How do you find the number of moles of copper in copper gluconate? In my experiment I used 1.4 g of copper gluconate, and ended up with 0.1 g of copper.
Answers
Answer: 0.0016 moles of copper are present in copper gluconate
Explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass.
The equation used is:
\(\text{Number of moles}=\frac{\text{Given mass}}{\text{Molar mass}}\) ......(1)
Given mass of copper = 0.1 g
Molar mass of copper = 63.55 g/mol
Plugging values in equation 1:
\(\text{Moles of copper}=\frac{0.1g}{63.55g/mol}=0.0016 mol\)
Hence, 0.0016 moles of copper are present in copper gluconate
Related Questions
which option describes the direction of thermal energy transfer in earth's oceans?
Answers
Answer:
Energy is moved from areas of surplus to those of deficit, with warm currents transporting warm water polewards and cold currents taking colder water to lower latitudes. It holds onto this heat for longer than the land does and the ocean currents move this heat around, from the tropics to higher latitudes.
From the equator to the poles
_________________________
 Write the chemical compounds and names for the following ionic compounds. Cesium and sulfur 
Answers
Answer:
Cesium is Cs.
Sulphur is S.
penetration of a solution into tissue is most dependent upon which characteristic of the solution
Answers
The penetration of a solution into tissue is most dependent upon its solubility or the ability to dissolve in the tissue.
The ability of a solution to penetrate tissue depends on several factors, but one of the most critical characteristics is its solubility. Solubility refers to the ability of a substance to dissolve in a given solvent, in this case, the tissue. When a solution has high solubility, it can readily mix and dissolve in the tissue, allowing for efficient penetration.
Solubility is influenced by various factors such as the nature of the solute and solvent, temperature, and concentration. A solution with a high solubility in tissue will have a greater affinity for the tissue components and can effectively permeate through the tissue barriers.
In summary, the solubility of a solution plays a crucial role in determining its ability to penetrate tissue. Higher solubility enhances the solution's ability to dissolve in the tissue, facilitating better penetration and distribution of the solution within the tissue.
Learn more about solubility here:
https://brainly.com/question/31493083
#SPJ11
the element with a completely filled p-subshell is:
Answers
The element with the completely filled p-subshell is Ar (Option C)
What is electronic configuration?This is the arrangement of elecartons in the atomic orbitals of an element. The modern electronic configuration makes use of the s, p, d, f orbital notation where
Sharp (s) = Maximum of 2 electronsPrincipal (p) = Maximum of 6 electronsDiffuse (d) = Maximum of 10 electrons Fundamental (f) = Maximum of 14 electrons How to determine the element with the completely filled p-orbitalTo obtain the correct answer, we shall write the electronic configuration of each element given in the question. This is illustrated below
Na (11) => 1s² 2s²2p⁶ 3s¹P (15) => 1s² 2s²2p⁶ 3s²3p³Ar (18) => 1s² 2s²2p⁶ 3s²3p⁶Al (12) => 1s² 2s²2p⁶ 3s²From the above illustrations, we can see that only Ar has completely filled p-subshell.
Thus, Ar (Option C ) is the correct answer to the question
Complete question
See attached photo
Learn more about electronic configuration:
https://brainly.com/question/14283892
#SPJ12

Ammonium (NH4+), carbonate (CO32−), and phosphate (PO43−) are all examples of: (a) multivalent metals (b) polyatomic ions (c) covalent molecules (d) molecular compounds. Help pleasee
Answers
Answer:
Option b) polyatomic ions
Explanation:
Polyatomic ions are ions consisting of two or more atoms.
From the question given above, we can see that each ions consist of more than one atom as shown below:
Ions >>>>>> Number of atom present
NH4+ >>>>> 2
CO32− >>>> 2
PO43− >>>> 2
Thus, we can say that the above ions are polyatomic ions.
Answer:
It's C.
Explanation:
The four types of bonding that are important in minerals are covalent, metallic, Van der Waals. Your answer 16. The property of is a mineral's resistance to scratching.
Answers
Minerals with covalent bonding, such as diamond, are typically very hard. Metallic bonding results in minerals that are malleable and ductile, but not necessarily hard.
Van der Waals bonding is weaker and results in minerals that are relatively soft and have a low melting point.
The four types of bonding that are important in minerals are covalent, metallic, Van der Waals. The property of a mineral's resistance to scratching is called hardness.
Hardness is a physical property of minerals that describes their resistance to scratching by other minerals or materials. The Mohs scale is a way of ranking minerals according to their hardness.
The scale runs from 1 (the softest mineral, talc) to 10 (the hardest mineral, diamond). Minerals with covalent bonding, such as diamond, are typically very hard. Metallic bonding results in minerals that are malleable and ductile, but not necessarily hard.
Van der Waals bonding is weaker and results in minerals that are relatively soft and have a low melting point.
To know more about necessarily visit:
https://brainly.com/question/32679605
#SPJ11
2.00 grams of epsom salts are allowed to dehydrate in a warm oven overnight. the mass of the remaining powder is 0.98 grams. how much water was lost?
Answers
The formula of epsom salt is MgSO4. 7H2O
Mass of water crystal = 2.00-0.98 = 1.02 g
moles of water = 1.02/18 = 0.0566 moles
Moles of mgso4 = 0.98/(24.31+32.07+64) = 0.0081 moles
So ratio is 0.0566/0.0081= approx 7
So the formula of epsom salt is MgSO4. 7H2O
Mass is a physical body's total amount of matter. It also serves as a gauge for the body's inertia, or resistance to acceleration (change in velocity) in the presence of a net force. The strength of an object's gravitational pull to other bodies is also influenced by its mass.
The kilogram is the primary mass unit in the SI (kg). Even though weight is frequently measured using a spring scale rather than a balance scale and directly compared with known masses, mass is not the same as weight in physics. Due to the lower gravity on the Moon, an object would weigh less than it does on Earth while maintaining the same mass.
To know more about mass,visit;
brainly.com/question/19694949
#SPJ4
Which immunization is available to prevent communicable disease and illness?WILL MARK BRAINLIEST 30 PTS
bronchitis
diabetes
measles
strep throat
Answers
Measles is a communicable disease that requires immunization to prevent illness when exposed to it.
Measles is a communicable disease and highly contagious. It is usually very serious in children. The disease can be contacted through droplets in the air, physical contact and through mother-to-child transmission.
It is a very serious disease as it is caused by a virus which replicates very fast in the throat and nose thereby creating symptoms such as runny nose, coughing, sneezing and is usually accompanied with a rash.
The severity and death rates is what gave rise to the creating of a measles vaccine which is usually given to babies after birth to make them immune to the potential exposure to the virus.
Diabetes, bronchitis and strep throat have no vaccine as they can always be treated or managed with over-the-counter drugs.
Read more on https://brainly.com/question/25249487
Please help fast! I will give brainliest!

Answers
Answer:(c)
Explanation:
Please help me!
Thank you.

Answers
And no problem!!
How many atoms are there in the repeating peptide backbone units of proteins? Select one: a. 1 b. 2 c. 3 d. 4 e. 5
Answers
The repeating peptide backbone units of proteins contain 3 atoms. By understanding the structure of the "backbone" for peptides and proteins.
The structure of a peptide may be written very simply without illustrating the entire amide synthesis step.
The N-H 2, CH, C double bond O; N-H 2, CH, C double bond O; etc. repeating units make up the peptide backbone.
Nothing more than the use of the amide synthesis process results in the creation of peptides. The amide bond in peptides is often formed in the same sequence that the amino acids are listed. An amino acid's amine end (N terminal) is always on the left and its acid end (C terminal) is always on the right. The diagram on the left illustrates how to write the reaction between glycine and alanine to create the dipeptide glyclalanine. A water molecule is created when hydrogens (red) on the amine and oxygen (red) from the acid combine. The amide bond is created by the joining of the carboxyl oxygen (green) with the amine nitrogen (green).
Learn more about Peptide here:
https://brainly.com/question/30332053
#SPJ4
Which of the following are physical changes? Choose all that apply.
A Gas burns in an engine.
B Sugar dissolves in a cup of water.
C Glass bends as it is heated.
D Water expands as it freezes.
Answers
Hope this helped
example of nitrogen fertilizer
Answers
Answer:
The most common forms of N fertilizer include anhydrous ammonia, urea, and urea-ammonium nitrate (UAN) solutions.
Explanation:
How many moles of O2 must react to form 4.67 moles of NO2?
Answers
The balanced chemical equation for the reaction between oxygen (O2) and nitrogen dioxide (NO2) is:
2NO2 + O2 → 2NO3
From this equation, we can see that for every one mole of O2 that reacts, two moles of NO2 are produced. Therefore, we can set up the following proportion to find the number of moles of O2 that must react to form 4.67 moles of NO2:
1 mole of O2 / 2 moles of NO2 = x moles of O2 / 4.67 moles of NO2
Solving for x, we get:
x = (1 mole of O2 / 2 moles of NO2) x 4.67 moles of NO2
x = 2.335 moles of O2
Therefore, 2.335 moles of O2 must react to form 4.67 moles of NO2.
Please help fasts thanks
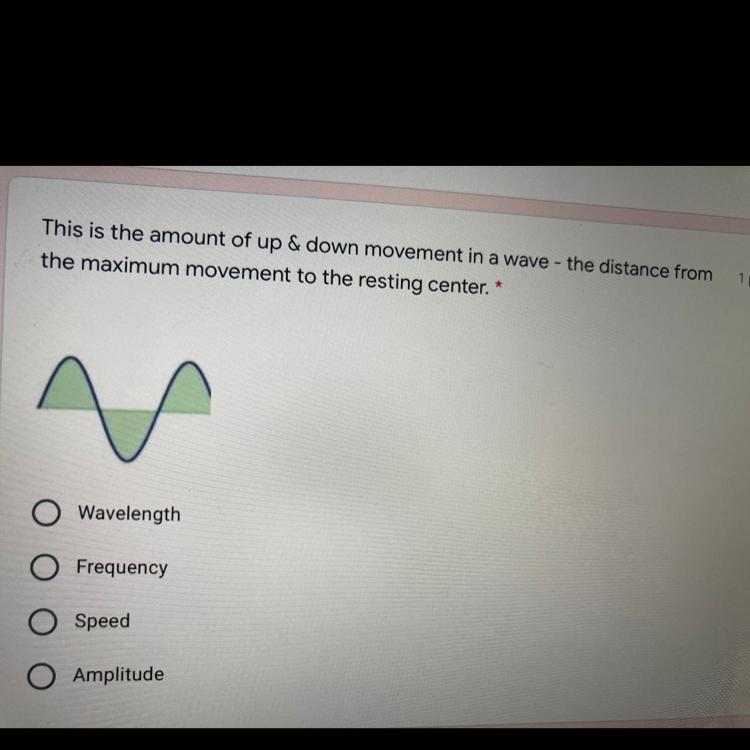
Answers
Answer:
amplitude
Explanation:
The amplitude of a wave is the distance from the centre line (or the still position) to the top of a crest or to the bottom of a trough
Answer:
I think it is wave length
Explanation:
yeah pretty sure
which forces are contact forces? which r non contact forces/
magnetic force,friction,air resistance,gravitational force eletrostatic force and upupthrust
Answers
How many electrons are contained in a neutral atom of an element with an atomic number of 19 and an atomic mass of 39. 9
Answers
Answer:19
Explanation:
A coffee cup calorimeter with a heat capacity of 6. 70 J/∘ C was used to measure the change in enthalpy of a precipitation reaction. A 50. 0 mL solution of 0. 360 M AgNO3 was mixed with 50. 0 mL of 0. 540 M KSCN. After mixing, the temperature was observed to increase by 4. 06∘C. Calculate the enthalpy of reaction, ΔHrxn, per mole of precipitate formed (AgSCN). Assume the specific heat of the product solution is 4. 11 J / (g⋅∘C) and that the density of both the reactant solutions is 1. 00 g/mL. Calculate the theoretical moles of precipitate formed from AgNO3 and KSCN. Moles of precipitate formed from AgNO3: mol moles of precipitate formed from KSCN: mol Calculate the heat change experienced by the calorimeter contents, ????contents. ????contents= J Calculate the heat change expierenced by the calorimeter contents, ????cal. ????cal= J Calculate the heat change produced by the solution process, ????solution. ????solution= J Calulate ΔHsolution for one mole of precipitate formed. ΔHsolution= kJ/mole
Answers
A coffee cup temperature with a heat capacity of 6. 70 J/∘ C was used to measure the change in enthalpy of a precipitation reaction.The value of ΔHrxn was found to be 61.9 kJ/mol.
Calculate the enthalpy of reaction, ΔHrxn, per mole of precipitate formed (AgSCN). Assume the specific heat of the product solution is 4. 11 J / (g⋅∘C) and that the density of both the reactant solutions is 1. 00 g/mL.1. Calculation of Moles of precipitate formed from AgNO3:To find the value of ΔHrxn, we used the formula ΔHrxn = Qsolution/n, where Qsolution is the heat change produced by the solution process and n is the number of moles of AgSCN formed.
To find the value of n, we first calculated the number of moles of AgNO3 and KSCN used in the reaction using the formula n = M × V.To find the heat change produced by the solution process, we used the formula
Q = m × c × ∆T,
where Q is the heat change, m is the mass of the product solution, c is the specific heat capacity of the product solution, and ∆T is the change in temperature of the solution.The value of ΔHrxn was found to be 61.9 kJ/mol.
To know more about temperature Visit;
https://brainly.com/question/29072206
#SPJ11
enter the net ionic equation for the reaction of aqueous sodium chloride with aqueous silver nitrate. express your answer as a chemical equation.
Answers
The net ionic equation for the reaction of aqueous sodium chloride (NaCl) with aqueous silver nitrate (AgNO3) is as follows:
Ag+ (aq) + Cl- (aq) → AgCl (s)
When sodium chloride and silver nitrate are mixed in water, they undergo a double displacement reaction, where the cations and anions switch partners. The silver cation (Ag+) from silver nitrate combines with the chloride anion (Cl-) from sodium chloride to form silver chloride (AgCl) precipitate.
In the complete ionic equation, we can write the reactants and products as their respective ions:
Na+ (aq) + Cl- (aq) + Ag+ (aq) + NO3- (aq) → AgCl (s) + Na+ (aq) + NO3- (aq)
However, the sodium cation (Na+) and nitrate anion (NO3-) ions remain unchanged throughout the reaction and are spectator ions. The net ionic equation removes these spectator ions to show only the species involved in the chemical change:
Ag+ (aq) + Cl- (aq) → AgCl (s)
The net ionic equation for the reaction of aqueous sodium chloride with aqueous silver nitrate is Ag+ (aq) + Cl- (aq) → AgCl (s). This equation represents the formation of silver chloride precipitate in the solution.
To learn more about molecular equation, visit;
brainly.com/question/14286552
#SPJ11
A 1.25 g sample of aluminum is reacted with 3.28 g of copper (II) sulfate. How much aluminum sulfate is produced?

Answers
Step 1
The reaction involved:
2 Al (s) + 3 CuSO4 (aq) => Al2(SO4)3 (aq) + 3 Cu (s) (completed and balanced9
---------------
Step 2
Information provided:
1.25 g Al
3.28 g CuSO4
---
Information needed:
The molar masses of:
Al) 27.0 g/mol
CuSO4) 160 g/mol
Al2(SO4)3) 342 g/mol
----------------
Step 3
The limiting reactant:
By stoichiometry:
2 Al (s) + 3 CuSO4 (aq) => Al2(SO4)3 (aq) + 3 Cu (s)
2 x 27.0 g Al ------ 3 x 160 g CuSO4
1.25 g Al ------ X
X = 1.25 g Al x 3 x 160 g CuSO4/2 x 27.0 g Al
X = 11.1 g CuSO4
For 1.25 g of Al, 11.1 g of CuSO4 is needed but there is 3.28 g, so the limiting reactant is CuSO4.
---------------
Step 4
The amount of Al2(SO4)3:
2 Al (s) + 3 CuSO4 (aq) => Al2(SO4)3 (aq) + 3 Cu (s)
3 x 160 g CuSO4 ------------ 342 g Al2(SO4)3
3.28 g CuSO4 ------------ X
X = 2.34 g
Answer: 2.34 g Al2(SO4)3
Explain how soil contributes to biodiversity, fighting disease and agriculture.
Answers
Answer:
In order to promote biodiversity, fight disease, and advance agriculture, soil is essential in the following ways:
Biodiversity: A variety of microbes, insects, and plants that make up intricate food webs live in soil. The diversity of the soil fosters the growth of plants, which in turn provide habitat and food for other organisms, and contributes to the stability of the ecosystem.
Fighting disease: Soil microorganisms compete with pathogenic organisms for resources and space and also produce antimicrobial chemicals, which helps to avoid plant diseases. Additionally, minimising tillage and increasing organic matter are two soil management techniques that can help lower the prevalence of plant diseases.
Agriculture: Soil supports crops physically, supplies them with vital nutrients, and controls the flow of air and water. Crop yields and quality can both rise with healthy soil and a balanced soil microbiota. Furthermore, sustainable agriculture can be supported by soil management techniques like cover crops and decreased tillage that preserve soil resources and maintain soil health.
The soil is home to a wide range of bacteria, insects, or plants that support complex food webs.
What is soil?The bioactive, porous media that has grown in the top layer of the Earth's crust is known as soil. Being a source of water as well as nutrients, a filter for harmful wastes, a site for their breakdown, and a collaborator in the cycle of carbon as well as other substances through the planet's ecosystem, soil constitutes one of the main substrates of life on Earth.
In the following way soil contributes to biodiversity, fighting disease and agriculture. Biodiversity: The soil is home to a wide range of bacteria, insects, or plants that support complex food webs. Disease prevention: Soil microorganisms create antimicrobial compounds and compete against pathogenic organisms for nutrients and space, which helps prevent plant diseases. Agriculture: Soil provides crops with physical support, essential nutrients, and control over air and water flow.
Therefore, the soil is home to a wide range of bacteria, insects, or plants that support complex food webs.
To know more about soil, here:
https://brainly.com/question/27588666
#SPJ2
In which pair of elements are the chemical properties of the elements most similar? Explain your reasoning.
a. sodium and chlorine
b. nitrogen and phosphorus
c. boron and oxygen
Answers
Answer:
b. nitrogen and phosphorus
Explanation:
Elements in the same column of the Periodic Table have similar chemical properties. Both N and P are in the same column.
Alice added sodium chloride to water and stirred the water for several minutes. Alice is most likely trying to demonstrate that ionic compounds.
Answers
Sodium chloride is an ionic compound. The chemical name of Sodium chloride is NaCl.
What are ionic compounds?Ionic compounds are made up of ions. They have charged particles. Ionic compounds when dissolved in solvents they form ions. Sodium chloride losses Na + and cl ions. Magnesium oxide will form mg2+ and O2 ions.
Ionic compounds dissolve in polar solvents. Examples are water, methanol and formamide. For ionic compounds to dissolve there will be ionic compounds will form.
Ionic bonds are not directional. There would be electrostatic or columbic attraction will be form in molecules. The bonding seen in ionic compounds is called ionic bonding. There are two types of ions seen in molecules such as positive ions and negative ions.
Therefore, Sodium chloride is an ionic compound. The chemical name of Sodium chloride is NaCl.
To learn more about ionic bonding, refer to the link:
https://brainly.com/question/11527546
#SPJ4
Answer: B
Explanation: JUST TOOK THE QUIZ
Energy is defined as...
A.
the ability to do work.
B.
the ability to dissolve in water.
C.
the mass of a substance times its acceleration.
D.
how radioactive a substance is.
Answers
Answer:
A
Explanation:
the ability to dissolve in water
Answer: B.
Explanation: The ability to do work
Use the Henderson-Hasselbalch equation to perform the following calculations. The Ka of acetic acid is 1. 8 * 10–5. Review your calculations with your instructor before preparing the buffer solutions. FW for sodium acetate, trihydrate (NaC2H302•3H20) is 136. 08 g/mol. • Buffer A: Calculate the mass of solid sodium acetate required to mix with 50. 0 mL of 0. 1 M acetic acid to prepare a pH 4 buffer. Record the mass in your data table. Buffer B: Calculate the mass of solid sodium acetate required to mix with 50. 0 mL of 1. 0 M acetic acid to prepare a pH 4 buffer. Record the mass in your data table
Answers
46.9 mg of solid sodium acetate is required to mix with 50.0 mL of 0.1 M acetic acid to prepare a pH 4 buffer. 470 mg of solid sodium acetate is required to mix with 50.0 mL of 1.0 M acetic acid to prepare a pH 4 buffer.
For Buffer A:
pH = 4.0
pKa = 4.74 (from the Ka of acetic acid)
[HA] = 0.1 M acetic acid = 0.1 mol/L
[A-] = unknown
Solving for [A-]:
pH = pKa + log([A-]/[HA])
4.0 = 4.74 + log([A-]/0.1)
-0.74 = log([A-]/0.1)
0.069 = [A-]/0.1
[A-] = 0.0069 M
Now that we know the concentration of sodium acetate required, we can calculate the mass of solid sodium acetate needed:
moles of \(NaC_2H_3O_2\)= [A-] x volume of solution
moles of \(NaC_2H_3O_2\)= 0.0069 mol/L x 0.05 L
moles of \(NaC_2H_3O_2\)= 0.000345 mol
mass of \(NaC_2H_3O_2\)= moles of \(NaC_2H_3O_2\) x FW of \(NaC_2H_3O_2\)
mass of \(NaC_2H_3O_2\)= 0.000345 mol x 136.08 g/mol
mass of \(NaC_2H_3O_2\)= 0.0469 g or 46.9 mg
For Buffer B:
pH = 4.0
pKa = 4.74 (from the Ka of acetic acid)
[HA] = 1.0 M acetic acid = 1.0 mol/L
[A-] = unknown
Solving for [A-]:
pH = pKa + log([A-]/[HA])
4.0 = 4.74 + log([A-]/1.0)
-0.74 = log([A-]/1.0)
0.069 = [A-]/1.0
[A-] = 0.069 M
Now that we know the concentration of sodium acetate required, we can calculate the mass of solid sodium acetate needed:
moles of \(NaC_2H_3O_2\)= [A-] x volume of solution
moles of \(NaC_2H_3O_2\)= 0.069 mol/L x 0.05 L
moles of \(NaC_2H_3O_2\)= 0.00345 mol
mass of \(NaC_2H_3O_2\)= moles of \(NaC_2H_3O_2\)x FW of \(NaC_2H_3O_2\)
mass of \(NaC_2H_3O_2\)= 0.00345 mol x 136.08 g/mol
mass of \(NaC_2H_3O_2\)= 0.470 g or 470 mg
pH is a measure of the acidity or basicity of a solution, commonly used in chemistry. It stands for "potential of hydrogen" and is defined as the negative logarithm of the hydrogen ion concentration in a solution. A solution with a pH of 7 is considered neutral, while a solution with a pH less than 7 is considered acidic, and a solution with a pH greater than 7 is considered basic.
The pH scale ranges from 0 to 14, with 0 being the most acidic and 14 being the most basic. Each unit on the scale represents a tenfold difference in the hydrogen ion concentration. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5. The pH of a solution can be measured using a pH meter or pH paper.
To learn more about pH visit here:
brainly.com/question/491373
#SPJ4
How many electrons are in the second shell of an atom of this element 7
N
15
O 2
O 5 O 7 O 15
Answers
The atomic number of nitrogen (N) is 7. Therefore, an atom of nitrogen has 7 electrons. There will be 5 electrons in the second shell of an atom of this element 7.
The electrons in an atom are distributed among its shells. The first shell, also known as the K shell, can hold a maximum of two electrons, while the second shell, also known as the L shell, can hold a maximum of eight electrons. To determine how many electrons are in the second shell of an atom of nitrogen, we need to write its electronic configuration.
The electronic configuration of nitrogen is 1s²2s²2p³. The first shell has two electrons (1s²) while the second shell has five electrons (2s²2p³). Therefore, there are 5 electrons in the second shell of an atom of nitrogen (N).
You can learn more about atomic numbers at: brainly.com/question/16858932
#SPJ11
3) Which of the following atoms has the smallest number of neutrons?
A) nitrogen-14
®) carbon-14
C) oxygen-16
D) neon-20
and third ene
Answers
Answer:
carbon-14..............
The atom which has the smallest number of neutrons is carbon-14 as it has the least atomic number.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ6
Which phrase best describes igneous rocks?
form when lava and magma cool
accumulate sediments following erosion
require heat and pressure to be formed
consist of many sediment layers
ctivity
Answers
Answer:
A) form when lava and magma cool
please give me brainliest :p
God bless!
Answer:
A: form when lava and magma cools
Explanation:
Got it right on Edge 2020 :)
Is my answer correct?

Answers
Yes the ANSWER is Correct-
On first half life the mass will be 10 gram
On second half life the mass will be 5 gram
On third half life the mass will be 2.5 gram
Gerald's science teacher mixed liquid X and liquid Y, both at room-temperature, in a large beaker. two beakers of clear solutions to the left of a right-pointed arrow, and a large beaker with a clear solution to the right of the arrow The mixture in the large beaker still looked clear like water, but when the students, one at a time, carefully touched the outside of the large beaker, it felt warm to the touch. Why did the large beaker most likely feel warm? A. The two liquids were not soluble in water. B. The release of a gas heated the solution. C. A chemical reaction produced a new substance. D. The energy of mixing warmed the liquids.
Answers
Answer:
c
Explanation:
A chemical reaction produced a new substance. In a chemical reaction, the atoms that make up the reactants are rearranged to produce various products.
What is chemical reaction?chemical reaction, the transformation of one or more chemicals (the reactants) into one or more distinct compounds (the products). Chemical elements or chemical compounds make up substances.
A chemical reaction is one in which two or more reactants will react to form a new substance. When new substance will form it will produce heat. Therefore, when science teacher was mixing two liquids it was generating heat.
Heating a substance allow molecules to move faster. When heat is given to the object it will move fast. When heat will apply to a solid it will turn into liquid. When heat is supplied to the liquid it will turn into gas.
Therefore, A chemical reaction produced a new substance. In a chemical reaction, the atoms that make up the reactants are rearranged to produce various products.
To learn more about chemical reaction, refer to the link:
https://brainly.com/question/22817140
#SPJ5