How can the crystal field splitting parameter Δoct be related to the wavelength of light absorbed in a transition metal complex?
Answers
Δoct represents the energy difference between the lower-energy d-orbitals (t2g) and the higher-energy d-orbitals (eg) in an octahedral complex.
The crystal field splitting parameter Δoct is related to the wavelength of light absorbed in a transition metal complex through the phenomenon of crystal field theory. In transition metal complexes, the metal ion is surrounded by a group of ligands that generate a crystal field, which causes the d-orbitals of the metal to split into two sets of orbitals. The energy difference between these two sets is represented by Δoct. When light is absorbed by a transition metal complex, an electron in one of the lower energy d-orbitals is excited to a higher energy d-orbital. The energy of the absorbed light corresponds to the energy difference between the two sets of d-orbitals, which is proportional toΔoct Therefore, the wavelength of light absorbed in a transition metal complex is directly related to the value of Δoct. As Δoct increases, the energy difference between the d-orbitals increases, and the absorbed wavelength shifts to the higher energy (shorter wavelength) end of the spectrum.
By using the equation λ = (hc) / Δoct, you can relate the crystal field splitting parameter (Δoct) to the wavelength of light absorbed in a transition metal complex.
To know more about crystal field splitting, click here
https://brainly.com/question/28304987
#SPJ11
Related Questions
a solution is made using 195.2 ml of methanol (density 0.792 g/ml) and 300.0 ml of water (density 1.000 g/ml). what is the molality methanol in water?
Answers
The molality of methanol in water is 16.105 mol/kg.
To calculate the molality of methanol in water, we first need to calculate the mass of methanol used in the solution.
Mass of methanol = volume x density = 195.2 ml x 0.792 g/ml = 154.6304 g
Next, we need to calculate the mass of water used in the solution.
Mass of water = volume x density = 300.0 ml x 1.000 g/ml = 300.0 g
Now, we can use the formula for molality:
Molality = moles of solute / mass of solvent in kg
To calculate moles of methanol, we first need to convert the mass of methanol to moles using its molar mass (32.04 g/mol).
Moles of methanol = 154.6304 g / 32.04 g/mol = 4.8316 mol
Next, we need to convert the mass of water to kg.
Mass of water in kg = 300.0 g / 1000 g/kg = 0.3 kg
Now we can calculate the molality:
Molality = 4.8316 mol / 0.3 kg = 16.105 mol/kg
Therefore, the molality of methanol in water is 16.105 mol/kg.
To know more about molality of methanol click here:
https://brainly.com/question/30527540
#SPJ11
Diamond is an allotrope of :
a. carbon b. sillica
c. phosphorus c. Nitrogen
Answers
Answer:
carbon
Explanation:
because it is an allotrope of carbon
Answer:
Carbon
Explanation:
its literaly carbon
How many km is 250 m?
Answers
a solution is prepared by mixing 694.0 ml of ethanol with 506.0 ml of water. the molarity of ethanol in the resulting solution is 10.32 m. the density of ethanol at this temperature is 0.7893 g/ml. calculate the difference in volume between the total volume of water and ethanol that were mixed to prepare the solution and the actual volume of the solution.
Answers
The difference in volume is -59.1 mL, indicating that the actual volume of the solution is slightly less than the initial volume of the water and ethanol that were mixed.
These are the following steps for calculation :-
First, we need to calculate the amount of ethanol in the solution:
moles of ethanol = molarity × volume of solution
moles of ethanol = 10.32 mol/L × 0.6940 L
moles of ethanol = 7.15488 mol
Next, we can calculate the mass of ethanol in the solution:
mass of ethanol = volume of ethanol × density of ethanol
mass of ethanol = 0.6940 L × 0.7893 g/mL
mass of ethanol = 0.547 grams
Now we can calculate the mass of water in the solution:
mass of water = volume of water × density of water
mass of water = 0.5060 L × 1 g/mL
mass of water = 0.5060 grams
The total mass of the solution is:
total mass = mass of ethanol + mass of water
total mass = 0.547 grams + 0.5060 grams
total mass = 1.053 grams
The density of the solution is:
density = total mass / total volume
total volume = total mass / density
total volume = 1.053 grams / (694.0 mL + 506.0 mL)
total volume = 1.053 grams / 1200 mL
total volume = 0.8775 g/mL
The difference in volume between the total volume of water and ethanol that were mixed to prepare the solution and the actual volume of the solution is:
difference in volume = actual volume - initial volume
difference in volume = 1 / 0.8775 - (694.0 mL + 506.0 mL)
difference in volume = 1.1409 L - 1200 mL
difference in volume = -59.1 mL
Therefore, the difference in volume is -59.1 mL, indicating that the actual volume of the solution is slightly less than the initial volume of the water and ethanol that were mixed.
To know more about volume visit :-
https://brainly.com/question/463363
#SPJ1
Flammable materials, like alcohol, should never be dispensed or used near Group of answer choices an open door. a sink. another student. an open flame.
Answers
Answer:
An open flame. Hope this helped! :)
What volume of water can be boiled by 3.0 kJ of energy? (Refer to table of
constants for water.)
A. 3.0 kJ x
1 mol
x 18.02 g/mol
1 mL
= 13 mL
1 g
4.186 kJ
B. 3.0 kJ x 1 mol
* 18.02 g/mol
6.03 kJ
1 mL
= 9.0 mL
1 g
C. 3.0 kJ x
1 mol
x 18.02 g/mol
(-285.83 kJ)
1 mL
1g
= 0.19 mL
1 mL
D. 3.0 kJ x
1 mol
40.65 kJ
* 18.02 g/mol
= 1.3 mL
SUBMIT
Answers
Answer: 3.0 kJ × 1 mol/40.65 kJ× 18.02 g/mol × 1 mL/1 g= 1.3 mL
Calculate the atomic mass of Nitrogen if the two common isotopes of Nitrogen have masses of 31.972 amu (85.00 % abundance) and 33.96
amu (15.00% abundance)
Answers
Explanation:
hope the picture helps you to understand:)
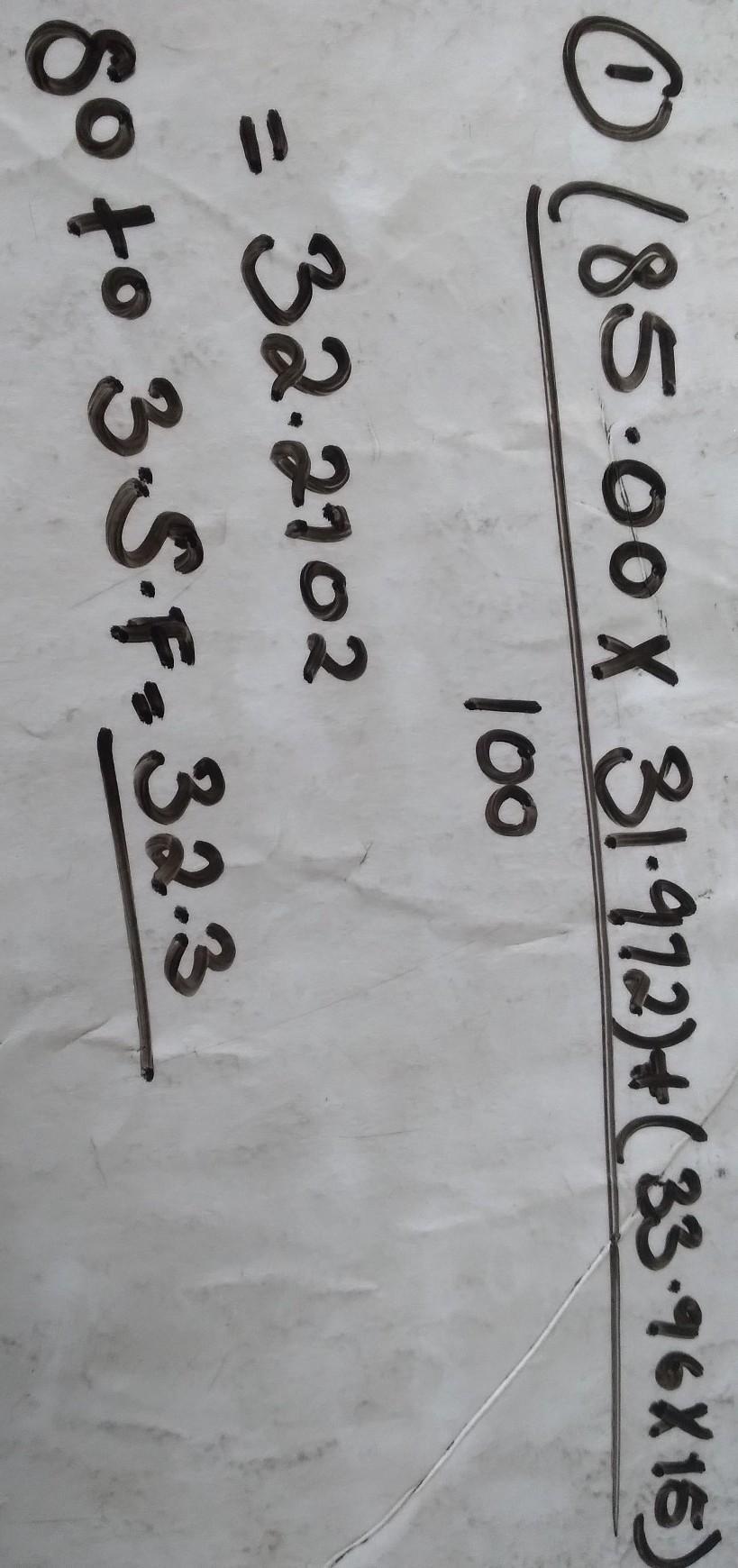
how to determine if a compound is polar or nonpolar
Answers
A molecule is nonpolar if it is symmetrical; that is, the electrons are distributed uniformly across the molecule. The polarity of a compound is determined by the electronegativity difference between its constituent atoms. When two atoms with significantly different electronegativities bond, they create a polar covalent bond.
A polar covalent bond is one in which electrons are not shared equally between the two atoms involved in the bond and, as a result, has a partial negative and a partial positive end. In a nonpolar covalent bond, however, the electrons are shared equally between the two atoms, and no partial charges are generated. Polar molecules are those that have an electronegativity difference between the constituent atoms, making them polar covalent molecules, whereas nonpolar molecules are those that lack a significant electronegativity difference between the atoms, resulting in nonpolar covalent molecules.
For example, O2, H2, and N2 are nonpolar because they contain identical atoms, whereas NH3, H2O, and CH3OH are polar because they have electronegativity differences between their constituent atoms.
To know more about electronegativity visit-
https://brainly.com/question/10531792
#SPJ11
how many atoms are centered on the [111] direction within the fcc unit cell?
Answers
In a face-centered cubic (fcc) unit cell, there are four atoms located at the corners of the unit cell and an additional atom located at the center of the unit cell.
These atoms are arranged in such a way that the [111] direction passes through the center of each face of the unit cell. Therefore, there are four atoms that are centered on the [111] direction within the fcc unit cell. This arrangement of atoms is characteristic of the fcc crystal structure, which is commonly found in metals such as aluminum, copper, and gold.
The [111] direction is an important crystallographic direction in fcc materials as it is the direction of densest packing of atoms and is often used as a reference direction in materials science.
To know more about face-centered cubic (fcc) unit cell refer here:
https://brainly.com/question/13002222
#SPJ11
A tree rooted into a hillide fall during a torm, the tree roll down the hill at a rate of 13m / how many km/hr i it traveling?
Answers
The speed of the fallen tree from the hill in km/hr would be 46.4 km/hr.
simple unit conversion is applied here so, The problem here is about converting the speed of an object from one unit to another. Specifically, we are to convert from m/s to km/hr.
Speed is the function of distance and time. Thus, the distance needs to be converted to km from m and the time to hr from s.
13 m/s is equivalent to 13 m distance and 1 second time.
remember that:
1000 m = 1 km
13 m = 13/1000
= 0.013 km
3600 seconds = 1 hr
1 second = 1/3600
= 0.00028 hour
The speed of the fallen tree in km/hr will be then:
0.013/ 0.00028 = 46.4 km/hr
To know more about speed click here
brainly.com/question/7359669
#SPJ4
A gas has a pressure of 4.62 atm when its volume is 2.33 L. If the temperature remains constant, what will the pressure be when the volume is changed to 1.03 L? Express the final pressure in torrs.
Answers
The final pressure would be 7905.6 torr when the volume is changed to 1.03 L.
Boyle's law states that
At a steady temperature, the item of weight and volume of a gas is steady.
which implies
P1V1 = P2V2
where
P1 = starting weight
V1 = starting volume
P2 = ultimate weight
V2 = ultimate volume
Substituting the given values into equation (1), we get:
P1 = 4.62 atm
V1 = 2.33 L
V2 = 1.03 L
Rearranging the equation (1)
P2 = P1V1/V2 = (4.62 atm x 2.33 L)/1.03 L = 10.41 atm
To convert atm to torr, ready to utilize the conversion factor:
As we know that 1 atm = 760 torr
so that, the ultimate weight in torr would be:
P2 = 10.41 atm x 760 torr/atm = 7905.6 torr
P2 = 7905.6 torr
Hence, the ultimate weight would be 7905.6 torr when the volume is changed to 1.03 L.
to know more about gas pressure problems refer to this :
https://brainly.in/question/49500660
#SPJ4
How much H₂ is needed to react with 6.58 g of O₂ in this reaction?
H₂ + O₂ --> H₂O
Answers
For this reaction the balanced chemical equation is
2
H
2
+
O
2
→
2
H
2
O
The mole ratios are determined using the coefficients of the substances in the balanced chemical equation.
2
m
o
l
H
2
:
1
m
o
l
O
2
:
2
m
o
l
H
2
O
The mole ratio for
O
2
H
2
O
is:
2
m
o
l
H
2
:
1
m
o
l
O
2
2
m
o
l
H
2
1
m
o
l
O
2
or
1
m
o
l
O
2
2
m
o
l
H
2
1
m
o
l
O
2
:
2
m
o
l
H
2
O
1
m
o
l
O
2
2
m
o
l
H
2
O
or
2
m
o
l
H
2
O
1
m
o
l
O
2
I hope this was helpful.
hellpppppoppopppppppp

Answers
Answer:
C
Explanation:
it's always colder at night and close to night especially near water and that's when wind tends to blow the most
importance of mole ratio in solvey process
Answers
Answer:
Sry i accidently clicked on ''SAVE''.
U can remove it or report it...
Explanation:
what does it mean when a dynamic equilibruim has been reached
Answers
Answer:
Dynamic equilibrium only occurs in reversible reactions, and it’s when the rate of the forward reaction is equal to the rate of the reverse reaction.
Explanation:
Does this answer your question?
If I have one mole of sulfur, how many atoms would that be?
Answers
Answer:
Atoms of sulfur = 9.60⋅g32.06⋅g⋅mol−1×6.022×1023⋅mol−1
Explanation:
because the units all cancel out, the answer is clearly a number, ≅2×1023 as required.
Indicate which alcohol in each pair will undergo an elimination reaction more rapidly when heated with h2so4.
Answers
Indicate which alcohol in each pair will undergo an elimination reaction more rapidly when heated with H-SO CH2OH CH CH2OH он OH.
What is alcohol ?Ketones, aldehydes, and carboxylic acids can be produced when alcohol is oxidized. These functional groups can be used to additional processes; for instance, carboxylic acids can be used for esterification, while ketones and aldehydes can be applied to subsequent Grignard reactions. When an organic compound is oxidized, more bonds from carbon to oxygen (or another electronegative element, like a halogen), and possibly fewer links to hydrogen, are formed.
Secondary alcohols can be efficiently oxidized up to the ketone stage without rupturing carbon-carbon bonds. Except in extremely restricted circumstances, no more oxidation is visible. Tertiary alcohols cannot be oxidized at all without rupturing carbon-carbon bonds, in contrast to primary alcohols, which can be converted to aldehydes or further converted to carboxylic acids.
To learn more about alcohol from the link:
https://brainly.com/question/19048777
#SPJ4
9.Magnetization of iron is a physical change.
A true
B false
Answers
Answer:
This is true.
Explanation:
Magnetization of iron is a physical change and not a chemical change as there is no change of state, no change of temperature, no smell and no evolution of gas.
What type of Chemical reaction is taking place in this
equation? BaCl₂ + Na₂(SO4)→ Ba(SO4) + 2NaCl
O Synthesis Reaction
O Double Replacement Reaction
O Neutralization Reaction
O Decomposition Reaction
Answers
BaCl₂ + Na₂(SO4)→ Ba(SO4) + 2NaCl the type of chemical reaction is taking place in this equation is double replacement reaction. Therefore, option 2 is correct.
What do you mean by the double replacement reaction ?The double replacement reaction is also called as double displacement reaction.
A general formula for a double replacement reaction is written as follows: AB + CD ⇒ AD + BC.
Examples of double replacement reactions are sodium hydroxide reacts with ammonium chloride to produced sodium chloride, ammonium hydroxide. NaOH + NH₄Cl ⇒ NaCl + NH₄OH.
When barium chloride solution reacts with sodium sulphate solution, a white precipitate of barium sulphate and sodium chloride are produced. This is double replacement reaction.
Thus, option 2 is correct.
To learn more about the double replacement reaction, follow the link;
https://brainly.com/question/19267538
#SPJ9
what is the number of molecules in the compound 4CaCO3
Answers
Answer:
The compound 4CaCO3 consists of 4 molecules of calcium carbonate, which has the chemical formula CaCO3.
Atoms are the smallest unit of matter which we see around us and molecules are found to be the combination of these atoms. Here the number of molecules of calcium carbonate present is 4.
What are molecules?Molecules are defined as the combination of one or more atoms which are combined with the help of chemical bonds. These bonds can be covalent, ionic, metallic or co-ordinate bonds. There exists covalent bond between the hydrogen and oxygen atoms in water molecule.
A group of two or more than two atoms of the same or different elements that are chemically bonded is known as the molecule. In the molecule CaCO₃, there are 'Ca', 'C' and 'O' atoms. Here there are 4 molecules of CaCO₃.
The properties of the molecules correlate with their structures. For example the molecule water is bent structurally and therefore has a dipole moment.
To know more about molecules, visit;
https://brainly.com/question/29254782
#SPJ2
Pseudoscience is best defined as ____ science.
factual
false
fun
feedback-based
Answers
Answer:
false
Explanation:
Pseudoscience is a field of study where researchers claim to be scientific in their research and adopt some of the procedures of science, but fail to fulfill the criteria effectively.
what will most likely happen to the United States population if reproduction patterns remain the same over the next 50 years?
Answers
Answer:
Explanation:
Mass Extinction
what is the percentage by mass of cyclohexane in the mixture?
Answers
The mass of the mixture is 95.8 g and the percentage by mass of cyclohexane in the mixture is 81.33%.
To calculate the percentage by mass of cyclohexane in the mixture, you need to use the formula:
Percentage by mass = (mass of component / total mass of mixture) × 100
Given that you have a mixture of cyclohexane and water and the density of the mixture is 0.958 g/mL,
1. To determine the mass of the mixture, you need to know the volume of the mixture and the density of the mixture. Since the density of the mixture is given, you can use the following formula to determine the mass of the mixture:
mass of mixture = density of mixture × volume of mixture
The mass of the mixture is: mass of mixture = 0.958 g/mL × 100 mL = 95.8 g
2. Since the density of cyclohexane is given as 0.779 g/mL, you can use the following formula to determine the mass of cyclohexane:
mass of cyclohexane = density of cyclohexane × volume of cyclohexane = 0.779 g/mL × 100 mL = 77.9 g
3. Using the formula given above, you can calculate the percentage by mass of cyclohexane in the mixture:
percentage by mass of cyclohexane = (mass of cyclohexane / mass of mixture) × 100
percentage by mass of cyclohexane = (77.9 g / 95.8 g) × 100 = 81.33%
To learn more about cyclohexane click here https://brainly.com/question/17019157
#SPJ11
What do all cations have in common? Check all that apply.
A. positive
B. negative
C. metals
D. non-metals
Answers
Answer:
B
Explanation:
All cations have more protons than electrons so they always have a positive charge.
What volume of a 10. 00 mol/L acetic acid stock solution is required to make
775. 0 ± 0. 5 mLof a 2. 500 mol/L acetic acid solution? How much water must you
add to make this standard dilution?
Answers
Thus, the volume of water required is approximately (775.0 mL - 193.75 mL) ≈ 581.25 mL.
We are given the following information: the concentration of the stock solution (C1) is 10.00 mol/L, the volume of the stock solution (V1) is unknown, the concentration of the desired solution (C2) is 2.500 mol/L, and the volume of the desired solution (V2) is 775.0 ± 0.5 mL.
Using the dilution formula, C1V1 = C2V2, we can rearrange it to solve for V1. Thus, V1 = (C2V2) / C1. Substituting the given values, we have V1 = (2.500 mol/L * 775.0 mL) / 10.00 mol/L.
By performing the calculation, we find that V1 ≈ 193.75 mL. Therefore, approximately 193.75 mL of the 10.00 mol/L acetic acid stock solution is required to make the 2.500 mol/L acetic acid solution.
To determine the volume of water needed to make the standard dilution, we subtract the volume of the stock solution from the total desired volume.
Learn more about dilution formula here:
https://brainly.com/question/31598121
#SPJ11
2. When a bubble escapes form a sunken ship, it has a volume of 12.0ml at a pressure of 400.0 atm. and a temp.. of -3.00°C. It reaches the surface where the pressure is 1.10 atm. and the temperature is 27.0°C. What is its new volume? ( round the answer to nearest tens for sig figs)
Answers
Answer: 4848.22 mL
Explanation:
V2= P1V1T2/(T1 P2) = (400.00 X 12.0 X 300.15)/(270.15 x 1.10)
how many oxygen atoms are in 3.00 g of sodium dichromate, na2cr2o7?
Answers
There are approximately 0.15 oxygen atoms in 3.00 g of sodium dichromate (Na₂Cr₂O₇).
The formula for sodium dichromate is Na₂Cr₂O₇. It contains two sodium (Na) atoms, two chromium (Cr) atoms, and seven oxygen (O) atoms. The atomic mass of oxygen is 16, so the molar mass of oxygen is 16 g/mol.
Therefore, the molar mass of Na₂Cr₂O₇ can be calculated as:
Molar mass of Na₂Cr₂O₇ = 2(23) + 2(52) + 7(16)
Molar mass of Na₂Cr₂O₇ = 142 g/mol
To determine the number of oxygen atoms in 3.00 g of Na₂Cr₂O₇,
we first need to calculate the number of moles of Na₂Cr₂O₇ in 3.00 g:
moles = mass / molar mass
moles = 3.00 g / 142 g/mol
moles = 0.02113 mol
Now, we can use Avogadro's number to determine the number of oxygen atoms in 0.02113 mol of Na₂Cr₂O₇:
Number of oxygen atoms = 7 atoms/mol × 0.02113 mol
Number of oxygen atoms = 0.14891 atoms ≈ 0.15 atoms (rounded to two significant figures)
Therefore, there are approximately 0.15 oxygen atoms in 3.00 g of sodium dichromate (Na₂Cr₂O₇).
Learn more about the molar mass from the given link-
https://brainly.com/question/21334167
#SPJ11
Which of the following organisms would NOT be in the first trophic level of an energy pyramid?
A. dog
B. tree
C. algae
D. grass
Answers
this is because the first energy comes from plants bc they can make their own food— therefore, energy :)
The dog would not be in the part of first trophic level of an energy pyramid.
What is energy pyramid?Energy pyramid is defined as a model that show the flow of energy from one tropic or feeding level to next tropic or feeding level in an ecosystem.
There are basically three energy pyramid.
Pyramid of numberPyramid of biomassPyramid of energyTrophic level is defined as the source based on their nutrition or food, organisms occupy a specific place in a food chain.
First trophic level contains only green plants and producers. Their plants and product where consumed by second level of organisms.
Thus, the dog would not be in the part of first trophic level of an energy pyramid.
To learn more about energy pyramid, refer to the link below:
https://brainly.com/question/2837831
#SPJ5
Baking soda is a common household chemical that is :
acidic
basic
neutral
Answers
А
Newton's Third Law of Motion
states:
Edit
Move with text
