Answers
Related Questions
C6H12O6 -> 2C2H5OH + 2CO2Determine the volume of carbon dioxide gas produced when 7.0 moles of glucose decomposes at STP
Answers
Answer:
313.6 L
Explanation:
use mole/mole ratio to find moles of CO2 (=14)
multiply by 22.4
=313.6 L
Diamond and graphite are two crystalline forms of carbon. At 1 atm and 25°C, diamond changes to graphite so slowly that the enthalpy change of the process must be obtained indirectly. Determine ΔHrxn for
C(diamond) → C(graphite)
with equations from the following list:
(1) C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ
(2) 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ
(3) C(graphite) + O2(g) → CO2(g) ΔH = −393.5 kJ
(4) 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJ
Answers
The enthalpy change of the reaction C(diamond) → C(graphite) is -2.9 kJ.
The given information is ΔHrxn for the reaction C(diamond) → C(graphite) can be calculated with the given equations:Equations: C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ C(graphite) + O2(g) → CO2(g) ΔH = −393.5 kJ 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJThe required reaction can be obtained by adding the equations (1) and (4), as follows:C(diamond) + O2(g) + 2CO(g) → C(graphite) + 3CO2(g)Addition of the two equations (1) and (4) results in a reaction whose products are C(graphite) and CO2.
To get the final equation that involves only the required reactants and products, the equation (2) should be added, which consumes CO2 and produces O2, as shown below:C(diamond) + O2(g) → CO2(g) ΔH = −395.4 kJ [eq. (1)] 2 CO(g) → C(graphite) + CO2(g) ΔH = −172.5 kJ [eq. (4)] 2 CO2(g) → 2 CO(g) + O2(g) ΔH = 566.0 kJ [eq. (2)] C(diamond) + O2(g) + 2CO(g) → C(graphite) + 3CO2(g) ΔHrxn=ΣΔHf(products)−ΣΔHf(reactants) ΔHrxn=[(3 mol CO2)(-393.5 kJ/mol) + (1 mol C(graphite))(0 kJ/mol)] − [(1 mol C(diamond))(0 kJ/mol) + (1 mol O2)(0 kJ/mol) + (2 mol CO(g))(−172.5 kJ/mol)] − [(2 mol CO2)(566.0 kJ/mol)] ΔHrxn=−2.9 kJ.
for such more questions on reaction
https://brainly.com/question/11231920
#SPJ8
Which of the following is NOT a natural resource? Check all that apply.
1. Air
2. Water
3. Plastic
4. Sunlight
5. Cotton
6. Glass
7. Coal
8. Copper
Answers
While being made from natural resources, electricity does not constitute a natural resource because it goes through several procedures to produce it.
What are the seven categories of natural resources?Oil, coke, nat gas, metals, stone, or sand are examples of natural resources. Other natural resources include water, soil, sunlight, air, and so on. Natural resources have value because they enable life and provide for human needs.
A natural resource is land?Financially referred to it as land and raw materials, land resources (also known as natural resources) are found naturally within ecosystems that are mostly unaltered by civilization. A natural resource's biodiversity levels across different habitats are frequently used to describe it.
To know more about several visit:
https://brainly.com/question/13758372
#SPJ1
Consider the reaction below.
HI + H2O Right arrow. H3O+ + I–
Which is an acid-conjugate base pair?
Answers
In the given reaction: \(HI + H_2O \rightarrow H_3O +I^-\), the acid-conjugate base pair is \(HI\)(hydroiodic acid) and \(I^-\) (iodide ion).
An acid-conjugate base pair consists of an acid and its corresponding conjugate base. In an acid-base reaction, an acid donates a proton (\(H^+\)) to a base, resulting in the formation of a conjugate base. The acid and its conjugate base are related by the transfer of a proton.
In the given chemical reaction, \(HI + H_2O \rightarrow H_3O +I^-\),
In the forward reaction, HI donates a proton (\(H^+\)) to water (\(H_2O\)), forming the hydronium ion (\(H_3O^+\)). In this process, HI acts as an acid by losing a proton.In the reverse reaction, the hydronium ion(\(H_3O^+\)) can accept a proton, reverting back to water (\(H_2O\)), while the iodide ion (\(I^-\)) remains unchanged. In this process,\(I^-\) acts as the conjugate base by accepting a proton.Thus, the acid-conjugate base pair in the given chemical reaction is \(HI\) and \(I^-\).
Learn more about acid-conjugate base pair here:
https://brainly.com/question/32826047
#SPJ2
17.1 grams of magnesium metal burns in sulphur dioxide to form magnesium oxide and sulphur write a balanced equation for the reaction and calculate the mass of magnesium oxide and the mass of sulphur that forms.
Answers
Mass of MgO = 28.35grams
Mass of Sulphur = 11.29 grams
Explanations:The balanced chemical equation between magnesium metal and sulphur dioxide is given as:
\(2Mg+SO_2\rightarrow2MgO+S\)Determine the moles of magnesium
Mole = mass/molar mass
Mole of Mg = 17.1/24.305
mole of Mg = 0.704moles
According to stoichiometry, 2 moles of Mg produces 2 moles of MgO, hence the required mass of MgO will be:
\(\begin{gathered} Mass\text{ of MgO}=0.704\times40.3 \\ Mass\text{ of MgO}=28.35grams \end{gathered}\)Similarly, 2moles of Mg produces 1 mole of sulphur, hence the mass of sulphur produced is;
\(\begin{gathered} Mass\text{ of S}=\frac{1}{2}\times0.704\times32.065 \\ Mass\text{ of S}=11.29grams \end{gathered}\)Hence the mass of magnesium oxide and the mass of sulphur that forms is 28.35grams and 11.29grams
What type of reaction is 2NaBr+Cl2-NaCl+Br2
Answers
Answer:
An anionic single replacement reaction.
Explanation:
Hope this helps!!!
Answer:
Substitution or Displacement maybe?
suppose the price elasticity of demand for heating oil is 0.2 in the short run and 0.7 in the long run.
Answers
Short run heating oil consumption will decrease by 4%, and long-term demand will decrease by 14%. Because people can react to changes in the price of heating oil more readily, the change is greater over time.
How elastic is the price both now and in the future?The demand elasticity for heating oil at the going rate = 0.2 in the foreseeable future
Price change over the long term is 0.7%; price change over the short term is 2.20 - 1.80 / 2 x 100 = 20%.
Both the short-term and long run demand for heating oil will decline by 4% each.
The shift in the price of heating oil is bigger over time because customers can respond to changes more quickly. The ratio of variations in quantity demanded and price over the short term is known as price elasticity of demand.
To get the percentage change in quantity required, divide 20 by 0.2; the result is 4%.
The ratio of long-term variations in quantity required and price is known as price elasticity of demand.
Change in demand as a percentage: 20 * 0.7 = 14% Change in demand as a percentage: 0.7 Demand change as a percentage: 20
Your question is incomplete but most probably your full question was
Suppose the price elasticity of demand for heating oil is 0.2 in the short run and 0.7 in the long run. if the price of heating oil rises from $1.80 to $2.20 per gallon, what happens to the quantity of heating oil demanded in the short run? in the long run? (use the mid point method in your calculations.)
To learn more about the short and long run here
brainly.com/question/16836331
#SPJ4
What effect does a catalyst have on a reaction?
A. A catalyst makes it possible for a reaction to happen.
B. A catalyst contributes energy to a reaction.
C. A catalyst speeds up the rate of a reaction.
D. A catalyst increases the concentrations of products.
SUBMIT
Answers
Answer:
it would increase
Explanation:
D
A catalyst speeds up the rate of a reaction. Hence, option C is correct.
What is a catalyst?A catalyst is a substance that allows the chemical reaction to occur at a faster rate or under different conditions (e.g. at a lower temperature) without being affected by the reaction.
A catalyst enables a chemical reaction to proceed at a usually faster rate or under different conditions.
Hence, option C is correct.
Learn more about the catalyst here:
https://brainly.com/question/18959742
#SPJ2
Elements in the same group of the periodic table have the same number of
Answers
Answer:
valence electrons
The elements in each group have the same number of electrons in the outer orbital. Those outer electrons are also called valence electrons. They are the electrons involved in chemical bonds with other elements. Every element in the first column (group one) has one electron in its outer shell.
A cylinder with a moveable piston contains 218 mL of nitrogen gas at a pressure of 1.32 atm and a temperature of 298 K.
Answers
Answer:
No question is being asked here.
Explanation:
If looking for the moles or grams of nitrogen gas you would have to use the ideal gas law: PV=nRT. If the movable piston moves and you are given more measurements you would use the combined gas law P1V1/T1=P2V2/T2
Rewrite this measurement with a simpler unit, if possible.
4.5·m2m2m
Answers
Answer:
why are people s opinions and prejudices her greatest obstacles
PLEASE HELP...
Balance this nuclear reaction by supplying the missing nucleus. Replace each question mark with an appropriate integer or symbol.
Cf98249 + ? ⟶Db105260+410n
Answers
The balanced form of the nuclear equation is as follows; 249/98 Cf + 15/7 N⟶ 260/105 Db + 4(1/0) n.
What is a nuclear equation?A nuclear equation is process such as the fission of an atomic nucleus, or the fusion of one or more atomic nuclei and/or subatomic particles in which the number of protons and/or neutrons in a nucleus changes.
According to this question, Californium element is a reactant to produce dubnium and a neutron as products.
However, the law of conservation of mass must be fulfilled by ensuring the mass and atomic numbers of elements in reactant and product side are the same.
249/98 Cf + 15/7 N⟶ 260/105 Db + 4(1/0) n
Learn more about nuclear equation at: https://brainly.com/question/13315150
#SPJ1
4. A puddle of coastal seawater, caught in a depression formed by some coastal rocks at high tide, begins to evaporate as the tide goes out. If the volume of a puddle decreases to 23% of its original volume, what is the sodium chloride concentration if it was initially 0.449 M?
Answers
Answer:
0.583M NaCl
Explanation:
Molarity is an unit of concentration defined as the ratio between moles of solute and liters of solution.
In the puddle, the solute is sodium chloride that is dissolved in water and you have 0.449 moles of NaCl per liter of water
When the solution begins to evaporate, amount of water decreases whereas moles of NaCl remain constant.
As 23% of the water evaporates, amount of water that remains is 100-23 = 77%, that means now you have 0.449 moles of NaCl per 77% of a liter, 0.770L. The new concentration is:
0.449 moles NaCl / 0.770L =
0.583M NaClCompare the weight of a 100-lb person on Mercury with the weight of that same person on Jupiter. What can you conclude about the mass and force of gravity of these planets?
A
Mercury has much less mass than Jupiter, so the force of gravity is weaker on Mercury and this causes the person to weigh less.
B
Mercury is closer to the sun than Jupiter so this causes the person to weigh less.
C
The mass of Mercury and Jupiter has no impact on the force of gravity.
D
Mercury has more mass than Jupiter, so the force of gravity is greater on Mercury and this causes the person to weigh less.
Answers
Answer:
if you weigh 100 pounds on Earth, you would weigh only 38 pounds on Mercury. That's because Mercury weighs less than Earth, and therefore its gravity would pull less on your body. If, on the other hand, you were on heavy Jupiter, you would weigh a whopping 253 pounds!
1. An electric iron has a
power rating of 750W
a. How many joules of
electric energy does it
change into heat energy
every second?
b. How many joules of
work can it do in 3
seconds
c. How long does it take
the iron to do 1500J of
work?
2. Use the kinetic particle
theory to explain why a
solid has a definite shape
and liquid has none.
Answers
Explanation:
a) E = P × t
E = 750 × 1 s = 750 J
b) E = P × t
E = 750 × 3s = 2250 J
c) E = P × t
1500 = 750 × t
t = 1500/750
t = 2 s
In the anaerobic fermentation of grain, the yeast Saccharomyces cerevisiae digests glucose from plants to form the products ethanol and propenoic acid by the following reactions: Reaction 1: C6H12O6 2 C2H5OH + 2 CO2 Reaction 2: C6H12O6 2 C2H3CO2H + 2 H2O In an open flow reactor 4000 kg of a 12% glucose-water solution flows in. During fermentation, 120 kg of carbon dioxide is produced together with 90 kg of unreacted glucose. What are the weight percents of ethyl alcohol and propenoic acid that exit the broth? Assume that none of the glucose is assimilated into the bacteria.
Answers
Answer:
Explanation:
The first step in order to solve this particular question is to make sure that the two reactions given in the question is balanced. Therefore, we have;
Reaction 1: C6H12O6 -----------------------------> 2 C2H5OH + 2 CO2.
Reaction 2: C6H12O6 ------------------------------> 2 C2H3CO2H + 2 H2O.
Next, we determine the number of moles of water and that of glucose. Recall that we are given from the question that the open flow reactor = 4000 kg of a 12% glucose-water solution flows in that is to say the percentage for water is [100% - 12% = 88%]. Also, the molar mass of water = H₂O = 18 kg/kmol and that for glucose =180 kg/kmol.
Number of moles of water = (4000 kg × 88%) ÷ 18 = 195.6 kmol.
Number of moles of glucose= (4000kg × 12%) ÷ 180 = 2.67 kmol.
Next thing to do is to determine the number of moles in the unreacted glucose . Therefore, the 90 kg of unreacted glucose ÷ 180kg/kmol = 0.5 kmol.
So, we have that During fermentation, 120 kg of carbon dioxide is produced. Thus, the number of kmol = 120kg÷ 44kg/mol = 2.73kmol.
For reaction 1, we have 2 moles of CO₂ that is to say the extent of the reaction = 2.73kmol / 2 moles of CO₂ = 1.365 kmol.
For reaction 2, we have 2 moles of CO₂ that is to say the extent of the reaction = 2.67kmol - 0.5 kmol - 1.365kmol = 0.805 kmol.
For both reaction, the kmol for outflow of glucose = 2.73 kmol.
Also, 2 × 0.805 + 1.365 × 0 = 1.61kmol.
Hence, 195.6 kmol + 1.61 =197.21 kmol.
The mass of ethanol = 46.1 kg/kmol × 2.73 kmol = 125.853 kg.
The weight percent of ethanol has been 29.72%, and the percent mass of propionic acid has been 47.79%.
The balanced chemical equations of the reactions have been:
\(\rm C_6H_1_2O_6\;\rightarrow\;2\;C_2H_5OH\;+\;2\;CO_2\)
\(\rm C_6H_1_2O_6\;\rightarrow\;2\;C_2H_3CO_2H\;+\;2\;H_2O\)
The solution has consisted of 12% glucose.
Water in the solution = 88%
The mass of the solution = 4000 kg.
The moles of glucose = 12% of 4000 kg
Moles of glucose = \(\rm \dfrac{12}{100}\;\times\;4000\;\times\;\dfrac{1}{180\;g/mol}\)
Moles of glucose = 2.67 kmol.
Moles of water = 88 % of 4000 kg
Moles of water = \(\rm \dfrac{88}{100}\;\times\;4000\;\times\;\dfrac{1}{18\;g/mol}\)
Moles of water = 195.6 kmol.
The unreacted glucose in the mixture = 90 kg
Moles of unreacted glucose:
Moles = \(\rm \dfrac{weight}{molecular\;weight}\)
Moles of unreacted glucose = \(\rm \dfrac{90\;\times\;1000\;g}{180\;g/mol}\)
Moles of unreacted glucose = 0.5 kmol
The mass of carbon dioxide produced = 120 kg.
Moles of carbon dioxide produced = \(\rm \dfrac{120\;\times\;1000\;g}{44\;g/mol}\)
Moles of carbon dioxide produced = 2.73 kmol
Since 1 mole of glucose produces 2 moles of carbon dioxide.
2.73 kmol of carbon dioxide has been produced from 1.365 kmol of glucose.
The moles of ethanol produced by reaction 1 = 2 moles/ mole glucose.
The glucose present has been 2.67 kmol.
The ethanol produced = 5.34 kmol.
Moles of propionic acid produced = 5.34 kmol.
The mass of 5.34 kmol ethanol = Moles × molecular weight
The mass of ethanol produced = 5.34 × 1000 × 46.07g/mol
The mass of ethanol produced = 246.0138 kg.
The mass of propionic acid produced = 5.34 × 1000 × 74.08 g/mol
The mass of propionic acid produced = 395.5872 kg.
The mass of water produced = 5.34 × 1000 × 18 g/mol
The mass of water produced = 96.12 kg.
The remained glucose = 90 kg
The total mass in the reactor:
= Mass of glucose + water + propionic acid + ethanol
= 90 + 96.12 + 395.5872 + 246.0138 kg
= 827.721 kg.
% Mass of ethanol = \(\rm \dfrac{Mass\;of\;ethanol}{Total\;mass}\;\times\;100\)
% Mass of ethanol = \(\rm \dfrac{246.0138}{827.721}\;\times\;100\)
% Mass of ethanol = 29.72%
% Mass of Propionic acid = \(\rm \dfrac{Mass\;of\;propionic\;acid}{Total\;mass}\;\times\;100\)
% Mass of Propionic acid = \(\rm \dfrac{395.5872}{827.721}\;\times\;100\)
% Mass of Propionic acid = 47.79 %.
The weight percent of ethanol has been 29.72%, and the percent mass of propionic acid has been 47.79%.
For more information about weight percent, refer to the link:
https://brainly.com/question/18204076
what did scientists mix to make the savanna cat
Answers
PLS HELP ME WITH MY CHEMISTRY

Answers
Answer:
Explanation:density=mass×volume
so your answer will be 14.7×6.6=97.02
Find the element that is oxidized and the one that is reduced 2 FeCl3 + SnF2 --> 2 FeF2 + SnCl4
Answers
Explanation
2 FeCl3 + SnF2 => 2 FeF2 + SnCl4
The half-reaction: oxidation
\(\text{ Sn}^{+2}=>\text{ 2e}^{-1}+\text{ Sn}^{+4}\)The half-reaction: reduction
\(2\text{ Fe}^{+3}+\text{ 2e}^{-1}=>\text{ 2Fe}^{+2}\)Answer: Sn is oxidized and Fe is reduced
10
The reaction in which ammonia is formed is N2(g) + 3H2(g) + 2NH3(). At equilibrium, a
1-L flask contains 0.15 mol H. 0.25 mol N, and 0.10 mol NH. Calculate K., for the reaction.
11.85
23.8
25.0
16
Answers
The equilibrium constant (K) : 11.85
Further explanationGiven
Reaction
N₂(g) + 3H₂(g) ⇒ 2NH₃(g)
Required
K(equilibrium constant)
Solution
The equilibrium constant (K) is the value of the concentration product in the equilibrium
The equilibrium constant based on concentration (K) in a reaction
pA + qB -----> mC + nD
\(\tt K=\dfrac{[C]^m[D]^n}{[A]^p[B]^q}\)
For the reaction above :
\(\tt K=\dfrac{[NH_3]^2}{[N_2][H_2]^3}\\\\K=\dfrac{0.1^2}{0.25\times 0.15^3}\\\\K=11.85\)
What type of reaction?
HCN,Na2So4
Mg3N2
Co2, H2O
Cu,Zn(NO3)2
Na,N2
Answers
HCN, Na2SO4: Combination of compounds.
Mg3N2: Chemical compound.
CO2, H2O: Dissolution or hydration reaction.
Cu, Zn(NO3)2: Single-replacement reaction.
Na, N2: Combination of elements.
Let's analyze each chemical combination to determine the type of reaction involved:
HCN, Na2SO4:
The combination of hydrogen cyanide (HCN) and sodium sulfate (Na2SO4) does not represent a specific chemical reaction. It is simply the combination of two compounds.
Mg3N2:
Mg3N2 represents a chemical compound, magnesium nitride. It does not indicate a specific reaction.
CO2, H2O:
The combination of carbon dioxide (CO2) and water (H2O) represents a chemical reaction known as hydration or dissolution. When carbon dioxide dissolves in water, it forms carbonic acid (H2CO3), which can further dissociate into hydrogen ions (H+) and bicarbonate ions (HCO3-).
Cu, Zn(NO3)2:
The combination of copper (Cu) and zinc nitrate (Zn(NO3)2) represents a single-replacement reaction. Copper displaces zinc from the compound, resulting in the formation of copper nitrate (Cu(NO3)2) and zinc metal (Zn).
Na, N2:
The combination of sodium (Na) and nitrogen gas (N2) does not represent a specific reaction. It is simply the combination of two elements.
For more question on Combination click on
https://brainly.com/question/28304510
#SPJ11
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
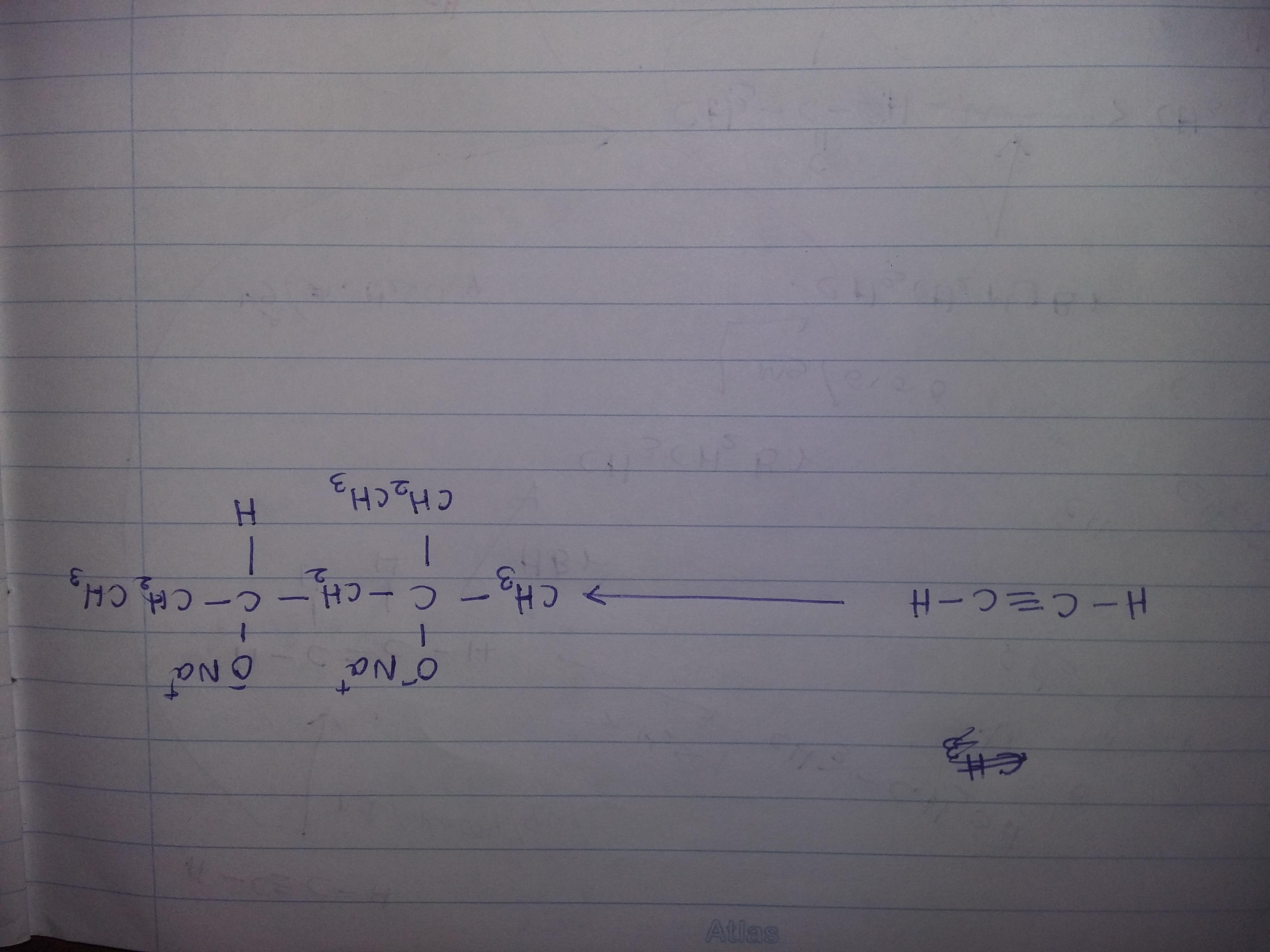
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
What is the structural equation for aspirin esterificarion
Answers
Answer:
The synthesis of aspirin is known in organic chemistry as an esterification reaction. This is a substitution reaction in which an alcohol (the –OH group in salicylic acid) reacts with acetic anhydride to form an ester, aspirin.
Explanation:
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
The half reaction with a more positive standard reduction potential will
Answers
The half reaction with a more positive standard reduction potential will proceed spontaneously in a redox reaction
The half reaction with a more positive standard reduction potential will undergo reduction when compared to the half reaction with a more negative standard reduction potential.
Oxidation-reduction reactions, often known as redox reactions, are a set of chemical reactions that involve electron transfer between reactants. In a redox reaction, one reactant is oxidized, losing electrons, while the other reactant is reduced, gaining electrons.
The oxidation half-reaction is the process of losing electrons and increasing the oxidation number, whereas the reduction half-reaction is the process of gaining electrons and decreasing the oxidation number. The total reaction is referred to as the redox reaction.
Half-reaction:Half-reaction refers to the two parts of an oxidation-reduction reaction that happen separately. A half-reaction must always be either an oxidation reaction or a reduction reaction. It also describes the movement of electrons and hydrogen ions in an equation.
Know more about redox reaction here:
https://brainly.com/question/21851295
#SPJ8
what is the original pressure of a 750 ml sample of He at 0 degrees Celsius if it exerts 2 atm at 25 degrees Celsius and 500 ml
Answers
To determine the original pressure of a 750 ml sample of helium (He) at 0 degrees Celsius, we can use the combined gas law, which relates the initial and final conditions of a gas sample. The combined gas law equation is:
(P1 × V1) / (T1) = (P2 × V2) / (T2)
Where:
P1 and P2 are the initial and final pressures, respectively.
V1 and V2 are the initial and final volumes, respectively.
T1 and T2 are the initial and final temperatures, respectively.
Let's assign the given values:
P1 = unknown (original pressure)
V1 = 750 ml (initial volume)
T1 = 0 degrees Celsius (initial temperature)
P2 = 2 atm (final pressure)
V2 = 500 ml (final volume)
T2 = 25 degrees Celsius (final temperature)
Before using the combined gas law equation, we need to convert the temperatures to Kelvin scale by adding 273.15 to both T1 and T2:
T1 = 0 + 273.15 = 273.15 K
T2 = 25 + 273.15 = 298.15 K
Now we can plug in the values into the combined gas law equation:
(P1 × 750 ml) / (273.15 K) = (2 atm × 500 ml) / (298.15 K)
To solve for P1, we can cross multiply and rearrange the equation:
P1 = (2 atm × 500 ml × 273.15 K) / (750 ml × 298.15 K)
P1 = 0.924 atm
Therefore, the original pressure of the 750 ml sample of helium at 0 degrees Celsius is approximately 0.924 atm.
.11 moles of water vapor contains how many liters?
Answers
Answer:
2.46 L
Explanation:
At standard temperature and pressure 1 mole of any ideal gas occupies 22.4 L of volume.
SO, at STP,
Volume = Mole × 22.4 L/mol
Volume = 0.11 mol × 22.4 L/mol
Volume = 2.46 L
SO2Cl2 rightarrow SO2 (g) + Cl2 (g)At 600 K, SO2Cl2 will decompose to form sulfure dioxide and chlorine gas via the above equation. If the reaction is found to be first order overall, which of the following will cause an increase in he half life of SO2CL2?A. Increasing the initial concentration of SO2,CI2.B. Increasing the temperature at which the reaction occurs.C. Decreasing the overall pressure in the container.D. None of these will increase the half life.
Answers
Answer: D. None of these will increase the half life.
Explanation:
Half life is the amount of time taken to decay to half of its original value. The half life is related to rate constant by the following relation for a first order reaction:
\(t_{\frac{1}{2}}=\frac{0.693}{k}\)
As half life is independent of concentration , temperature and pressure, none of these factors would affect the half life.
Thus in the reaction, \(SO_2Cl_2 \rightarrow SO_2(g)+Cl_2(g)\) following first order kinetics, none of the factors will increase the half life.
What is the pigment that absorbs light energy and make glucose?
Answers
Answer: chlorophyll
Explanation: Photosynthetic cells contain chlorophyll and other light-sensitive pigments that capture solar energy.
A container holds one mole of a gas if the amount of gas is tripled how many molecules of gas will be in the container?
Answers
Answer:At the molecular level, the pressure of a gas depends on the number of collisions its molecules have with the walls of the container. If the pressure on the piston is doubled, the volume of the gas decreases by one-half. The gas molecules, now confined in a smaller volume, collide with the walls of the container twice as often and their pressure once again equals that of the piston.
Explanation:
Answer:
thanks
Explanation: