How does newtons second law of motion apply to grocery shopping with a shopping cart?
Answers
When grocery shopping with a shopping cart, the second law of motion applies in several ways.
Newton's second law of motion states that the acceleration of an object is directly proportional to the net force acting on it and inversely proportional to its mass. In other words, F=ma, where F is the net force, m is the mass, and a is the acceleration.
First, when you push on the shopping cart with a certain force, the cart will accelerate in the direction of the force. The greater the force you apply, the greater the acceleration of the cart.
Second, the mass of the shopping cart also affects its acceleration. The more groceries you add to the cart, the greater its mass, and the less it will accelerate for a given force. This is why it is more difficult to push a full shopping cart than an empty one.
In conclusion, Newton's second law of motion applies to grocery shopping with a shopping cart because the acceleration of the cart depends on both the force applied to it and its mass.
You can learn more about Newton's 2nd law of motion at: brainly.com/question/29227915
#SPJ11
Related Questions
Will give brainly NEED CHEM HELP

Answers
Answer:
X is hydrochloric acid solution, Z is sodium chloride solution and Y is sodium hydroxide solution.
Explanation:
I will try be as detailed as possible.
Litmus paper:
-Short summary: Bases will turn blue and red litmus papers to blue and acids will turn blue and red litmus papers to pink while the pink will remain pink.(Simple Mnemonic: You can say B from base turns blue litmus blue and the others are the opposite).
-Also, neutral solutions will have not cause any color change on litmus papers. This is because litmus papers have a color change in the presence of H+ from acids and Oh- from bases. So, blue litmus paper remains blue and red litmus paper remains pink.
-Hydrochloric acid solution is a strong monoprotic acid because it fully dissociates to produces H+ ions. Therefore, in the presence of blue and pink litmus papers it will turn the blue to pink and the pink will remain pink.
-Sodium chloride solution is a neutral solution because it dissociates to Na+ and Cl- which do not play a role in the color change.
-Sodium hydroxide solution is a strong monoprotic base hence it fully dissociates to produce OH- no wonder the high pH value.
pH value:
-Acids have a pH value is <7 while pH of neutral solutions is 7 and basic solutions is >7.
-So from this analogy we can say that hydrochloric acid solution is 2,sodium chloride solution is 7 and sodium hydroxide solution is 10.
Universal indicator:
-A universal indicator gives a more precise data as compared to litmus because it not only identifies the type of the solution (acidic, basic or neutral) it also tells us the strength of the solution by comparing the color of the solution when the universal indicator is added against the pH universal indicator scale.
-Unfortunately ,the colors are quite a lot and I cannot type all of them but you could search for 'pH universal indicator scale' to clearly see the whole thing.
-In the scale, you can see that after addition of the universal indicator, sodium chloride solution neutral solutions (pH7) will give a green color, hydrochloric acid solution (pH=2) which is a strong acidic solution will give a red color and sodium hydroxide solution (pH=10) which is a strong base will give a blue solution.
Reaction with Magnesium:
-I'd like to recommend also that you search for 'metal reactivity' so that you can be able to see the trend .
-A highly reactive metal can displace a low reactive metal but a low reactive metal cannot displace a high reactive metal under normal conditions.
-So, for sodium hydroxide and sodium chloride solutions with magnesium ,magnesium cannot displace sodium because sodium is one of the most reactive metals therefore there will be no change.
-For hydrochloric acid solution, the reaction will take place because Magnesium is more reactive than hydrogen thus can be able to displace hydrogen ions and form Magnesium chloride solution.
~I hope this will help. All the best:)
list the four different sublevels and (given that only a maximum of two electrons can occupy an orbital) determine the maximum number of electrons that can exist in each sublevel.
Answers
l = 0 → s = 2 electrons, l = 1 → p = 6 electrons, l = 2 → d = 10 electrons, l= 3 → f = 14 electrons.
What is electron?The smallest elemental component of an atom, the electron has a negative charge. The smallest elemental component of an atom, the electron has a negative charge. In a negative ion, there are an adequate amounts of both electrons and protons.
Briefing:According to the subatomic particles, the orbit region of space has a higher likelihood of harboring an electron. It is impossible to simultaneously detect the electron's location and velocity (uncertainty principle). Therefore, the theory establishes five electron density to represent one electron, making it simple to recognize it:
→n is the principal quantum number and identify the shell where the electron is.It ranges from 1 to 7, and the consonants K, L, m actually, N, O, P, etc Q stand in for it;
→l is the azimuthal quantum number and identify the subshell (or sublevel) where the electron is. The consonants s, p, d, or f stand in for it, which ranges from 0 to 3;
→ml is the magnetic quantum number, and it represents the orbital. It varies from -l to +l, passing by 0. There can be at least two electrons in each orbital;
→ms is the spin number and represents the spin of the electrons. The range is +1/2 to -1/2.
The sublevel s (l = 0) only has one orbital, enabling it to have at least two electrons; the subarea p (l = 1) has three orbitals, so it can and has at least six electrons; the subarea d (l = 2) has five orbitals, so it can would have had at least ten electrons; and the basement level f (l = 3) has seven orbitals, enabling it to have at least fourteen electrons.
To know more about Electrons visit:
https://brainly.com/question/18367541
#SPJ4
n2o5 (m) .92 1.23 1.79 2.00 2.21 gaous decomposition of dinitrogen pentoxide into nitrogen dioxide and oxygen has been studies in carbon tetrachoridesolvent at a certain temperature
Answers
The reaction order (m) is 1, and the rate constant (k) is approximately 1.34 for the decomposition of N₂O₅ in carbon tetrachloride at the specified temperature.
To determine the reaction order and rate constant for the decomposition of dinitrogen pentoxide (N₂O₅) in carbon tetrachloride solvent at a certain temperature, we need to use the given data points.
The general rate equation for a reaction can be written as:
Rate = k[N₂O₅]^m
Where:
Rate is the rate of the reaction,
k is the rate constant,
[N₂O₅] is the concentration of N₂O₅, and
m is the reaction order with respect to N₂O₅.
The given data points to determine the reaction order and rate constant:
[N₂O₅] Rate
0.92 1.23
1.23 1.79
1.79 2.00
2.00 2.21
2.21
To determine the reaction order (m), we can examine the ratio of rate values for two different concentrations of N₂O₅:
(1.79/1.23) = (2.00/1.79) = (2.21/2.00) = 1.46
Since the ratio of rate values is approximately constant at 1.46, it indicates that the reaction is first-order with respect to N₂O₅. Therefore, m = 1.
Now, we can use any of the data points to calculate the rate constant (k). Let's use the first data point ([N₂O₅] = 0.92 and Rate = 1.23) and plug the values into the rate equation:
1.23 = k(0.92^1)
Simplifying:
k = 1.23 / 0.92
k ≈ 1.34
Therefore, the reaction order (m) is 1, and the rate constant (k) is approximately 1.34 for the decomposition of N₂O₅ in carbon tetrachloride at the specified temperature.
To know more about carbon tetrachloride:
https://brainly.com/question/30459999
#SPJ4
An ad claims that a supplement helps a person lose weight, helps curb appetite to snack less, raises levels of serotonin to make a person feel less hungry, blocks new fat from forming, and increases metabolism to have more energy and burn existing fat. The manufacturer of the supplement published the data from the study of the product.
The study of the supplement used 100 subjects between the ages of 20 and 65. Of the 100 participants, 50 were male and 50 were female. Once the participants were selected for the study, each had the visceral fat measured. After all 100 participants were measured, it was determined that each had a measurement of more than 90 centimeters.
The testing subjects were divided into two groups. One group of 50 received the supplement and one group of 50 received a placebo. The study lasted for 16 weeks, but after 12 weeks, both groups were given the placebo.
After 16 weeks, men in both groups had a slight decrease in body weight. At the end of the study, the group receiving the supplement had reduced visceral fat when compared to the placebo.
After reading this data summary, what do you think? Answer the following questions in a post. Each question is worth 2 points.
1.Based on the testing data of the supplement, were any of the product's claims, proven correct? If so, identify the claims.
2.What data led you to that conclusion?
3.Which of the product's claims, if any, were not proven to be correct?
4.What type of testing data would you need to see in support of those claims to believe they were true?
5.When you see a product advertised on social media, how can you determine whether it is making reliable claims?
Answers
Answer:
If FDA finds that the evidence supporting the proposed claim is credible and the claim can be qualified to prevent it from misleading consumers,
fda.gov
Explanation:
balance the following skeleton reaction and identify the oxidizing and reducing agents. so32−(aq) cl2(g) → so42−(aq) cl−(aq) [basic]
Answers
The balanced chemical equation is 2SO₃²⁻(aq) + Cl₂(g) → SO₄²⁻(aq) + 2Cl⁻(aq). The oxidizing agent is Cl₂(g) and the reducing agent is SO₃²⁻(aq).
SO₃²⁻(aq) + Cl₂(g) → SO₄²⁻(aq) + Cl⁻(aq)
We can start by balancing the atoms other than hydrogen and oxygen. In this case, we have sulfur (S), chlorine (Cl), and oxygen (O) atoms.
Sulfur: There is 1 sulfur atom on each side, so it is already balanced.
Chlorine: There are 2 chlorine atoms on the left side and 1 chlorine atom on the right side. To balance chlorine, we need to put a coefficient of 2 in front of Cl⁻ on the right side:
SO₃²⁻(aq) + Cl₂(g) → SO₄²⁻(aq) + 2Cl⁻(aq)
Oxygen: There are 3 oxygen atoms on the left side (1 from SO₃²⁻ and 2 from Cl₂) and 4 oxygen atoms on the right side (all in SO₄²⁻). To balance oxygen, we need to put a coefficient of 2 in front of SO₃²⁻ on the left side.
2SO₃²⁻(aq) + Cl₂(g) → SO₄²⁻(aq) + 2Cl⁻(aq)
Finally, the equation is a balanced chemical equation.
To identify the oxidizing and reducing agents, we look at the change in oxidation states of the elements involved.
In this reaction, the oxidation state of sulfur changes from +4 in SO₃²⁻ to +6 in SO₄²⁻. Therefore, sulfur is being oxidized, making Cl₂ the oxidizing agent.
The oxidation state of chlorine changes from 0 in Cl₂ to -1 in Cl⁻. Therefore, chlorine is being reduced, making SO₃²⁻ the reducing agent.
The oxidizing agent is Cl₂(g) and the reducing agent is SO₃²⁻(aq).
Learn more about the oxidation state here:
https://brainly.com/question/31315172
#SPJ11
tin has 10 stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occurring tin atoms. how many atoms of 124sn are present in 23.0 g of naturally occurring tin?
Answers
Atoms of 124sn are present in 23.0 g of naturally going on the tin: The average atomic mass of Sn is 118. seventy-one g/mole percentage.
This is explained as the Atomic mass of Tin (Sn) = 118.71 g/mol. 1 mol of Sn = 118.71 g.
An atom is the smallest unit of everyday count that bureaucracies a chemical detail. each solid, liquid, gas, and plasma consists of neutral or ionized atoms. Atoms are extraordinarily small, commonly around one hundred picometers throughout.
An atom is a particle of count that uniquely defines a chemical detail. An atom includes a principal nucleus that is surrounded by one or extra negatively charged electrons. Atoms are built of two kinds of elementary particles: electrons and quarks. Electrons occupy an area that surrounds an atom's nucleus. each electron has an electrical rate of -1. Quarks make up protons and neutrons, which, in flip, make up an atom's nucleus.
Learn more about atoms here:
https://brainly.com/question/6258301
#SPJ4
a student determined the empirical formula of magnesium oxide by heating a known mass of magnesium in air and weighing the product after the magnesium had burned. which factors would result in her calculated formula having a higher ratio of magnesium to oxygen than 1:1? i. not all the magnesium reacted ii. some of the product escaped before it was weighed iii. some of the product was magnesium nitride, mg3n2
Answers
All the magnesium do not reacted and some of the product was magnesium nitrite, Mg₃N₂ are the factors . Option B is correct.
The empirical formula, which is defined as the ratio of subscripts of the smallest possible whole number of the elements in the formula, is the simplest formula for a compound.
Mg + O₂( air) ⇒ MgO.
assume mass of magnesium [Mg]= w₁ g
mass of the product [MgO] = w₂ g
Thus, mass of oxygen in MgO = w₂-w₁
= x g
Now, to explain the experimental result, which indicates a higher Mg to oxygen ratio rather than a 1:1 ratio, this x g is less than w₁ g.
i) x< w₁ If all of the w₁ is not reacted, less w₂ is formed, and the ratio will be greater than 1:1.
ii) If a side effect occurs and some magnesium nitride is heated in the air, [Mg₃N₂] also formed.
Mg + N₂( air) ⇒ Mg₃N₂ ,
Because it contains both the product MgO and Mg₃N₂, x g will yield a higher Mg ratio.
iii) Option II is incorrect because both MgO and Mg₃N₂ are white amorphous solids that are stale and inert. So it can't be evaded during assortment.
Learn more about Empirical formula:
brainly.com/question/1603500
#SPJ1
Complete question as follows:
A student determined the empirical formula of magnesium oxide by heating a known mass of magnesium in air and weighing the product after the magnesium had burned. Which factors would result in her calculated formula having a higher ratio of magnesium to oxygen than 1:1? I 1. Not all the magnesium reacted II. Some of the product escaped before it was weighed III. Some of the product was magnesium nitrite, Mg, N,
A. I and II only
B. I and Ill only
C. Il and Ill only
D. I, II and III
Where is the air pressure greater-- at sea level or on a mountaintop? Explain
Answers
Answer:
Air pressure is greater at sea level than at the top of a mountain. The amount of air at sea level is greater than that on top of a mountain.
Explanation:
Please give thanks to all my answers and please mark as brilliant and please follow me
What step in the water treatment process involves the removal of sediment?
A.
aeration
B.
desalination
C.
filtration
Answers
What is the primary result of a fission
reaction?
(1) conversion of mass to energy
(2) conversion of energy to mass
(3) binding together of two heavy nuclei
(4) binding together of two light nuclei
Answers
Answer:
(1) conversion of mass to energy
Explanation:
Nuclear fission: In nuclear fission, an unstable atom splits into two or more smaller pieces that are more stable, and releases energy in the process. The fission process also releases extra neutrons, which can then split additional atoms, resulting in a chain reaction that releases a lot of energy.
The primary result of a nuclear fission reaction is the conversion of energy to mass.
What is nuclear fission reaction?Nuclear fission reaction is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The nuclear fission reaction produces gamma photons and releases a very large amount of energy even by the energetic standards of radioactive decay.Hence, option 2 is the answer.
To learn more about nuclear fission reaction here
https://brainly.com/question/19721281
#SPJ2
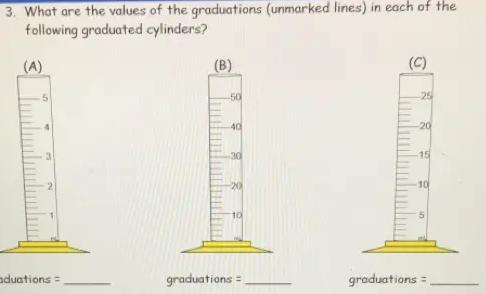
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
which one of these is correct? and how do I find out?

Answers
Answer:
I'm not really advanced at these type of problems, but it looks like it's 1. If I'm wrong, sorry.
If a sample of air in a closed container was heated would the total pressure of the air in the container increase decrease or remain unchanged. Explain your reasoning.
Answers
According to the Gay-Lussac's law, as the container is heated that is as temperature increases pressure also increases as they are directly proportional to each other.
What is Gay-Lussac's law?Gay-Lussac's law is defined as a gas law which states that the pressure which is exerted by the gas directly varies with its temperature and at a constant volume.The law was proposed by Joseph Gay-Lussac in the year 1808.
The pressure of the gas at constant volume reduces constantly as it is cooled till it undergoes condensation .The nature of graph of pressure versus temperature is linear.
Learn more about Gay-Lussac's law,here:
https://brainly.com/question/2683502
#SPJ1
Which of the following statements on HPLC modes is true? A. Increasing the polarity of the mobile phase decreases the elution time of polar compounds in normal-phase HPLC B. A non-polar stationary phase is used in normal-phase HPLC C. Compounds have a lower attraction to the mobile phase than to the stationary phase in displacement development D. A polar stationary phase is used in reversed-phase HPLC E. More polar compounds elute first in normal-phase HPLC
Answers
The following statements on HPLC modes are true is more polar compounds elute first in normal-phase HPLC (Option E).
The liquid chromatography (HPLC) is a technique in analytical chemistry employed for the separation, identification, and quantification of elements. It is considered a highly sensitive method, and it works by separating the components in a mixture with the assistance of a solvent under high pressure.
There are two modes of HPLC: Reversed-Phase HPLC (RP-HPLC) and Normal-Phase HPLC (NP-HPLC). In RP-HPLC, a nonpolar stationary phase, such as C18, is used, and polar solvents, such as water, are used as mobile phases. Polar stationary phases, such as silica gel, are used in NP-HPLC, while nonpolar solvents, such as hexane, are used as mobile phases.
More polar compounds have a greater affinity for the polar stationary phase than less polar compounds, which have a higher affinity for the nonpolar mobile phase in NP-HPLC. As a result, less polar compounds elute first in normal-phase HPLC.
Thus, the correct option is E.
Learn more about HPLC: https://brainly.com/question/13490391
#SPJ11
This atom can form up to _____ single covalent bond(s). An atom with two electron shells. There is one electron pair in the inner electron shell and four unpaired electrons in the outer electron shell. 0 1 4 2 3
Answers
Answer:
4
Explanation: it's a carbon atom right....soo it'll form 4 bonds(single)
An atom can form 4 single covalent bonds is carbon with two electron shell.
What are covalent bonds?The bonds are defined as a strong bond that binds atoms, ions, or molecules together and promotes the production of molecules.
There are three types of bonds.
Ionic bondCovalent bondMetallic bondCovalent bonds are defined as the bond formed by sharing of electrons with each other.
Covalent bonding is a stable equilibrium of the attractive and repulsive forces between two atoms that occurs when they share electrons. Bonding pairs or sharing pairs are other names for these electron pairs.
It can also be defined as a type of chemical link where atoms share electrons to create electron pairs.
Covalent bonds has low melting point, boiling point, enthalpy of fusion and enthalpy of vaporization.
Thus, an atom can form 4 single covalent bonds is carbon with two electron shell.
To learn more about covalent bonds, refer to the link below:
https://brainly.com/question/10777799
#SPJ12
why polymer melting point transitions are broader than low molecular compounds?
Answers
Polymer melting point transitions are broader than those of low compounds because of their molecular structure and the forces holding them together.
Low molecular compounds have a simple, defined structure and are hmoleculareld together by intermolecular forces such as van der Waals forces, dipole-dipole interactions, and hydrogen bonding. When these forces are overcome, the compound transitions from solid to liquid.
Polymers, on the other hand, have a much more complex molecular structure with long chains of repeating units. These chains are held together by covalent bonds, which require much more energy to break than the intermolecular forces in low molecular compounds. As a result, polymers have a higher melting point than low molecular compounds.
Furthermore, the long chains in polymers are not perfectly aligned, meaning that some parts of the chains will require more energy to break than others. This leads to a broader melting point transition.
Additionally, some polymers may have different types of covalent bonds, resulting in different melting points for different parts of the polymer. These factors contribute to the broader melting point transition observed in polymers compared to low molecular compounds.
Learn more about, transitions.
https://brainly.com/question/29795678
#SPJ11
an experiment to compare the tension bond strength of polymer latex modified mortar (portland cement mortar to which polymer latex emulsions have been added during mixing) to that of unmodified mortar resulted in x
Answers
In an experiment comparing the tension bond strength of polymer latex modified mortar (portland cement mortar with added polymer latex emulsions) to that of unmodified mortar, the result was x.
To conduct this experiment, you would need to follow these steps:
1. Prepare the materials: Gather all the necessary materials, including polymer latex emulsions, portland cement, sand, water, and any other additives required for the mortar mixture.
2. Prepare the polymer latex modified mortar: Mix the portland cement, sand, water, and polymer latex emulsions according to the specified proportions. Ensure thorough mixing to achieve a homogeneous mixture.
3. Prepare the unmodified mortar: Mix the portland cement, sand, and water according to the specified proportions. Again, ensure thorough mixing for a consistent mixture.
4. Perform the tension bond test: Apply both the polymer latex modified mortar and the unmodified mortar onto separate test surfaces, such as concrete blocks or metal plates. Make sure the surfaces are clean and free from any contaminants.
5. Allow the mortar to cure: Let both the modified and unmodified mortar cure for a specific duration, following the manufacturer's instructions or established industry standards.
6. Test the bond strength: Use a tension bond strength test apparatus, such as a hydraulic or mechanical testing machine, to apply a force perpendicular to the bond interface of the mortar. Apply increasing force until the bond fails and records the maximum force required for bond failure in both cases.
7. Compare the results: Analyze the data obtained from the tension bond test for both the polymer latex modified mortar and the unmodified mortar. Determine the maximum force or stress required for bond failure in each case.
To know more about polymer visit:-
https://brainly.com/question/1443134
#SPJ11
3 facts about the core
Answers
Answer:
The inner core is a hot, dense ball of (mostly) iron. It has a radius of about 1,220 kilometers (758 miles). Temperature in the inner core is about 5,200° Celsius (9,392° Fahrenheit). The pressure is nearly 3.6 million atmosphere (atm).
Explanation:
There are two things that activate the leavening agent. What are they?
Answers
Answer:
Leavening agent is a material that causes doughs and batters to expand by releasing gases from inside the mixture, resulting in porous baked goods. Air, steam, yeast, baking powder, and baking soda are examples of such agents.
Explanation:
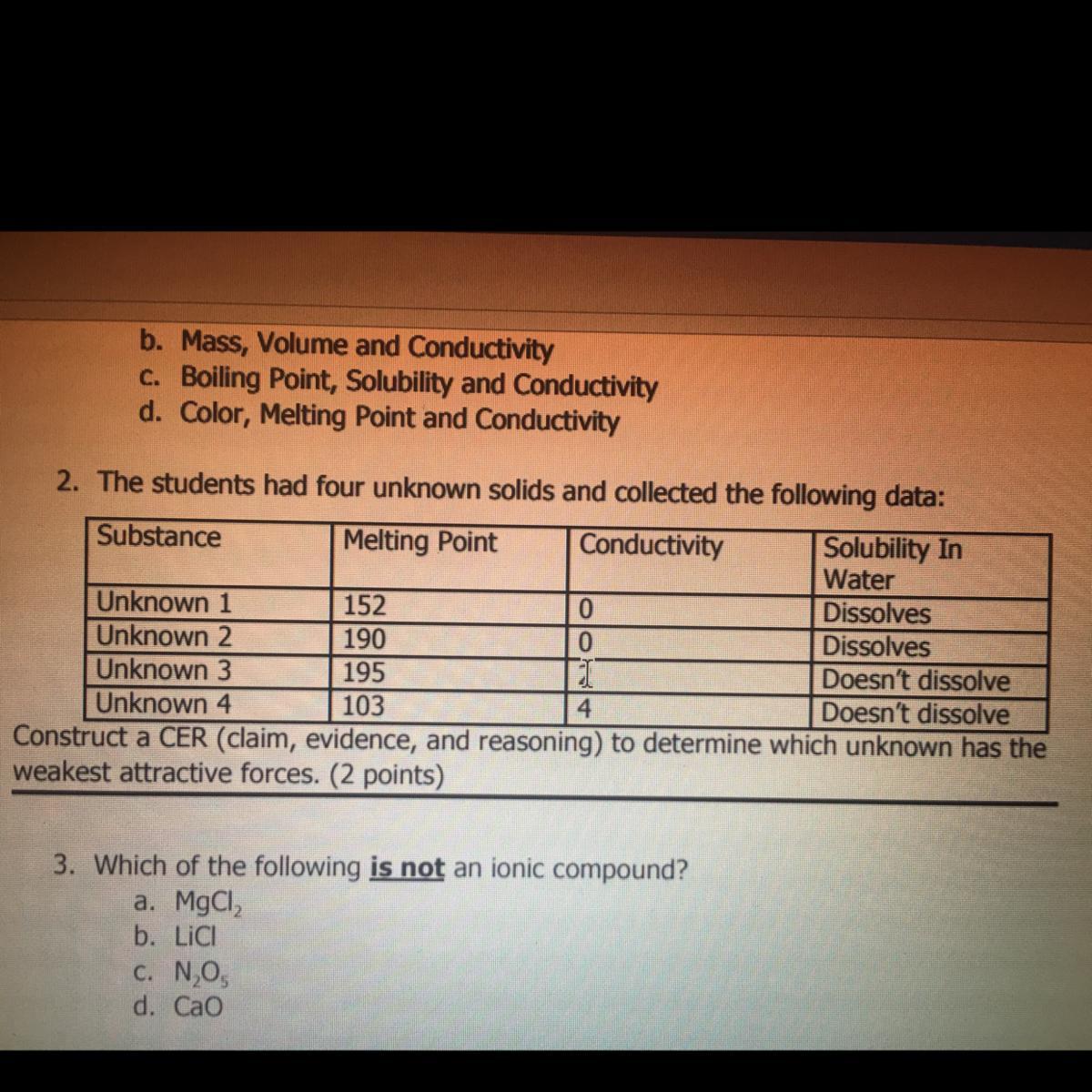
i just need #2, its due any minute. giving extra points, and will mark brainliest!!!!!!!!
which unknown has the weakest attractive forces?
Answers
Answer:
Unknown 4
Explanation:
when 25 g of a hydrate of nickel chloride was heated, 11.37 g of water was released. determine the name and formula of the hydrate
Answers
The correct formula of the compound can now be written as \(NiCl_{2} .9H_{2} O\)
What is the formula of the hydrate?We know that when we talk about a hydrate, we are talking about a compound that has some molecules of the water of crystallization in it. Thus we have that in this case, the compound that we are considering is nickel chloride.
Given that;
Number of moles of anhydrous salt = Number of moles of hydrated salt
Number of moles of hydrated salt = 25/130 + 18x
Number of moles of anhydrous salt = 11.37 g/130 g/mol
= 0.087 moles
When we now apply the formula, we have;
25/130 + 18x = 0.087 moles
0.087 (130 + 18x) = 25
11.31 + 1.566x = 25
x = 25 - 11.31 /1.566
x = 9
We now get;
\(NiCl_{2} .9H_{2} O\)
Learn more about hydrated salt:https://brainly.com/question/5586505
#SPJ1
name the complex ion [cu(nh3)4(h2o)2]2+. the oxidation number of copper is +2.
Answers
The complex ion is called tetraammineaquacopper(II) and has the formula [Cu(NH3)4(H2O)2]2+. In this complex, copper has an oxidation number of +2, which means it has lost two electrons and has two fewer negative charges than it does in its neutral state.
The complex is formed by the coordination of four ammonia (NH3) molecules and two water (H2O) molecules to a central copper ion. The ammonia molecules act as ligands, meaning they donate a pair of electrons to the copper ion, while the water molecules act as counter ions, balancing the charge of the complex.
This coordination results in a highly stable complex with unique properties and reactivity, making it an important compound in many chemical reactions.
Learn more about reactivity here:
brainly.com/question/30843855
#SPJ11
Name the layer where the pressure is 3.5 million atmospheres:
Answers
Answer:
The Inner Core
hope it was useful
stay at home stay safe
keep rocking
pls mark me as brani
........
Ksp for ZnS is 1.1 x 10-21 At what s2- concentration will ZnS precipitate for a 0.20 M solution of Zn(NO3)2? Zn(NO3)2 is a very soluble salt. 1.3.3 x 10-11 M 2. 2.2 x 10-20 M 3. 5.5 10-21 M 4. 5.5 x 10-20 M 5. 2.4 x 10-10 M
Answers
The equilibrium concentration of S2−, can be x,x[ S 2 − ]=[ Zn 2 + ]=0.20 MKsp=[Zn2+][S2−]=1.1×10−21=0.20x20x=sqrt(1.1×10−21/0.20)=5.5×10−20 M[Zn2+]=[S2−]=5.5×10−20 MTherefore, the precipitating concentration of ZnS is 5.5 × 10−20 M.
Zinc sulfide is a compound that is colorless, transparent, and refractive. The mineral wurtzite is its most common form, although sphalerite occurs as a red, yellow, greenish, or black color. It is a chemical compound made up of the elements zinc and sulfur, and its chemical formula is ZnS.What is Ksp?Ksp (solubility product constant) is the equilibrium constant for a solid substance dissolving in an aqueous solution. It reflects the degree of saturation of a solution with a solute. For a compound that is ionically dissociated, it is equivalent to the product of the concentrations of the ions, each raised to the power of their stoichiometric coefficient. Zn(NO3)2 is the chemical formula for zinc nitrate. Zinc nitrate is a salt with a colorless or white crystalline appearance that is easily soluble in water and ethanol.What is the formula for Zinc sulfide?ZnS is the chemical formula for zinc sulfide.What is the formula for sulfide?The sulfide ion is a negatively charged polyatomic ion with the chemical formula S2-. It can be made by reacting an acid with a sulfide salt or by reducing sulfur with an appropriate reducing agent.ZnS will precipitate when the ion product is greater than the solubility product constant, which is equal to 1.1 x 10-21. Therefore, let's compute the equilibrium constant for the reaction ZnS(s)⇌Zn2+(aq)+S2-(aq)The equilibrium expression for this reaction isKsp=[Zn2+][S2−]The equilibrium concentration of Zn2+ can be computed from the concentration of Zn(NO3)2:0.20 M Zn(NO3)2⇌0.20 M Zn2+The equilibrium concentration of S2−, can be x,x[ S 2 − ]=[ Zn 2 + ]=0.20 MKsp=[Zn2+][S2−]=1.1×10−21=0.20x20x=sqrt(1.1×10−21/0.20)=5.5×10−20 M[Zn2+]=[S2−]=5.5×10−20 MTherefore, the precipitating concentration of ZnS is 5.5 × 10−20 M.
Learn more about equilibrium concentration here:
https://brainly.com/question/32029862
#SPJ11
What will happen if a catalyst is added to the system
at equilibrium?
A+B <-> AB
A) The equilibrium concentration of AB will
increase.
B) The equilibrium concentration of AB will
decrease.
C) The rates of the forward and reverse reactions
will change.
D) The equilibrium constant for the reaction will
change.
Answers
When a catalyst is added to a system at equilibrium, the rates of the forward (A + B -> AB) and reverse (AB -> A + B) reactions will change (Option C).
A catalyst lowers the activation energy for both the forward and reverse reactions, thereby increasing their rates. However, the catalyst affects both reactions equally, maintaining the equilibrium constant (K) unchanged.
Although the reaction rates increase, the equilibrium concentrations of the reactants (A and B) and product (AB) do not change (neither Option A nor Option B). This is because the relative concentrations of reactants and products required to maintain equilibrium are determined by the equilibrium constant, which remains the same.
In conclusion, adding a catalyst to a system at equilibrium will change the rates of the forward and reverse reactions but will not affect the equilibrium concentrations of the reactants and products or the equilibrium constant for the reaction. Hence, the correct answer is Option C.
Learn more about catalyst here: https://brainly.com/question/26976764
#SPJ11
When copper sulphate is dissolved in water in a beaker, a bright blue liquid or solution is formed. If copper sulphate is added until no more will dissolve, a saturated solution is formed. Some blue crystals will remain at the bottom of the beaker?
Please answers quick
Answers
Answer:
ok i dont get your question fully but i'll answer
When copper sulfate is dissolved in water in a beaker, a bright blue liquid or solution is formed. If copper sulfate is added until no more will dissolve, a saturated solution is formed. And some blue crystals will remain at the bottom of the beaker due to crystallization reaction. It is the process by which a solid form, where the atoms or molecules are highly organized into a structure known as a crystal.
Write and balance a combustion reaction for the complete combustion of each molecule C9H16, C10H20, and C8H20.
Can someone show me how to do this? Thanks.
Answers
Answer:
1) C9H16 + 13O2 → 9CO2 + 8H2O
2) C10H20 + 15O2 → 10CO2 + 10H2O
3) C8H20 + 13O2 → 8CO2 + 10H2O
Explanation:
Complete combustion is when a substance burns and reacts with oxygen fully to produce carbon dioxide and water.
1) With the first molecule C9H16, let's write the equation:
C9H16 + O2 → CO2 + H2O
This equation is not balanced. First, let's balance the carbons in the reactants (left side) and products (right side):
We have 9 carbons on the left and 1 carbon on the right, so let's add a coefficient of 9 in front of the CO2 to make it balanced:
C9H16 + O2 → 9CO2 + H2O
Now that the number of carbon is balanced on both sides, let's balance the number of hydrogen:
The equation we have now:
C9H16 + O2 → 9CO2 + H2O
We have 16 hydrogens on the left and 2 hydrogens on the right, so let's add a coefficient of 8 in front of H20 because 8 × 2 = 16:
C9H16 + O2 → 9CO2 + 8H2O
All we need to do now is balance the oxygen:
We have 2 oxygens on the left and 26 oxygens on the right ( because in 9CO2 there are 9×2=18 oxygens and in 8H2O there are 8×1=8 oxygens, so 18+8=26)
Current equation:
C9H16 + O2 → 9CO2 + 8H2O (unbalanced)
To make the number of oxygens equal, add a coefficient of 13 in front of O2 because 13×2=26, so both sides are equal:
This is the final equation:
C9H16 + 13O2 → 9CO2 + 8H2O
2) Doing the same thing to C10H20:
C10H20 + O2 → CO2 + H2O
The number of carbons is not the same on both sides, so add a coefficient of 10 to CO2 so that there are 10 carbons on each side:
C10H20 + O2 → 10CO2 + H2O
Now let's balance the number of hydrogens. There are 20 hydrogens on the left and 2 hydrogens on the right, so add a coefficient of 10 in front of H2O because 10×2=20, so both sides have 20 hydrogens:
C10H20 + O2 → 10CO2 + 10H2O
Lastly balancing the number of oxygens. There are 2 oxygens on the left and 30 oxygens on the right (because in 10CO2 there are 10×2=20 oxygens and in 10H2O there are 10×1=10 oxygens, so 20+10=30)
Add a coefficient of 15 in front of O2 because 15×2=30, making both sides of oxygen equal to 30
Final equation:
C10H20 + 15O2 → 10CO2 + 10H2O
3) Now for C8H20:
C8H20 + O2 → CO2 + H2O (unbalanced)
Let's balance the number of carbons by adding a coefficient of 8 in front of CO2 to make both sides of carbon equal to 8:
C8H20 + O2 → 8CO2 + H2O
There are 20 hydrogens on the left and 2 hydrogens on the right, so add a coefficient of 10 to H2O because 10×2=20, so both sides of hydrogen are equal to 20:
C8H20 + O2 → 8CO2 + 10H2O
Lastly, balancing the oxygens. There are 2 oxygens on the left and 26 oxygens on the right (because in 8CO2 there are 8×2=16 oxygens and in 10H2O there are 10×1=10 oxygens, so 16+10=26)
Add a coefficient of 13 in front of O2 because 13×2=26, so there will be 26 oxygens on both sides.
Final equation:
C8H20 + 13O2 → 8CO2 + 10H2O
During which change of state would the volume of a substance increase the most?
O deposition
O melting
O vaporization
O sublimation

Answers
Which property of water makes it a good choice for use in heat exchangers? Choose the correct answer.
A)high entropy
B)low entropy
C)low specific heat
D)high specific heat
Answers
Answer:
The correct answer is D)high specific heat
Explanation:
The specific heat indicates the amount of heat required to raise the temperature of a substance in 1 unit of temperature. The specific heat of water is around 4.18 J/g.°C. That means that 1 gram of water is able to retain a high amount of heat (4.18 J) when the temperature is raised to 1°C. Compared with other substances, this specific heat capacity is higher. Thus, water is a good choice for use in a heat exchanger.
Is milk going sour caused by heating

Answers
Answer:
Both are chemical changes and both are physical changes.
Explanation:
Milk can go sour in the fridge and I don't really know about the conserve mass one.