How do Δoctahedral splitting parameter (Δoct) and Δtetrahedral splitting parameter (Δtet) compare?
Answers
The Δoctahedral splitting parameter (Δoct) and Δtetrahedral splitting parameter (Δtet) are both used to describe the energy difference between the d-orbitals in transition metal complexes. The octahedral splitting parameter (Δoct) is generally larger than the tetrahedral splitting parameter (Δtet).
Δoct is the energy difference between the d orbitals in an octahedral complex, where the ligands are arranged around the metal ion in an octahedral shape. Δtet, on the other hand, is the energy difference between the d orbitals in a tetrahedral complex, where the ligands are arranged around the metal ion in a tetrahedral shape. In general, the relationship between these two parameters is Δtet ≈ 4/9 Δoct, with the octahedral splitting parameter being approximately 2.25 times larger than the tetrahedral splitting parameter.
The values of Δoct and Δtet are related, but they are not equal. Δtet is always smaller than Δoct because the ligands are closer to the metal ion in an octahedral complex, leading to a greater repulsion between the electrons in the d orbitals. This results in a larger energy difference between the d orbitals in an octahedral complex compared to a tetrahedral complex.
In summary, Δoct and Δtet are both used to describe the energy difference between the d orbitals in transition metal complexes, but Δoct is larger than Δtet due to the arrangement of the ligands around the metal ion.
Learn more about ligands here:
https://brainly.com/question/2980623
#SPJ11
Related Questions
How many significant figures are in 0.00102?
Answers
Answer:
3
Explanation:
102 are significant figures
Answer:
3 digits
Explanation:
The first nonzero digit is counted as a significant digit and the zero inbetween 2 nonzero digits count too.
Therefore, the significant digits are 0.00102.


At a certain temperature, 2.50 g Ca reacts completely in 30.0 seconds. The rate of consumption of Ca is
Answers
The rate of consumption of Ca is 0.0833 g/s.
The rate of consumption of Ca can be determined by dividing the mass of Ca consumed (2.50 g) by the time taken for the reaction to occur (30.0 seconds). This gives us a rate of 0.0833 g/s, indicating that 0.0833 grams of Ca are consumed every second during the reaction at the given temperature.
In chemical reactions, the rate of consumption or production of a substance is typically expressed in terms of the change in concentration over time. In this case, since the mass of Ca consumed is given, we can directly calculate the rate of consumption.
It's important to note that the rate of consumption of Ca may vary with temperature and other reaction conditions. The given rate applies specifically to the given temperature and the specific reaction conditions mentioned in the problem.
for such more questions on rate
https://brainly.com/question/24749252
#SPJ8
If 75.0 mg of potassium-42 was administered to a patient at 10 am on monday, how many mg will remain at 10 am on thursday of that same week? the half life of k-42 is 12 hours.
Answers
The half-life of potassium-42 is 12 hours, from Monday 10 am to Thursday 10 am, a total of 72 hours have passed (3 days).
Since each half-life is 12 hours, there are 6 half-lives in 72 hours. The amount remaining mg at 10 am on Thursday is approximately 2.34 mg.
To calculate the amount of potassium-42 remaining at 10 am on Thursday, we need to determine how many half-lives have passed since Monday. Since the half-life of potassium-42 is 12 hours, from Monday 10 am to Thursday 10 am, a total of 72 hours have passed (3 days).
Since each half-life is 12 hours, there are 6 half-lives in 72 hours.
To find the amount remaining, we can use the formula:
Amount Remaining = Initial Amount * (1/2)^(Number of Half-Lives)
Given that the initial amount is 75.0 mg, we can calculate the amount remaining as follows:
Amount Remaining = 75.0 mg * (1/2)^6
Calculating this, we find that the amount remaining at 10 am on Thursday is approximately 2.34 mg.
To know more about remaining mg visit:
https://brainly.com/question/20216163
#SPJ11
If you have 60 moles of HCl, what should the volume of solution be to make a 10 M solution?
Answers
Answer:10
Explanation:
Multiply the volume by the density to get the mass.
Divide the mass by the molar mass to get the number of moles.
Ocean currents bring warm from the equator towards earth?
Answers
Answer:Ocean currents act much like a conveyor belt, transporting warm water and precipitation from the equator toward the poles and cold water from the poles back to the tropics.
Explanation:
Answer:
Explanation:
Ocean currents act much like a conveyor belt, transporting warm water and precipitation from the equator toward the poles and cold water from the poles back to the tropics.
hope it helps!
12. What are two different ways to turn a turbine to generate electricity without using fossil
fuels?
HELPP
Answers
Answer:
two different ways are 1st by using water and 2nd by using wind these also not harm our environment
Based on this chart, what percentage of energy comes from fossil fuels?
Sources of Energy
Petroleum
37%
Other
1%
Natural Gas
24%
Renewable
Energy
7%
Coal
23%
Nuclear
Electric Power
8%
O A. 60%
OB. 23%
Ο Ο Ο Ο
C. 84%
O D. 37%
Answers
Answer:
37%+24%+23%=84% , i wish my answer is correct
write a net ionic equation to show why solid potassium hydroxide, koh (s), forms a basic solution when it dissolves in water.
Answers
a net ionic equation to show why solid potassium hydroxide, koH (s), forms a basic solution when it dissolves in water
Equation
KOH (s) [H2O ] → K+(aq)+OH−(aq)
The Arrhenius theory, the Brnsted-Lowry theory, and the Lewis theory are the three ideas that have contributed to the definitions of acids and bases over time. According to Arrhenius, an acid is a chemical that, when ionised, releases protons (hydrogen ions) into the solution, whereas a base releases hydroxide ions.
According to Brnsted-Lowry, a base is a proton acceptor and an acid is a proton giver. According to Lewis, a base is an electron-pair donor, while an acid is an electron-pair acceptor
KOH satisfies the Arrhenius theory's definition of a base by producing hydroxide ions when ionised. The hydroxide ion is the base component from a Brnsted-Lowry perspective since it can accept a proton to create water. Although it is a little more difficult to understand from a Lewis perspective why this is a base, the oxygen in the hydroxide has three pairs of non-bonding electrons on it. When a proton (acid) is present, it lacks the electrons necessary to create a covalent link, therefore hydroxide donates an electron pair to the proton in order to build a coordinate covalent bond, which produces water. The three theoretical requirements for a base are thus satisfied by the aforementioned net ionic equation.
To know more about acid,
https://brainly.com/question/25148363
#SPJ4
What is the molecular formula?
A) C₂H₂O
B) CO2
C) HCI
D) NaOH

Answers
Answer:
B) Co2 cuz in the diagram I see two similar atoms it might be of (oxygen) and the one atom of (carbon)
A calorimeter contains 39.1g of water at 22.0°C. When a sample of silver at 100°C was placed in it the final temperature was 27.0°C. Determine the mass of the silver sample
Answers
The mass of the silver sample is approximately 39.9 grams.
m_silver = (39.1 × 4.18 × (27.0 - 22.0)) / (0.235 × (27.0 - 100))
m_silver ≈ 39.9g
To determine the mass of the silver sample, we can use the principle of heat transfer and the specific heat capacity equation.
First, we need to calculate the heat lost by the silver and gained by the water using the equation:
Q (heat lost by silver) = Q (heat gained by water)
The formula for calculating heat is:
Q = m × c × ΔT
Where Q is the heat, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
The specific heat capacity of water is approximately 4.18 J/g°C, and for silver, it is 0.235 J/g°C.
The initial temperature of the water is 22.0°C, the final temperature is 27.0°C, and the mass of water is 39.1g.
Using the equation, we have:
(m_silver × c_silver × ΔT_silver) = (m_water × c_water × ΔT_water)(m_silver × 0.235 × (27.0 - 100)) = (39.1 × 4.18 × (27.0 - 22.0))
Solving this equation, we find:
m_silver = (39.1 × 4.18 × (27.0 - 22.0)) / (0.235 × (27.0 - 100))
m_silver ≈ 39.9g
Therefore, the mass of the silver sample is approximately 39.9 grams.
For more question on mass
https://brainly.com/question/28078240
#SPJ11
What is the best definition of solar radiation?Storing Storing of of energy energy in in Earth'Earth's s oceansoceansEnergy Energy from from the the Sun Sun that that reaches reaches EarthEarthMoisture Moisture and and heat heat energy energy in in the the atmosphereatmosphereIncreased Increased evaporation evaporation due due to to warm warm waterwater
Answers
Answer:The solar energy that we receive through solar radiation is directly or indirectly responsible for aspects as important to life as: Photosynthesis in plants; maintaining a planet temperature compatible with life. of the wind. The solar energy that reaches the earth's surface is 10,000 times greater than the energy currently consumed by all
Explanation:
identify the first step in preparing a spectrophotometer for use.
Answers
We have that the first step is to put the Power source into an on state,Thereby powering the Light point and the Spectrophotometer.
From the question we are told
identify the first step in preparing a spectrophotometer for use.
Generally
A SpectrophotometerThis is a device is out there to help scientist i the mostly in the field of chemistry.
This device is used to Know or arcertain particle with light consuming properties.
The Spectrophotometer is Mostly found in laboratories.
And usually in the use of a Spectrophotometer the first step is to put the Power source into an on state.Thereby powering the Light point and the Spectrophotometer
Therefore
The first step is to put the Power source into an on state,Thereby powering the Light point and the Spectrophotometer.
For more information on this visit
https://brainly.com/question/14379882
Plz help asap What can you conclude about the iron(ii) and iron(iii) ions?

Answers
Answer:
The chemistry of iron is dominated by the +2 and +3 oxidation states i.e. iron(II) and iron(III) complexes e.g. Fe2+ and Fe3+ complex ions with selected ligands, usually of an octahedral shape, a few tetrahedral iron(III) complexes are mentioned too. The reactions of the aqueous ions iron(II) and iron(III) with ammonia, sodium hydroxide and sodium carbonate are described and explained as are complexes of iron(III) with the chloride ion and cyanide ion.
principal oxidation states of iron, redox reactions of iron, ligand substitution displacement reactions of iron, balanced equations of iron chemistry, formula of iron complex ions, shapes colours of iron complexes, formula of compoundsExplanation:
A wave's frequency is 3.60x10^14 s^-1
What is the wavelength?
__ nm
Answers
Answer: 9
Explanation:
2. Use Avogadro's number to determine the number of particles found in 20.0 g of NaCl. Show your
work. (3 pts)
Answers
Answer:
That is the correct explaination for the question

Calculate molar solubility in mol/l and solubility in g/l of sodium stearate (c17h35coona) in pure water.
Answers
The molar solubility of sodium stearate in pure water is 0.00163 mol/L, and the solubility is 0.5 g/L.
To calculate the molar solubility and solubility of sodium stearate (C17H35COONA) in pure water, we need to consider the balanced equation for its dissolution and use the given molecular formula and molar mass.
First, let's write the balanced equation for the dissolution of sodium stearate in water:
C17H35COONA(s) ⇌ C17H35COO−(aq) + Na+(aq)
The molar solubility is defined as the number of moles of solute (sodium stearate) that dissolve per liter of solvent (water). To calculate it, we need to determine the concentration of the sodium stearate ions in the saturated solution.
The molecular formula of sodium stearate (C17H35COONA) indicates that for every molecule that dissolves, one C17H35COO− ion and one Na+ ion are produced. Therefore, the concentration of both ions will be the same in the saturated solution.
To find the molar solubility, we need to calculate the concentration of either the C17H35COO− ion or the Na+ ion. Let's use the C17H35COO− ion.
The molar mass of sodium stearate (C17H35COONA) is 306.44 g/mol.
Now, we can calculate the molar solubility:
Molar solubility = (mass of sodium stearate / molar mass of sodium stearate) / volume of water
Since we are working with pure water, the volume is 1 liter (1000 mL).
To find the solubility in grams per liter (g/L), we multiply the molar solubility by the molar mass of sodium stearate.
Example calculation:
If the mass of sodium stearate dissolved is 0.5 grams, the molar solubility would be:
Molar solubility = (0.5 g / 306.44 g/mol) / 1 L = 0.00163 mol/L
Solubility = 0.00163 mol/L * 306.44 g/mol = 0.5 g/L
Learn more about molar solubility here:-
https://brainly.com/question/31043999
#SPJ11
Sodium-24 has a half-life of 15 h. How many hours is three half-lives?
a. 60 h
b. 45 h
c. 30 h
d. 15 h
e. 7.5 h
Answers
So if we want three half lives we multiply 15 by 3
The number of hours in three half lives is 45
Answer:
(B)
Explanation:
If 1 half-life is 15 hrs, then 3 half-lives = 3×15 = 45
Therefore, there are 45 hrs in 3 half-lives (b)
which four territories provide the ""bottomless reserve of cheap labor"" (187)? what is ironicabout the exploited labor (188)
Answers
The four territories that provide the "bottomless reserve of cheap labor" were China, India, southeast Asia and parts of Africa. The irony of the exploited labor is that despite being the backbone of the global economy and contributing to the wealth of developed countries.
The four territories that provide the "bottomless reserve of cheap labor" referred to in 187 are likely to be countries or regions in the developing world with large populations and low wages, such as China, India, Southeast Asia, and parts of Africa.
These territories are often exploited by multinational corporations seeking to maximize profits by outsourcing production to these regions where labor is cheap and often unprotected by strong labor laws or unions.
The irony of the exploited labor is that despite being the backbone of the global economy and contributing to the wealth of developed countries, the workers in these regions often live in poverty and face harsh working conditions. They are paid low wages, subjected to long hours, and lack basic benefits such as health insurance and job security. The irony lies in the fact that the workers who are contributing to the wealth and prosperity of others are often themselves unable to enjoy a decent standard of living.
To know more about Territory click here:
https://brainly.com/question/21152582#
#SPJ11
How does chemical activity change in Group 17?
Answers
Answer:
halogen is the name of group 17 on the table of elements
Answer:
As the atomic number increases, the atoms get bigger. Their chemical properties change just a little bit when compared to the element right above them on the table.
Explanation:
yes
i need a short paragraph (about two sentences) describing the geologic time scale.
Answers
The geologic time scale is the “calendar” for events in Earth history. It subdivides all time into named units of abstract time called—in descending order of duration—eons, eras, periods, epochs, and ages.
What are some examples which show that matter is made up of tiny particles?
Answers
The level of water does not increase after the addition of salt
true or false: nutrition is the acquisition of chemical substances by organisms for use as an energy source or as building blocks of cellular structures.
Answers
The statement " nutrition is the acquisition of chemical substances by organisms for use as an energy source or as building blocks of cellular structures" is true.
Nutrition is indeed the acquisition of chemical substances by organisms for use as an energy source or as building blocks of cellular structures. Organisms require various nutrients, including carbohydrates, proteins, fats, vitamins, minerals, and water, to carry out their metabolic processes, maintain growth and development, and support overall health and well-being.
These chemical substances obtained through nutrition are utilized by organisms to provide energy for cellular processes, such as respiration, synthesis of biomolecules, and movement. They also serve as building blocks for the construction and repair of cellular structures, including proteins, nucleic acids, lipids, and carbohydrates.
Nutrition is a fundamental process that ensures the availability of essential nutrients for organisms to carry out their life functions effectively. Different organisms have varied nutritional requirements depending on their specific metabolic needs, lifestyles, and physiological characteristics.
Learn more about nutrition from the link given below.
https://brainly.com/question/2044102
#SPJ4
Calculate the volume of oxygen produced at 298K and 100 kPa by the decomposition of 30 cm3 of 0.1 mol dm_3 H2O2.
Answers
2 H2O2 (aq) → 2 H2O (l) + O2 (g)
From this equation, we can see that for every 2 moles of hydrogen peroxide that decompose, 1 mole of oxygen is produced.
We can start by calculating the number of moles of H2O2 that are present in 30 cm3 of 0.1 mol dm-3 H2O2:
30 cm3 = 30/1000 dm3 = 0.03 dm3
number of moles of H2O2 = concentration × volume = 0.1 mol dm-3 × 0.03 dm3 = 0.003 moles
Since 2 moles of H2O2 decompose to produce 1 mole of O2, we can calculate the number of moles of O2 produced:
number of moles of O2 produced = 0.003 moles H2O2 × (1 mole O2 / 2 moles H2O2) = 0.0015 moles O2
Now we can use the ideal gas law to calculate the volume of O2 produced:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant (0.0821 L atm mol-1 K-1), and T is the temperature in Kelvin.
We can rearrange this equation to solve for V:
V = nRT / P
Plugging in the values, we get:
V = (0.0015 mol)(0.0821 L atm mol-1 K-1)(298 K) / (100 kPa)(101.325 kPa atm-1)
V = 0.0377 L
Therefore, the volume of oxygen produced at 298K and 100 kPa by the decomposition of 30 cm3 of 0.1 mol dm-3 H2O2 is 0.0377 L.
You are practicing classification by sorting pictures into groups based on the characteristics of the kingdoms. While you are sorting, your little brother asks why you placed the above picture in the fungi kingdom. Which of the following explanations should you give your brother?
A.
It belongs in the fungi kingdom because it is unicellular and heterotrophic
B.
It belongs in the fungi kingdom because it is multicellular and can perform photosynthesis
C.
It belongs in the fungi kingdom because it can perform photosynthesis and does not have cell walls
D.
It belongs in the fungi kingdom because it has cell walls and is heterotrophic
Answers
Answer: D
Explanation: fungi kingdom is Hetrotropiv and is unicellular
A solution of NaOH had a concentration of 20 g/dm3 What mass of NaOH would there be in 250 cm3 of the solution?
Answers
Answer:
5g NaOH
Explanation:

Question 4 of 10
What is the molarity of a solution of 3 moles (mol) of FeBr3 in 1/2 L water?
A.
3 mol/2L
B.
2L/3 mol
C.
0.5L/3 mol
D.
3 mol/0.5 L
SUBMIT
Answers
Answer:
B
Explanation:
Answer:
D. 3 mol/0.5 L
Explanation:
Just took the quiz
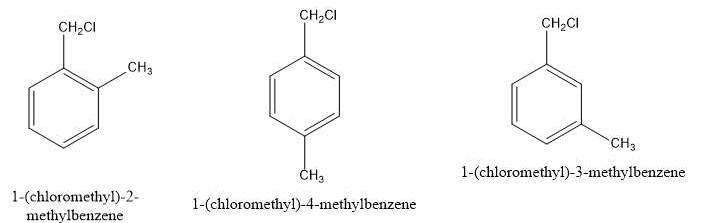
There are several aromatic compounds with the formula C8H9Cl. Draw those that have a disubstituted ring where the chlorine is attached to the ring. You do not have to consider stereochemistry. Draw one structure per sketcher. Add additional sketchers using the dropdown menu in the bottom right corner. Separate structures with + signs from the dropdown menu.
Answers
Draw the substituted ring structure in which the chlorine is attached to the ring. (seen the picture)
The benzene structure contains only 6 carbons and 6 hydrogens. The ring is replaced where the chlorine is attached to the ring. Therefore, the hydrogen-1 on the benzene ring is replaced by an ethyl group, and another hydrogen on the benzene ring is replaced by a chlorine group. Concerning the chlorine bond, there are 3 possible structures grouped called ortho, meta, and para.
Learn more about benzene structure at https://brainly.com/question/29692235
#SPJ4
The electrons between atoms in metallic bonds
allow for bonding metals to be reactive.
allow for bonding metals to be stable.
are stationary and provide durability to the metal.
are attracted to the neutrons of the metal.
Answers
Answer:
Metallic bonding is the type of chemical bonding that occurs between atoms of metals. In a metallic bond, atoms share their electrons in a way that allows them to form a “sea” of free electrons. This electron sea is responsible for the unique physical and electrical properties of metals.
Explanation:
Answer:
B. allow for bonding metals to be stable.
Explanation:
edge 2022
Tornadoes form so readily in Dixie Alley and Tornado Alley because different ___________________________ meet or collide here. In Tornado Alley, the ________, _________ air from Canada and the Rocky Mountains collides with ________, _________ air from the __________________________________. In Dixie Alley, the __________, _________ air from _______________________________ collides with the __________, _________ air from the _________________________________.
Answers
atmospheric conditions; warm, moist; hot, humid; Gulf of Mexico; cool, dry; Gulf Coast; warm, moist; Gulf of Mexico
Tornadoes are powerful, destructive columns of air that rotate rapidly and move across the ground. When warm, humid air and cold, dry air collide, they are created.Dixie Alley and Tornado Alley are where many atmospheric variables meet or clash, which explains why tornadoes originate here so easily. In Tornado Alley, hot, humid air from the Gulf of Mexico collides with warm, wet air from Canada and the Rocky Mountains. The Gulf Coast's cool, dry air and the Gulf of Mexico's warm, humid air meet in Dixie Alley.
learn more about Tornadoes Refer:brainly.com/question/28285636
#SPJ1
a 180.3 mci sample of a radioactive isotope is purchased by a medical supply house. if the sample has a half-life of 14 d, how long will it keep before its activity is reduced to 18.03 mci?
Answers
The symptoms of intense inflammation and shock occur in some gram-positive bacterial infections due to a group of toxins called superantigens. So the answer is a.
Superantigens are a type of toxin produced by some gram-positive bacteria that can cause an exaggerated immune response in the host. They are different from other bacterial toxins, such as A-B toxins, membrane-disrupting toxins, and lipid A, because they do not specifically target a particular cell type or receptor. Instead, they bind to the MHC-II molecules on antigen-presenting cells and to the T cell receptor, leading to the activation of a large number of T cells. They are able to activate a large number of T cells, which results in the release of a large amount of cytokines, such as interleukin-1, interleukin-2, and tumour necrosis factor. This can cause symptoms such as fever, nausea, vomiting, diarrhoea, and even shock.
Superantigens are different from other bacterial toxins, such as A-B toxins, membrane-disrupting toxins, and lipid A, because they do not specifically target a particular cell type or receptor. Instead, they bind to the MHC-II molecules on antigen-presenting cells and to the T cell receptor, leading to the activation of a large number of T cells. One example of a superantigen is the erythrogenic toxin produced by Streptococcus pyogenes, which causes scarlet fever. This toxin is responsible for the characteristic rash and fever seen in this disease.
To know more about Superantigens visit:
https://brainly.com/question/29453830
#SPJ11
The radioactive isotope will keep for 56 days before its activity is reduced to 18.03 mci.
The half-life of the radioactive isotope is 14 days, which means that every 14 days, the activity of the isotope will be reduced by half. Therefore, after the first 14 days, the activity of the isotope will be reduced to 90.15 mci (180.3/2). After another 14 days, the activity will be reduced to 45.075 mci (90.15/2).
After a total of 42 days (3 half-lives), the activity will be reduced to 10.03 mci (45.075/2). Finally, after 56 days (4 half-lives), the activity will be reduced to 18.03 mci (10.03/2).
It is important to consider the half-life of a radioactive isotope when working with it, as this information can be used to determine how long the isotope will remain active and at what point it may no longer be useful for its intended purpose.
For more information on radioactive isotope kindly visit to
https://brainly.com/question/28039996
#SPJ11