30 mL / 1520 torr
( C ) is correct
A fine of 50.0 mL of 0.0900 M CaCl2 reacts with excess sodium carbonate to give 0.366 g of calcium carbonate precipitate. What is the percent yield?
Answers
Answer:
81.26% is the percent yield
Explanation:
Based on the reaction:
CaCl₂ + Na₂CO₃ → 2NaCl + CaCO₃
Where 1 mole of CaCl₂ in excess of sodium carbonate produces 1 mole of calcium carbonate.
To solve this question we must find the moles of CaCl2 added = Moles CaCO₃ produced (Theoretical yield). The percent yield is:
Actual yield (0.366g) / Theoretical yield * 100
Moles CaCl₂ = Moles CaCO₃:
0.0500L * (0.0900moles / L) = 0.00450 moles of CaCO₃
Theoretical mass -Molar mass CaCO₃ = 100.09g/mol-:
0.00450 moles of CaCO₃ * (100.09g / mol) = 0.450g of CaCO₃
Percent yield = 0.366g / 0.450g * 100
81.26% is the percent yield
Which of the following is an example of how chemicals can both hurt and
help the environment?
A. Chemicals in a volcano's lava can harm plants, and some
chemicals can be used to determine what is in lava.
B. Chemicals are used to protect skin from the sun and also to help
skin tan.
C. Chemicals can pollute water, but they can also be used to clean
water.
D. Chemicals are used in artists' paints and also are used to clean
artists' paintbrushes.
Answers
Answer: C
Explanation: Because I just did the test lol.
Chemicals are substances that can be natural or man-made. It can both hurt and help the environment as they pollute water and can also clean water. Thus, option C is correct.
What are the effects of chemicals?Chemicals are compounds that are synthesized artificially and have various uses and applications in various fields. They can be both beneficial and detrimental to the environment and the ecosystem.
Chemicals like that from factories, pesticides run-off from agricultural fields, drugs, microorganisms, fertilizers, radioactive substances, etc, cause water pollution by acting as contaminants.
On the contrary, chemicals can be used to clean water pollution. Chloramine and chlorine are the most common chemical disinfectants that cleanse contaminated water to make it useful.
Therefore, option C. chemicals can both clean and pollute water.
Learn more about chemicals, here:
https://brainly.com/question/28222646
#SPJ2
The temperature of a 0.65L sample of carbon dioxide gas is 580K. If the pressure remains constant, what is the new volume of the gas if the temperature increases to 1300K?
Answers
Calculate: A. Mercury has a specific Heat Capacity of 0.14 J/goC. How much heat is needed to raise the thermometer temperature 15 oC to 95 oC. There is 5 g of mercury in the thermometer.
Answers
Answer:
\(\boxed {\boxed {\sf 56 \ Joules}}\)
Explanation:
We are given the mass, specific heat, and temperature, so we must use this formula for heat energy.
\(q=mc \Delta T\)
The mass is 5 grams, the specific heat capacity is 0.14 Joules per gram degree Celsius. Let's find the change in temperature.
ΔT= final temperature - initial temperature ΔT= 95°C - 15°C = 80°CWe know the variables and can substitute them into the formula.
\(m= 5 \ g \\c= 0.14 \ J/ g \ \textdegree C \\\Delta T= 80 \ \textdegree C\)
\(q= (5 \ g )( 0.14 \ J/ g \ \textdegree C ) ( 80 \ \textdegree C)\)
Multiply the first numbers. The grams will cancel.
\(q= 0.7 \ J/ \textdegree C(80 \ \textdegree C )\)
Multiply again. This time the degrees Celsius cancel.
\(q= 56 \ J\)
56 Joules of heat are needed.
according to the given reaction, if you want to produce 5.85 grams nacl of how much grams of hcl and nahco3 must you start with?
Answers
The correct answer to the given question about reaction is option b) 3.65 g of HCl and 8.40 of NaHCO3 is needed to start to produce 5.85 g of NaCl.
Although sea salt also contains other chemical salts, sodium chloride, generally known as salt, is an ionic substance with the chemical formula NaCl, which denotes a 1:1 ratio of sodium and chloride ions. 100 g of NaCl comprises 39.34 g Na and 60.66 g Cl, with molar weights of 22.99 and 35.45 g/mol, respectively. For both seawater and the extracellular fluid of many multicellular organisms, sodium chloride is the salt that contributes most to the saltiness. Salt, usually referred to as table salt, is frequently used as a seasoning and food preservation in its edible form. Sodium chloride is a primary source of the sodium and chlorine compounds used as feedstocks for further chemical synthesis and is utilized in large quantities in many industrial processes.
Question
according to the given reaction, if you want to produce 5.85 grams nacl of how much grams of hcl and nahco3 must you start with?
a) 5.85 g of HCl and 5.85 g of NaHCO3
b) 3.65 g of HCl and 8.40 of NaHCO3
c) 0.003 g of HCl and 0.001 of NaHCO3
d) 36.5 g of HCl and 84.0 of NaHCO3
To learn more about NaCl click here
brainly.com/question/4487559
#SPJ4
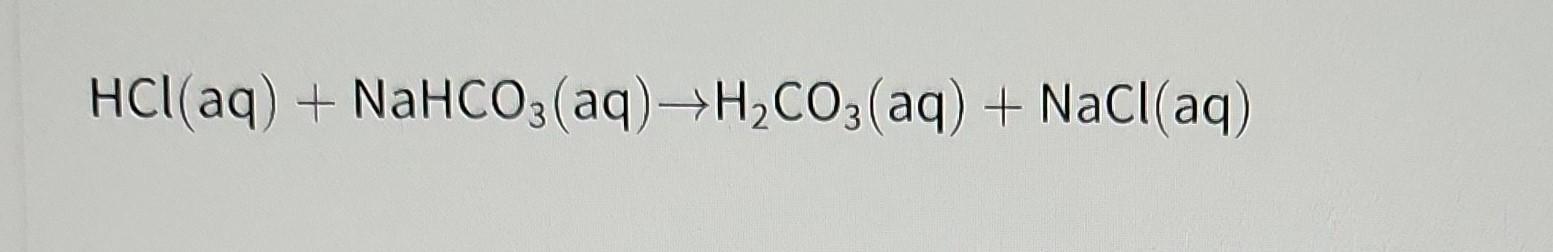
lodine (1) , What are the correct number of valance electrons
Answers
Answer: Iodine,
, is located in period 5, group 17 of the periodic table, and has an atomic number equal to
53
.
plss brainleist
Explanation: