Answers
Answer:
Hurricanes and tropical storms gain their power from heated water evaporating from the ocean.
Explanation:
Brown Water Effect The brown ocean effect is an observed weather phenomenon involving some tropical cyclones after landfall. Normally, hurricanes and tropical storms lose strength when they make landfall, but when the brown ocean effect is in play, tropical cyclones maintain strength or even intensify over land surfaces.Related Questions
10.Give the possible values for the magnetic quantum number for each of the following orbitals (a) 4p: (b) 3d: (c) 2s (d) 5P
Answers
Answer:
(a) 4p: ml = -3, -2, -1, 0, +1, +2, +3
(b) 3d: ml = -2, -1, 0, +1, +2
(c) 2s: ml = -1, 0, +1
(d) 5p: ml = -4, -3, -2, -1, 0, +1, +2, +3, +4
Explanation:
Manetic quantum numbers determine the spatial orientation and number of orbitals in the subshell. ml is from -l to +l.
Untitled Section
Which of the following is not a physical change? *
10
A. crushing
B. sublimation
C. rotting
D. deposition
Next
Page 29
Answers
Answer:
B.
Explanation:
Hope this helps.
In a climatological sense, dryness is a function of both annual rainfall and ________.
This a geology question.
Answers
Answer:
In a climatological sense, dryness is a function of both annual rainfall and evaporation
How much energy is required to raise the temperature of 4.0 g of mercury metal from 9.3 oC to 83.0 oC.
Answers
From the specific heat capacity of mercury, the amount of heat energy required to raise the temperature of 4.0 g of mercury metal from 9.3 °C to 83.0 °C is 77.792 J.
What is the specific capacity of mercury?The specific heat capacity of a substance is the amount of heat required to raise the temperature of a unit mass of the substance by one degree Celsius or kelvin.
The specific heat capacity of a substance is a constant that can be used to calculate the amount of heat required to raise the temperature of a given mass of a substance to any temperature.
The specific heat capacity of mercury is 0.140 J/g/k.
The formula for calculating specific heat capacity is given below:
Specific heat capacity, c = Δq/mΔT
where;
Δq = heat change
m = mass of the substance
ΔT = temperature change
The Heat required, Δq, will then be:
Δq = m * c * ΔT
Heat required, Δq = 4.0 * 0.140 * (83.0 - 9.3)
Heat required, Δq = 77.792 J
Lear more about specific heat capacity at: https://brainly.com/question/26866234
#SPJ1
Calculate the heat change in kilocalories for condensation of 6.5 kg of steam at 100 ° C
Answers
Answer:
6,500 gm of steam require= 3,510 kilo calories (approx)
Explanation:
Every 1 gram of water at 100° C absorb 540 calories
So,
Total water = 6.5 kg = 6,500 gram
So,
6,500 gm of steam require = 6,500 x 540
6,500 gm of steam require= 3,510 kilo calories (approx)
According to the question,
Total water,
6.5 kg or 6,500 gTemperature,
100°CNow,
Required steam will be:
= \(6500\times 540\)
= \(3510 \ kilo \ calories\)
Thus the above answer is right.
Learn more about heat here:
https://brainly.com/question/2907493
Element Name that’s has 7 protons and electrons and 3 neutrons
Answers
Answer:
Nitrogen - 10
Explanation:
7 Protons and 7 electrons = neutral charge
7 Protons = Element N (Nitrogen)
Atomic Mass = # of Protons + # of Neutrons
Atomic Mass = 7 + 3
Atomic Mass = 10
Nitrogen - 10
PROBLEM 19.12 Draw the structure of a triacylglycerol that fits each description: a. a saturated triacylglycerol formed from three 12-carbon fatty acids b. an unsaturated triacylglycerol that contains three cis double bonds c. a trans triacylglycerol that contains a trans double bond in each hydrocarbon chain
Answers
b. An unsaturated triacylglycerol that contains three cis double bonds would have three different unsaturated fatty acids attached to a glycerol backbone. Each fatty acid would contain a cis double bond.
c. A trans triacylglycerol that contains a trans double bond in each hydrocarbon chain would have three different trans fatty acids attached to a glycerol backbone. Each fatty acid would contain a trans double bond.
what is the formula for co3+ and se2-?
Answers
The formula for Co3+ is Co3+ because it represents the ion of cobalt that has lost three electrons, leaving it with a 3+ charge.
What is chemical formula and how they are formed ?
A chemical formula is a symbolic representation of a chemical compound that shows the types of elements present in the compound and the relative number of atoms of each element. For example, the chemical formula for water is H2O, which indicates that it is made up of two hydrogen atoms and one oxygen atom.
Chemical formulas are formed by identifying the elements that make up a compound and determining the relative number of each element in the compound. The number of each element is represented by a subscript following the chemical symbol of the element. For example, the chemical formula for methane is CH4, which indicates that there is one carbon atom and four hydrogen atoms in each molecule of methane.
The formula for Se2- is Se2- because it represents the ion of selenium that has gained two electrons, giving it a 2- charge.
To know more about reaction visit :-
https://brainly.com/question/11231920
#SPJ1
Calculate the amount of heat, in calories, that must be added to warm 89.7 g
of brick (0.20) from 22.0 °C
to 44.1 °C.
Assume no changes in state occur during this change in temperature.
Answers
The formula for calculating heat is:
Q = m * c * ΔT
Where Q is the heat energy required, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
Substituting the values given:
m = 89.7 g
c = 0.20 cal/g°C
ΔT = 44.1°C - 22.0°C = 22.1°C
Q = 89.7 g * 0.20 cal/g°C * 22.1°C
Q = 394.926 cal
Therefore, the amount of heat required to warm 89.7 g of brick from 22.0°C to 44.1°C is 394.926 calories.
The following balanced equation shows the formation of ammonia.
N2 + 3H2 Right arrow. 2NH3
How many moles of nitrogen are needed to completely convert 6.34 mol of hydrogen?
Answers
Answer:
For completely converting 6.34 moles of hydrogen to ammonia, 2.11 moles of Nitrogen is required.
The chemical reaction for the formation of ammonia by nitrogen and hydrogen reaction has been as follows:
For the formation of 2 moles of ammonia, 3 moles of hydrogen, and 1 mole of nitrogen s required.
The utilization of 3 moles of hydrogen requires 1 mole of Nitrogen.
So, the utilization of 6.34 moles of hydrogen requires:
3 moles Hydrogen = 1 -mole Nitrogen
6.34 moles hydrogen = moles of Nitrogen
6.34 moles of hydrogen requires = 2.11 moles of Nitrogen.
For completely converting 6.34 moles of hydrogen to ammonia, 2.11 moles of Nitrogen is required.
For more information about the ammonia formation, refer to the link:
brainly.com/question/20986532
rating answer section
Answer rating5.0
(20 votes)
The ion with the smallest diameter is ________. The ion with the smallest diameter is ________. Be2 Sr2 Ca2 Ba2 Mg2
Answers
Answer:
Be2^+
Explanation:
Ionic diameter increases down the group. This implies that Be2^+ will have the smallest diameter.
This extremely small diameter makes Be2^+ to differ considerably from other ions of group 2 elements.
For instance, the compounds of beryllium are mostly covalent in nature.
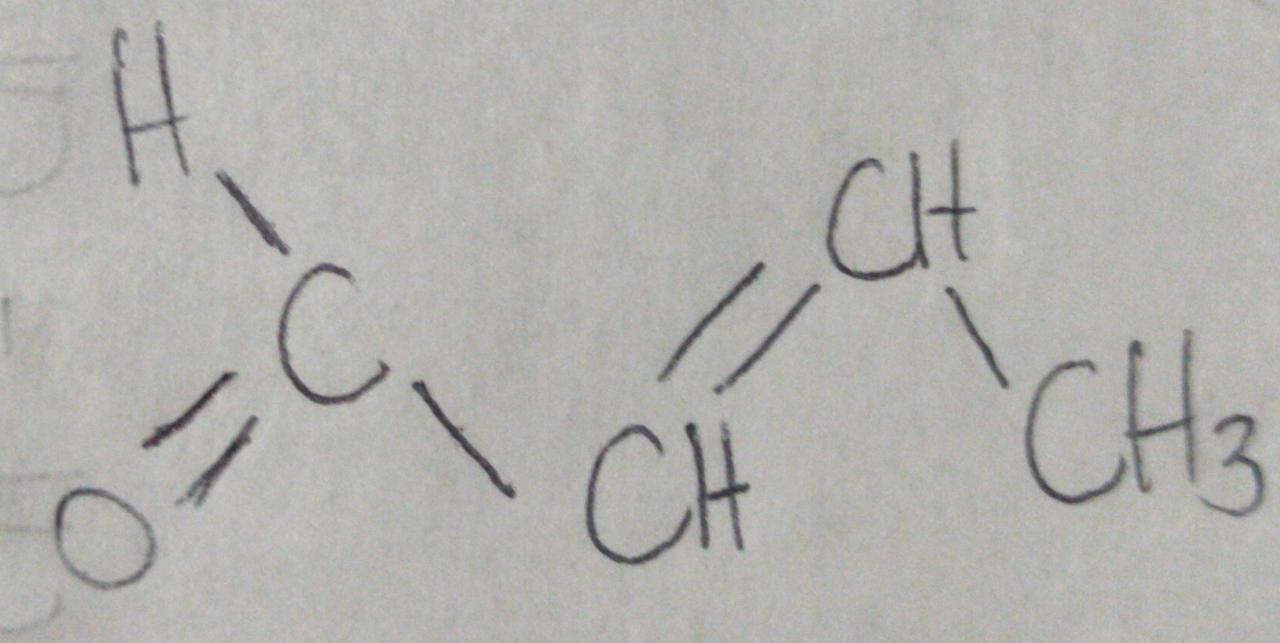
Identify the structure of (E)-2-butenal
Answers
Answer:
See attached picture.
Explanation:
Hello,
In this case, the E-Z nomenclature for alkenes is also related with the trans-cis ones, being the Z (zusammen) same as cis and E (entgegen) same as trans, therefore, for the structure of the (E)-2-butenal which has the double bond at the second carbon is shown on the attached picture considering it is an aldehyde with the carbonyl group due to the -al- suffix.
Regards.
Chlorine reacts with sodium and with hydrogen.
Compare the structure and bonding in sodium chloride and hydrogen chloride.
Answers
Answer:
Sodium Chloride has Ionic bond while Hydrogen Chloride has covalent bond.
Explanation:
Na has 11 electrons (2, 8, 1) and need to give away 1 electron to be stable
Cl has 17 electrons ( 2, 8, 7) and needs 1 electron to be stable.
Na transfers 1 electron to CL to form Ionic bond.
While
Hydrogen has 1 electron and shares with Chlorine to be stable.
Covalent bond involves sharing.


Chlorine reacts with sodium to form ionic sodium chloride while chlorine reacts with hydrogen to form covalent hydrogen chloride.
The nature of bonding between atoms depends on their relative difference in electronegativity.
Chemical bonding is the process by which atoms come together to form compounds.
There are different kinds of chemical bonds;
Ionic or electrovalent bondCovalent bondMetallic bondAn ionic bond is formed when there is high electronegativity difference between the bonding atoms. Typically, this type of bond is formed when the electronegativity difference is about 2.0 and above.
For a covalent bond, an electronegativity difference of about 0.4 - 1.7 is considered a polar covalent bond. Lower values of electronegativity difference corresponds to a nonpolar bond.
The electronegativity difference between sodium and chlorine is about 2.23. This corresponds to a pure ionic bond. The compound is composed of chlorine and sodium ion pairs alternately located in a crystal lattice as shown in the image attached.
The electronegativity difference between chlorine and hydrogen is about 0.96. This corresponds to a polar covalent bond. The negative end of the dipole points towards chlorine while the positive end of the dipole points towards hydrogen.
https://brainly.com/question/6071754

describe how you can determine the total change in empathy and activation energy from the diagram and if each is positive or negative 
Answers
The difference between the products point on the reaction profile and the energy of the reactants is the reaction's activation energy. The change is negative
What is a diagram of potential energy?The energy change between the reactants and the products can be seen in a potential energy diagram or reaction profile.
When we examine the reaction profile, we see that the energy difference between the reactants and products indicates that the process is endothermic. Enthalpy is calculated by deducting the energy of the reactants from the energy of the products.
Learn more about energy profile: brainly.com/question/11256472
#SPJ1

Question 3 of 10
Often, personal data are transmitted using digital signals. What might go
wrong with these transmissions?
A. The signal might become too weak to detect because it slows
down
B. The data might be altered, or changed, during the transmission
C. The data might be misinterpreted because of noise,
D. The data might be stolen or accessed by hackers.
Answers
Answer: D
Explanation:
The personal data are transmitted using digital signals. The data might be stolen or accessed by hackers to go wrong with these transmissions. Therefore, option D is correct.
What is digital signal ?A digital signal is one that can only ever take on one of a few discrete values at any given time. It represents data as a sequence of discrete values.
A continuous signal that represents physical measurements is an analog signal. Digital modulation is used to create time-separated signals known as digital signals. Sine waves serve as its signal. The square waves serve as a marker.
It makes use of a continuous range of values to aid in the representation of data. Digital technologies are any techniques, systems, equipment, or resources that produce, store, or process data electronically.
Thus, option D is correct.
To learn more about digital signal, follow the link;
https://brainly.com/question/22717116
#SPJ5
What is the generic name of this molecule? (Ph stands for phenyl)

Answers
The proper name of the compound is now 1,1 - dihydroxy-3-phenyl propene.
What is the name of the compound?Firstly we know that the parent chain is based on propane. Now we have three substituents on that parent chain. Two hydroxy moieties and one phenyl moiety.
The proper name of the compound is now 1,1 - dihydroxy-3-phenyl propene.
Learn more about names of molecules:https://brainly.com/question/26196615
#SPJ1
Identify whether each of the following pairs of compounds are enantiomers or diastereomers: they are diastereomers they are enantiomers they are neither enantiomers nor diastereomers they are enantiomers they are diastereomers they are neither enantiomers nor diastereomers they are neither enantiomers nor diastereomers they are diastereomers they are enantiomers they are enantiomers they are diastereomers they are neither enantiomers nor diastereomers they are enantiomers they are diastereomers they are neither enantiomers nor diastereomers they are enantiomers they are diastereomers they are neither enantiom
Answers
Hello. You did not show the pairs of compounds to which the question refers, which makes it impossible for them to be classified as enantiomers or diastereomers. However, I will try to help you in the best possible way.
To find out if the compounds are enantiomers or diastereomers, you must evaluate their structure in relation to each other. This is because enantiomers, will be the pairs of compounds that have very similar structures, reaching the point of looking like mirrored structures of each other, but they cannot overlap. On the other hand, diastereomers are neither able to overlap, nor have such similar structures.
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
The decomposition of SO2Cl2 is first order in SO2Cl2 and has a rate constant of 1.42 x 10-4
s
-1 at a
certain temperature.
a. If the reaction started with 75.6 g of SO2Cl2 dissolved in 1.25 L, what would be the
concentration of SO2Cl2 after 3.00 hours?
b. A student claims that the half life of SO2Cl2 changes as the reaction progresses. Do you agree
or disagree with the students claim? Explain your answer. Note: The experiment was
preformed at a constant temperature.
Answers
Answer:
a. 13.0g/L is the concentration of SO2Cl2 after 3.00h
b. FALSE
Explanation:
The first order reaction follows the equation:
ln[SO2Cl2] = -kt + ln[SO2Cl2]₀
Where [] is the concentration after time t, k is rate constant = 1.42x10⁻⁴s⁻¹
[]₀ is initial concentration:
a. []₀ = 75.6g/1.25L = 60.48g/L
t in seconds: 3h * (3600s / 1h) = 10800s
Replacing:
ln[SO2Cl2] = -1.42x10⁻⁴s⁻¹*10800s + ln[60.48g/L]₀
ln[SO2Cl2] = 2.5687
[SO2Cl2] = 13.0g/L is the concentration of SO2Cl2 after 3.00h
b. The rate constant of a reaction remains constant if temeprature remains constant.
9. Nitric acid, HNO3, is extensively used in the manufacture of fertilizer. A bottle containing 75.0 ml of nitric acid solution is labeled 6.0 M HNO3.
a. How many moles of HNO3 are in the bottle?
b. A reaction needs 5.00g of HNO3. How many ml of solution are required?
(Molar mass of HNO3 = 63.01 g /mol)
Answers
a)There are 0.45 moles of HNO₃ in the bottle.
b)Approximately 13 ml of the solution are required for the reaction.
a. To find the number of moles of HNO₃ in the bottle, we can use the formula:
moles = concentration (M) x volume (L)
Given that the concentration of the nitric acid solution is 6.0 M and the volume is 75.0 ml, we need to convert the volume to liters:
75.0 ml = 75.0 ml x (1 L/1000 ml) = 0.075 L
Now, we can calculate the number of moles:
moles = 6.0 M x 0.075 L = 0.45 moles
So, there are 0.45 moles of HNO₃ in the bottle.
b. To determine the volume of solution required for 5.00 g of HNO3, we need to use the molar mass of HNO3 to convert grams to moles:
moles = mass (g) / molar mass (g/mol)
Given that the molar mass of HNO₃ is 63.01 g/mol and the mass required is 5.00 g, we can calculate the number of moles:
moles = 5.00 g / 63.01 g/mol ≈ 0.079 moles
Now, we can use the concentration to find the volume:
volume (L) = moles / concentration (M)
volume (L) = 0.079 moles / 6.0 M ≈ 0.013 L
Finally, we need to convert the volume to milliliters:
volume (ml) = 0.013 L x (1000 ml/1 L) ≈ 13 ml
So, approximately 13 ml of the solution are required for the reaction.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
Liquid octane (CH3(CH2)6CH3) will react with gaseous oxygen (O2) to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). Suppose 17. g of octane is mixed with 112. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.
Answers
The maximum mass of water that could be produced by the chemical reaction is 162 g.
The given chemical equation is: 2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(l)In the chemical reaction of liquid octane with gaseous oxygen, the products are gaseous carbon dioxide and gaseous water.According to the balanced chemical equation, 2 moles of C8H18 react with 25 moles of O2 to form 18 moles of H2O.So, 1 mole of C8H18 react with 25/2 = 12.5 moles of O2 to form 9 moles of H2O.The molar mass of C8H18 is 114 g/mol. So, the number of moles in 17 g of C8H18 is:17 g / 114 g/mol = 0.149 molThe molar mass of O2 is 32 g/mol. So, the number of moles in 112 g of O2 is:112 g / 32 g/mol = 3.5 molFrom the balanced chemical equation, 1 mole of C8H18 react with 12.5 moles of O2 to form 9 moles of H2O.So, the number of moles of O2 required to react with 0.149 mol of C8H18 to form H2O is:(12.5 mol / 1 mol) × (0.149 mol / 2 mol) = 0.935 molThe maximum number of moles of H2O that can be produced from 0.149 mol of C8H18 and 0.935 mol of O2 is 9 mol.So, the mass of water produced from 17 g of C8H18 and 112 g of O2 is:9 mol × 18 g/mol = 162 g
for more questions on chemical reaction
https://brainly.com/question/25769000
#SPJ8
Laboratory balances that measure to the hundredths (0.01g) are calleda) centigram balanceb) milligram balancec) analytical balance
Answers
Answer:
\(A\)Explanation:
Here, we want to select the balance used to measure mass to a hundredth accuracy
The kind of balance used in this case is the centigram balance. The measure the mass of a substance to an accuracy of a hundredth which is 0.01g
what quality is represented by the metric system prefix deci-?
Answers
The prefix "deci" is used to express one tenth of the unit of any measurement. For example decimeter is the one tenth of the one meter length.
What is metric system ?Metric system is a n international system of units. Under the direction of an international standards body, the historical evolution of these systems culminated in the definition of the International System of Units (SI) in the middle of the 20th century. Metrication is the process of converting to the metric system.
There are many prefix that indicates the exact measurement of a variable under different situations. There are prefix like, centi, deci, micro, milli etc. The prefix deci is used to express one tenth (1/10) of the unit.
One decimeter is one tenth of 1 m. That is 10⁻¹ m. Similarly we can use the prefix deci to other variables such as deciliter, decigram etc. Usually used to express small length in metric system.
Find more on metric system:
https://brainly.com/question/25966695
#SPJ9
Into what genre does a novel fit that
takes place in a made-up world and
has events that couldn't really
happen?
A. non-fiction
B. mystery
C. fantasy

Answers
Answer:
A. non fiction or a ajami to download
Please solve this worksheet of chemical reactions.

Answers
Answer:
hgvghhshshh3hrurudus
Which candle burned the longest time why
Answers
Answer:
The one that burned longer because it burned longer
Explanation:
Why: slowest due to the hardness of the wax and the high temperature needed to melt the wax
what does the word interact
Lift
Fly
Discuss
Work with
Answers
Answer:
The answer would be work with.
Explanation:
Because, if we know what the word interact means then we can look for a word similar to interact. hope this makes sense <3.
Balance the following reaction: NH3 + I2 --> N2I6 + H2
Answers
The balanced equation will be \(2NH_3 + 3I_2 -- > N_2I_6 + 3H_2\)
What are balanced equations?They are chemical equations that obey the law of conservation of atoms.
In other words, they are equations in which the number of atoms before and after reactions are the same.
Thus, the balanced equation for the reaction will be \(2NH_3 + 3I_2 -- > N_2I_6 + 3H_2\)
More on balancing equations can be found here: https://brainly.com/question/7181548
#SPJ1
Question 5:
Two liquids A and B, are each poured into seperate 20 mL containers completely filling
them. You weigh both liquids and find that A has a mass of 15 grams and B has a mass
I
of 22.8 grams. When those liquids are put into the same container, one of them will
float on the other. Based on your knowledge of Density, which liquid will float on the
other?
Answers
Answer:
A will float on top of B.
Explanation:
B is more dense. example A is oil and B is water.
A 32.65-g sample of a solid is placed in a flask. Toluene, in which the solid is insoluble, is added to the fla sk so that the total volume of solid and liquid together is 50.00 mL. The solid and toluene together weigh 58.58 g. The density of toluene at the temperature of the experiment is 0.864 g/ mL. What is the density of the solid?
Answers
Answer:
Density of the solid is 1.63g/mL
Explanation:
Hello,
Data;
Mass of solid = 32.65g
Mass of toluene = ?
Mass of solid + toluene = 58.58g
Density of toluene = 0.864g/mL
Mass of toluene = (mass of solid + toluene) - mass of solid
Mass of toluene = 58.58 - 32.65
Mass of toluene = 25.93g
But density = mass / volume
Density of toluene = mass of toluene / volume of toluene
Volume of toluene = mass of toluene / density of toluene
Volume of toluene = 25.93 / 0.864
Volume of toluene = 30.01mL
Volume of solid = total volume of the mixture (solid + toluene) - volume of toluene
Volume of solid = 50 - 30.01
Volume of solid = 19.99mL
Density of the solid = mass of solid / volume of solid
Density of the solid = 32.65 / 19.99
Density of the solid = 1.63g/mL
Therefore the density of the solid is 1.63g/mL